Preparation method and application of modified cyclodextrin polymer hydrogel
A cyclodextrin polymer, hydrogel technology, applied in the directions of gel preparation, alkali metal compounds, chemical instruments and methods, etc., can solve the problems of small application range and low efficiency, and achieve convenient operation, wide application range, The effect of wide application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
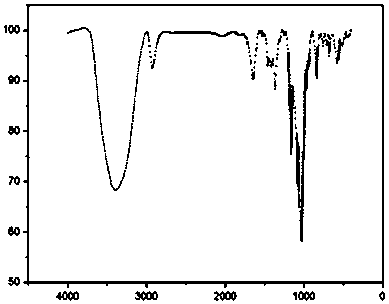
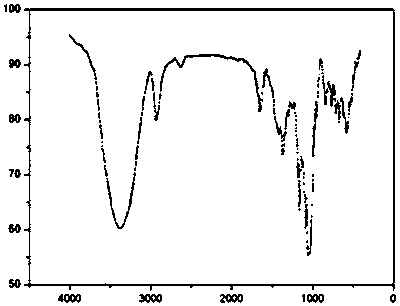
[0025] (1) Mix 8g (equivalent to 0.007mol) of β-cyclodextrin with 250mL of 0.45mol / L sodium hydroxide solution, stir and react in a water bath at 3°C for 5h, and add 1.1g (0.005mol) of p-toluenesulfonyl chloride every 1h, Add a total of 0.025 mol. After the reaction is completed, filter under reduced pressure, adjust the pH of the filtrate to 7.0 with 10% hydrochloric acid solution, crystallize at 3°C for 10 hours, filter under reduced pressure, recrystallize with deionized water, and dry under vacuum at 50°C to obtain the intermediate p-toluenesulfonate Acyl-β-cyclodextrin, infrared spectrum as shown in figure 1 shown.
[0026] (2) Mix 4g of p-toluenesulfonyl-β-cyclodextrin, 4.4g of thiourea, and 200mL of 95% ethanol aqueous solution, reflux at 70°C for 24 hours, add 150mL of 20% sodium hydroxide solution, reflux at 50°C for 5h, and 10% hydrochloric acid Adjust the pH of the filtrate to 2.0, recrystallize with deionized water, and then dry it in vacuum at 50°C to obtain ...
Embodiment 2
[0029] (1) Mix 8g (equivalent to 0.007mol) of β-cyclodextrin with 250mL of 0.6mol / L sodium hydroxide solution, stir and react in a water bath at 0°C for 5h, and add 2.2g (0.01mol) of p-toluenesulfonyl chloride every 1h, A total of 0.040 mol was added, after the reaction was completed, suction filtration under reduced pressure, 10% hydrochloric acid solution adjusted the pH of the filtrate to 6.0, crystallization at 0°C for 12 hours, suction filtration under reduced pressure, recrystallization with deionized water, and vacuum drying at 40°C to obtain the intermediate p-toluenesulfonate Acyl-β-cyclodextrin, infrared spectrum as shown in figure 1 shown.
[0030] (2) Mix 4g p-toluenesulfonyl-β-cyclodextrin, 4g thiourea, and 200mL 90% ethanol aqueous solution, reflux reaction at 65°C for 36h, add 150mL15% sodium hydroxide solution, reflux reaction at 50°C for 4h, 10% hydrochloric acid solution to adjust the pH of the filtrate to 3.0, recrystallize with deionized water, and then dr...
Embodiment 3
[0033] (1) Mix 8g (equivalent to 0.007mol), β-cyclodextrin and 250mL 0.8mol / L sodium hydroxide solution, stir in a water bath at 5°C for 3 hours, and add 2.2g (0.01mol) p-toluenesulfonyl chloride every 1h , add a total of 0.03mol, vacuum filtration after the reaction, 10% hydrochloric acid solution to adjust the pH of the filtrate to 8.0, crystallization at 5 ° C for 8 hours, vacuum filtration under reduced pressure, recrystallization with deionized water and vacuum drying at 45 ° C to obtain the intermediate p-toluene Sulfonyl-B-cyclodextrin, infrared spectrum as shown in figure 1 shown.
[0034] (2) Mix 4g of p-toluenesulfonyl-β-cyclodextrin, 8g of thiourea, and 200mL of 80% ethanol aqueous solution, reflux at 60°C for 48 hours, add 150mL of 10% sodium hydroxide solution, and reflux at 60°C for 3h, 10% Adjust the pH of the filtrate to 3.0 with hydrochloric acid solution, recrystallize with deionized water, and then dry it in vacuum at 40°C to obtain a mercapto-grafted β-cyc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com