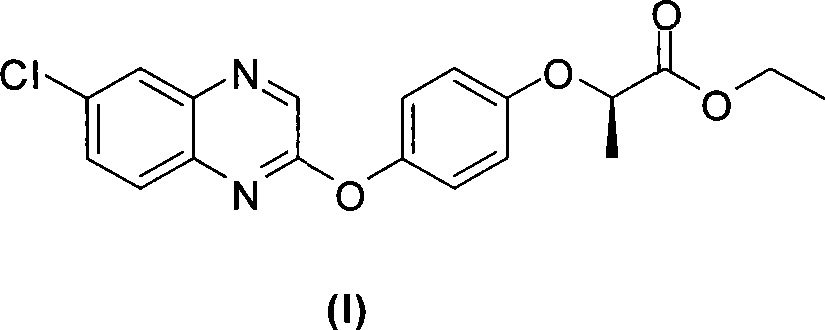
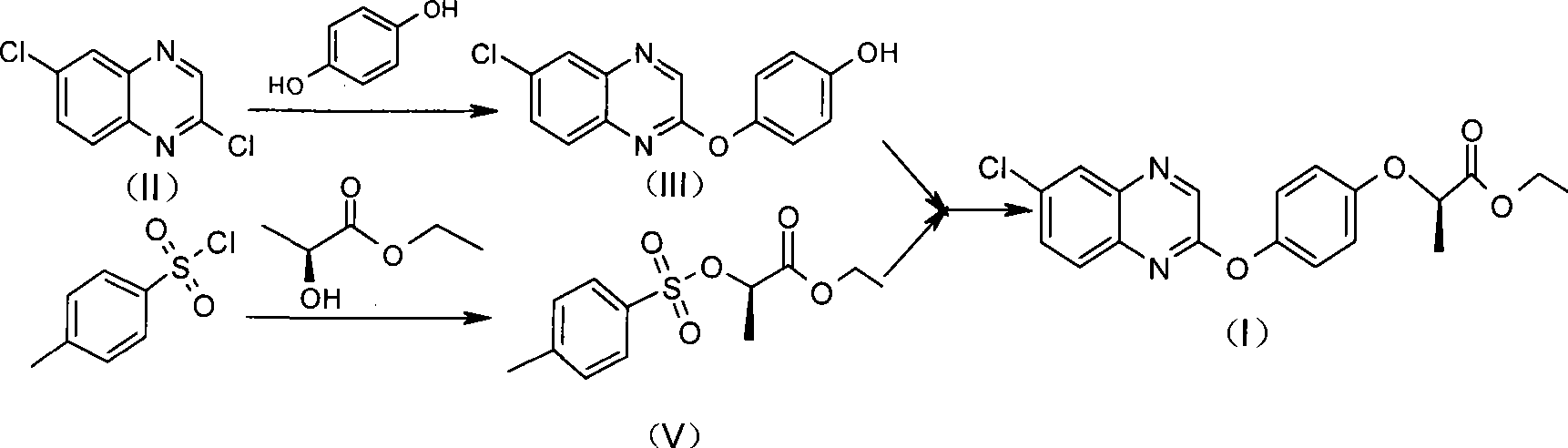
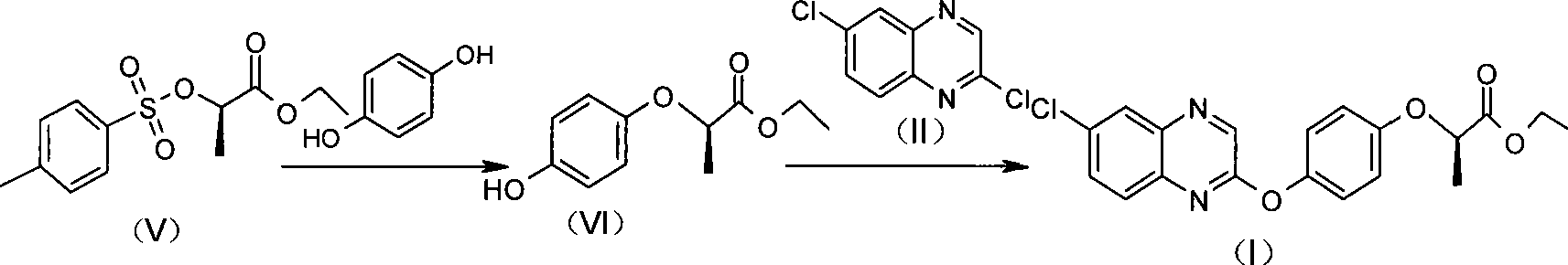
Preparation method of Quizalofop-p-ethyl with high optical content
An optical technology of quizalofop-p-ethyl is applied in the field of preparation of quizalofop-p-ethyl with high optical content, and can solve the problems of low yield, inevitable racemization, high toxicity of sulfate ester and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1 Preparation of quizalofop-p-ethyl with petroleum ether at 90°C-120°C as solvent
[0028] Under the environment of blowing nitrogen gas, take 50 mL of petroleum ether at 90°C-120°C and place it in a 100mL four-necked bottle, add 0.1g of tetrabutylammonium bromide, 3.7g (26.7mmol) of 200-300mesh potassium carbonate, 6.2 g (22.3mmol) 6-chloro-2-(4-hydroxyphenoxy) quinoline, 6.8g (24.5mmol) S (-) ethyl p-toluenesulfonyl lactate, reflux reaction for 6h. Cool down to 60°C, add 30g of water to wash, let stand to separate the liquid, remove the lower aqueous phase, add 1g of activated carbon to the organic phase, keep warm at 65°C-75°C for 1h, filter while hot, and the filtrate is precipitated under negative pressure to obtain a crude oily product 8.3g.
[0029] 30 g of absolute ethanol was added to the crude product for recrystallization to obtain 7.4 g of light yellow crystals with a purity of 99% and a yield of 87%.
[0030] mp=76°C-77°C, [α] 20 =+35.1 (EtOH, c=...
Embodiment 2
[0031] Example 2 Preparation of quizalofop-p-ethyl with petroleum ether at 60°C-90°C as solvent
