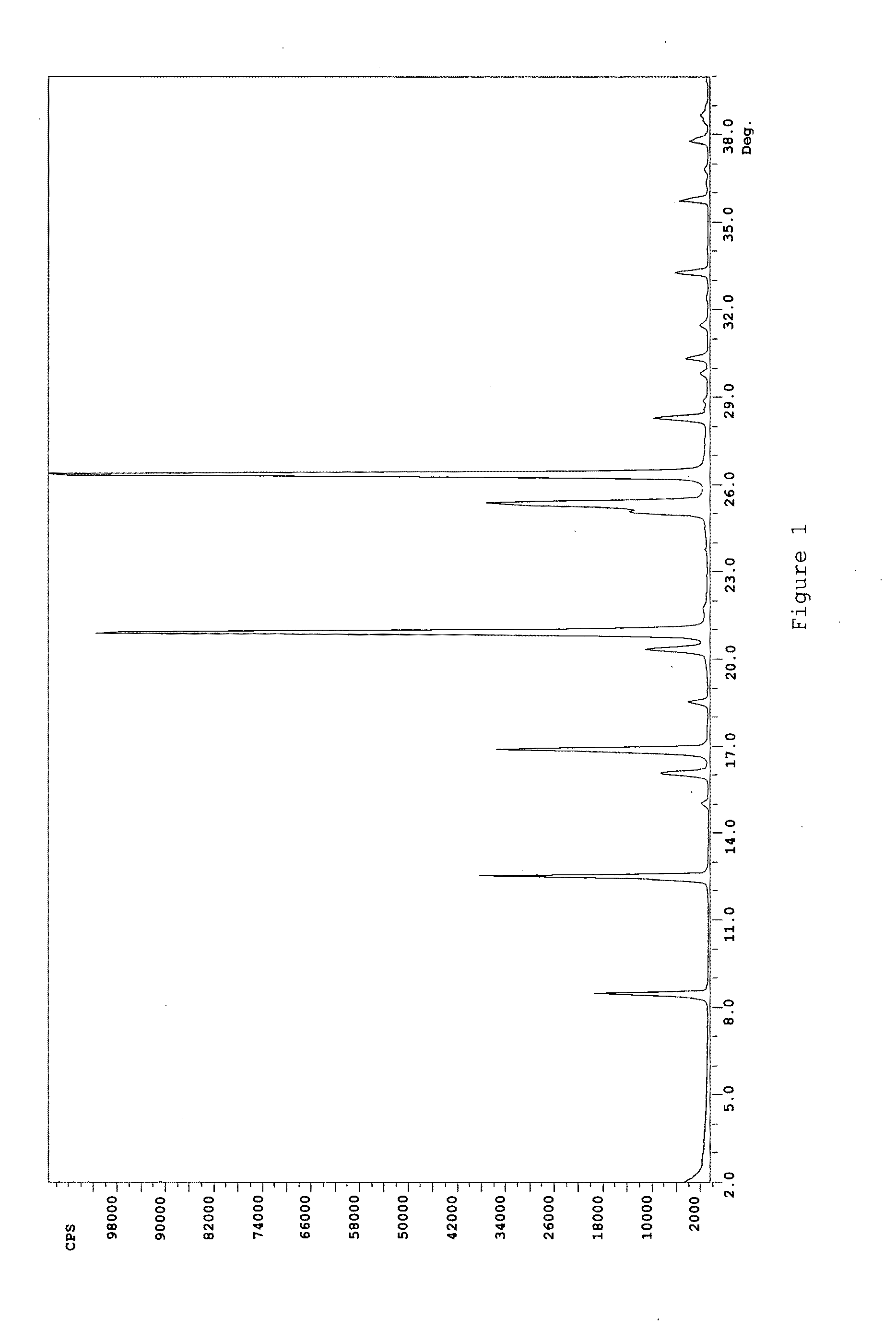
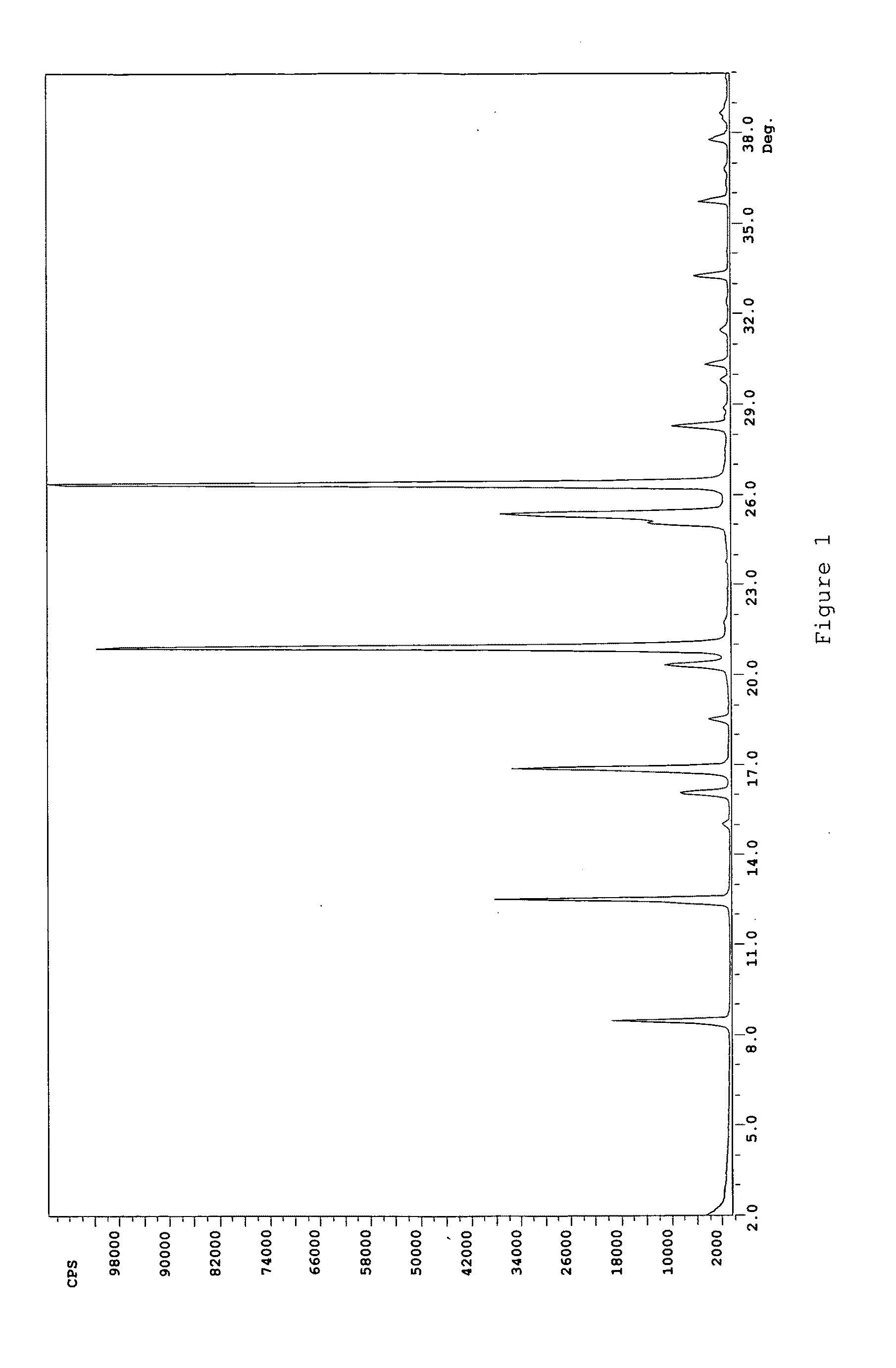
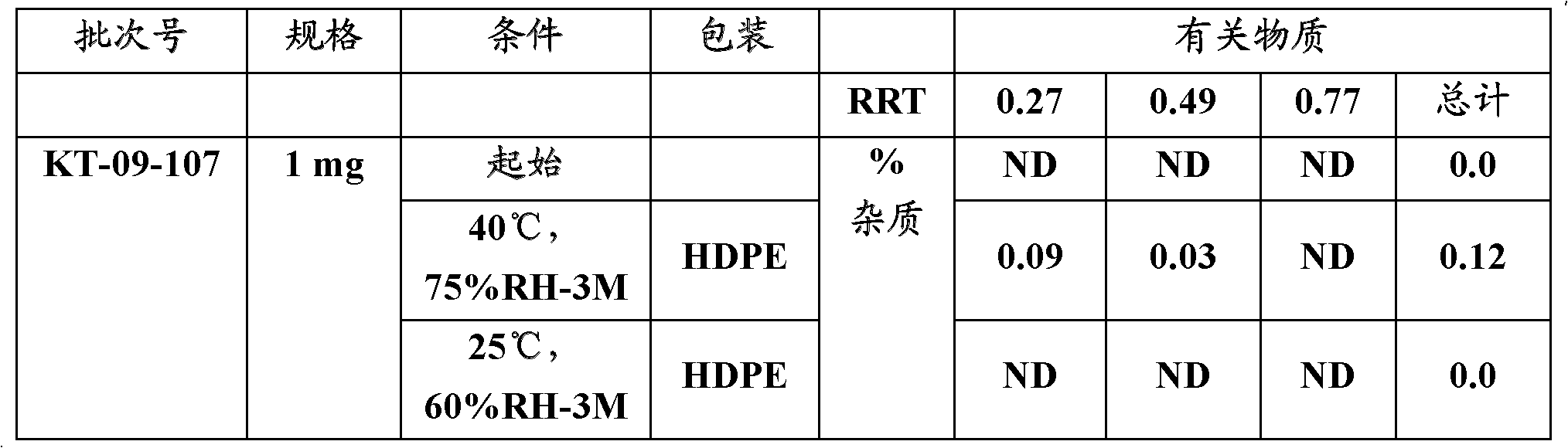
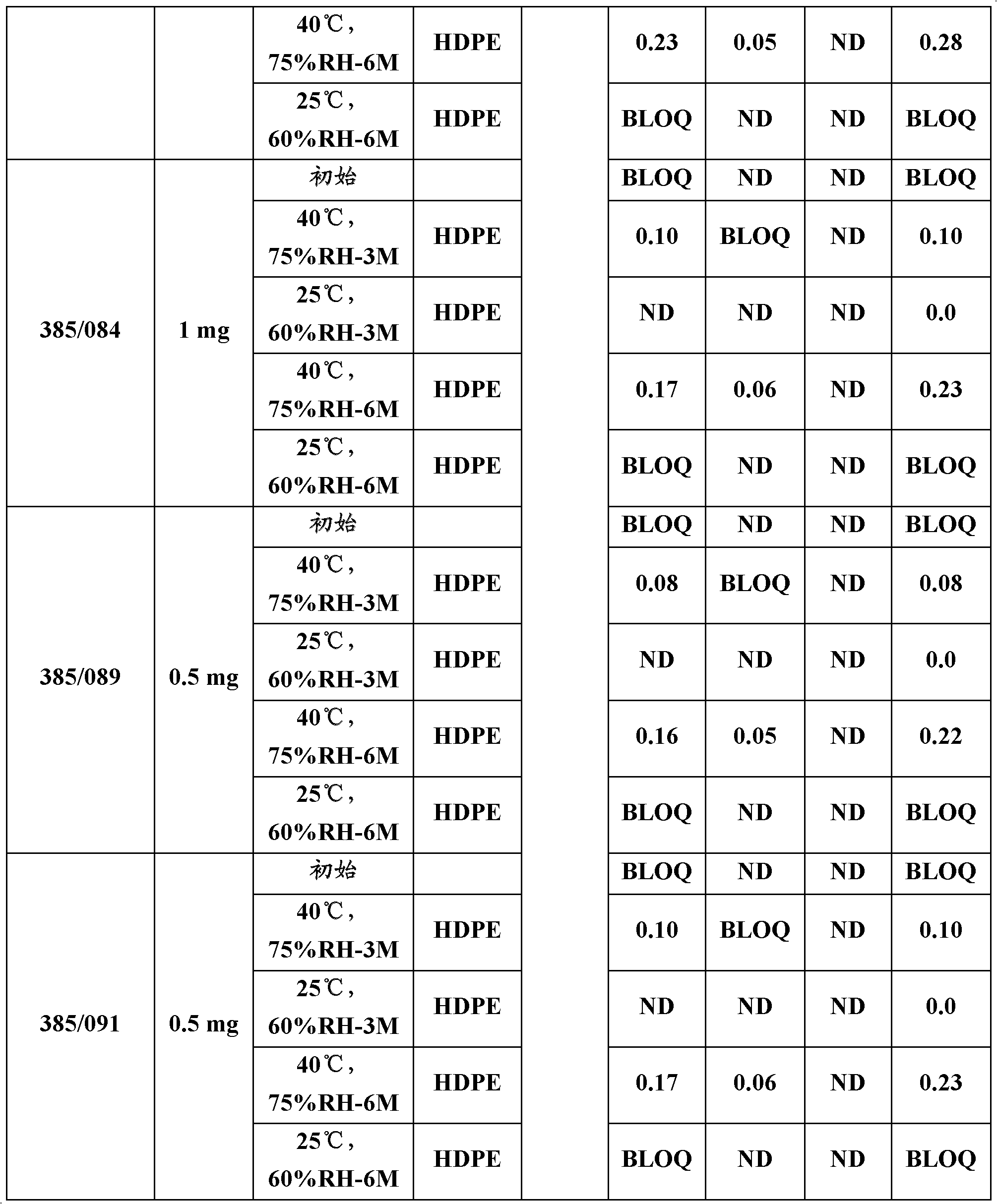
Patents
Literature
60 results about "1-aminoindan" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
From our library of Articles, Sigma-Aldrich presents HPLC Analysis of 1-Aminoindan Enantiomers on LARIHC™ CF6-P Keywords: Asymmetric synthesis, Chromatography, Clinical, Forensic, High performance liquid chromatography, Separation, Solvents, Supercritical fluid chromatography. Peer-Reviewed Papers. 15.
