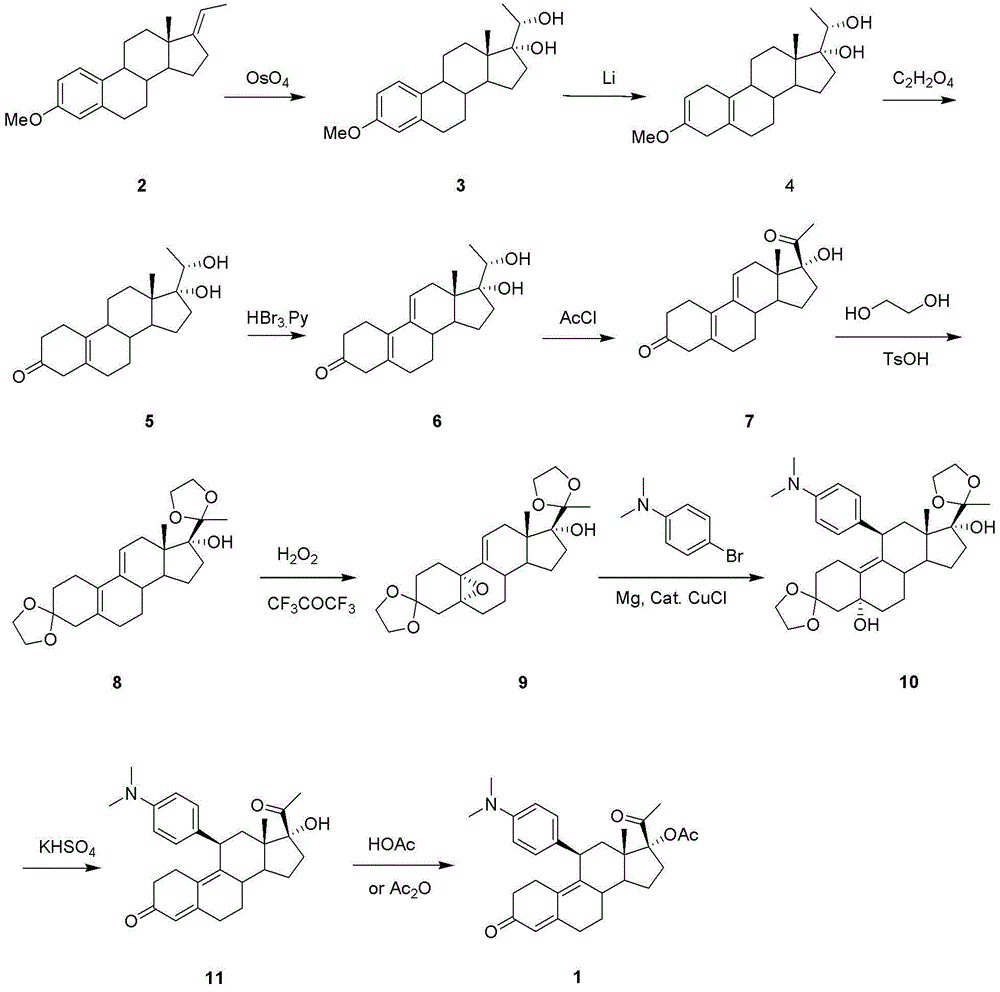
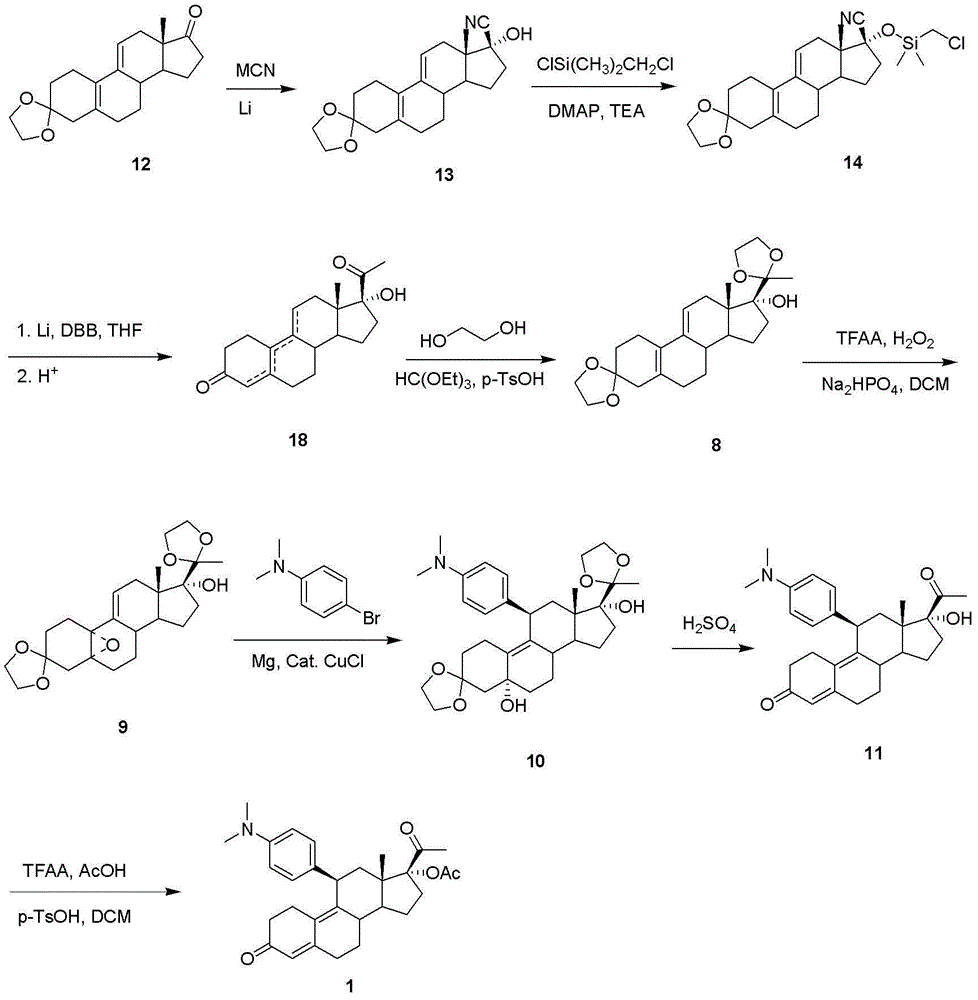
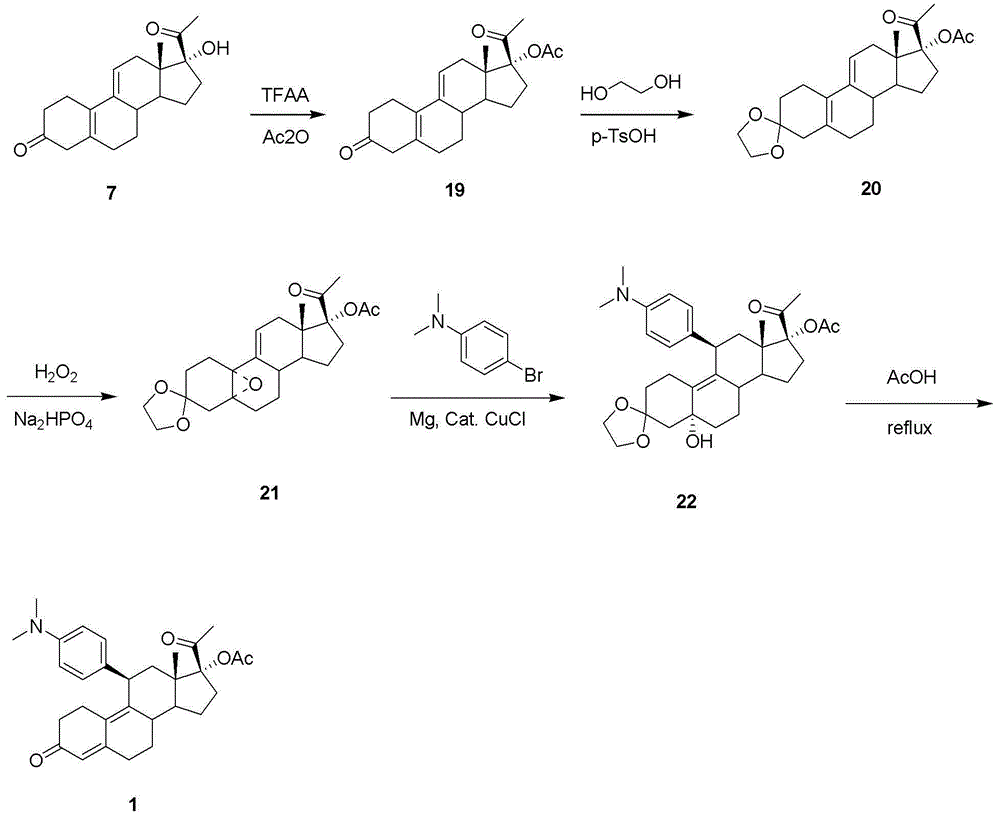
Synthetic method of ulipristal acetate
A technology of ulipristal acetate and a synthesis method, applied in the directions of steroids, organic chemistry, etc., can solve the problems of unsuitability for large-scale production, unfriendly reagents, troublesome post-processing, etc., and achieves low cost, operation and post-processing. Convenience and simple route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0035] The first step: the synthesis of compound 27
[0036] Add raw material 12 (3.14 g, 10 mmol) and potassium tert-butoxide (11.2 g, 100 mmol) to a mixed solvent of 150 ml ethylene glycol dimethyl ether and 63 ml tert-butanol at room temperature and stir to dissolve; Under nitrogen protection, a solution of p-toluenesulfonylmethyl isocyanide (3.9 g, 20 mmol) in 50 ml of ethylene glycol dimethyl ether was slowly dropped in, and the mixture was stirred at room temperature until the reaction was complete. After adding 100 ml of saturated ammonium chloride aqueous solution, the reaction solution was separated, and the aqueous layer was extracted once with ethyl acetate. The organic layers were combined, washed with water and brine respectively, dried over anhydrous magnesium sulfate, filtered and spin-dried to obtain a brown oily crude product, which was subjected to column chromatography to obtain light yellow oily compound 27 (2.5 g, yield 75%).
[0037] 1 HNMR (400MHz, CDC...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com