[0032] Under a nitrogen atmosphere, take 60mL of petroleum ether at 60°C-90°C and place it in a 100mL four-neck flask, add 0.2g of triethylamine, 1.0g (11mmol) of 200-250mesh sodium bicarbonate, 1.5g (5.4 mmol) 6-chloro-2-(4-hydroxyphenoxy) quinoline, 1.9g (7.0mmol) S(-) ethyl p-toluenesulfonyl lactate, reflux for 7h. Cool down to 55°C, add 30g of water to wash twice, let stand to separate the liquid, separate the lower aqueous phase, and extract the aqueous phase with petroleum ether twice, each time with an amount of 20mL. Combine the organic phases, add 0.5 g of activated carbon, and keep warm at 65°C-75°C for 1h. Suction filtration while it was hot, and the filtrate was precipitated under negative pressure to obtain 2.0 g of crude oily product.
[0033]15 g of absolute ethanol was added to the crude product for recrystallization to obtain 1.8 g of light yellow crystals with ...
Embodiment 3
[0035] Example 3 Using naphtha as a solvent to prepare quizalofop-p-ethyl
[0036] Under a nitrogen atmosphere, take 50 mL of naphtha and place it in a 100 mL dry four-neck flask, add 4.3 g (12.9 mmol) of strontium carbonate of 200 mesh to 300 mesh, 3.0 g (11 mmol) of 6-chloro-2-(4 -Hydroxyphenoxy)quinoxaline, 3.3g (12mmol) S(-) ethyl p-toluenesulfonyl lactate. Heat to 100°C-105°C, keep the reaction for 8h, cool down to 90°C, add 20g of water and stir for 0.5h, let stand to separate layers, add 1g of activated carbon to the organic phase, keep stirring at 80°C-85°C for 1h. Suction filtration while it was hot, the filtrate was cooled to below 10°C, filtered, the filter cake was rinsed with cold toluene and ethanol, and dried to obtain 3.5 g of light yellow crystals with a yield of 85%. [α] D20 = +35.0, enantiomeric ratio R / S = 98.7 / 1.3 (optical purity 97.4% e.e.). Analysis conditions: chiral column: Chiralpak AS-H, 254nm; mobile phase: n-hexane / isopropanol=98 / 2).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com