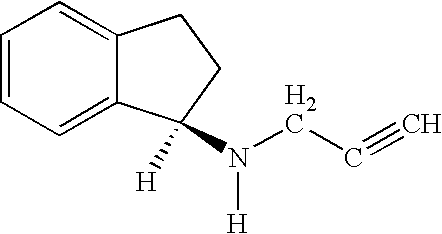
Pharmaceutical compositions comprising S-(-)-N-propargyl-1-aminoindan
InactiveUS6277886B1Enhance memoryBiocideOrganic active ingredientsBULK ACTIVE INGREDIENTNeurological disorder
Pharmaceutical compositions for the treatment of a neurological disorder of neurotrauma or for improving memory in a patient comprising a therapeutically effective amount of S-(-)-N-proparygl-1-aminoindan or a pharmaceutically acceptable salt thereof as active ingredient, and a pharmaceutically active carrier. The pharmaceutical compositions are adapted, in particular for treating a neurological hypoxia or anoxia, neurodegenerative diseases. Parkinson's Disease, Alzheimer's Disease, neurotoxic injury, head trauma injury, spinal trauma injury or any other form of nerve damage.
Owner:TECHNION RES & DEV FOUND LTD +1
Use of R-enantiomer of N-propargyl-1-aminoindan, salts, compositions and uses thereof
InactiveUS20060094783A1Avoid nerve damageBiocideOrganic active ingredientsMemory disorderAttention deficits
The subject invention provides methods of treating a subject afflicted with Parkinson's disease, memory disorder, depression, hyperactive syndrome, Attention Deficit Disorder, dementia, brain ischemia, stroke, head trauma injury, spinal trauma injury, neurotrauma, neurodegenerative disease, neurotoxic injury, multiple sclerosis, nerve damage, affective illness, schizophrenia or symptoms of withdrawal from an addictive substance, using the mesylate salt of R(+)-N-propargyl-1-aminoindan.
Owner:TEVA PHARMA IND LTD +1
Rasagiline formulations of improved content uniformity
ActiveUS20060188581A1Good content uniformityImprove uniformityOrganic active ingredientsBiocideN-propargyl1-aminoindan
Owner:TEVA PHARMA IND LTD
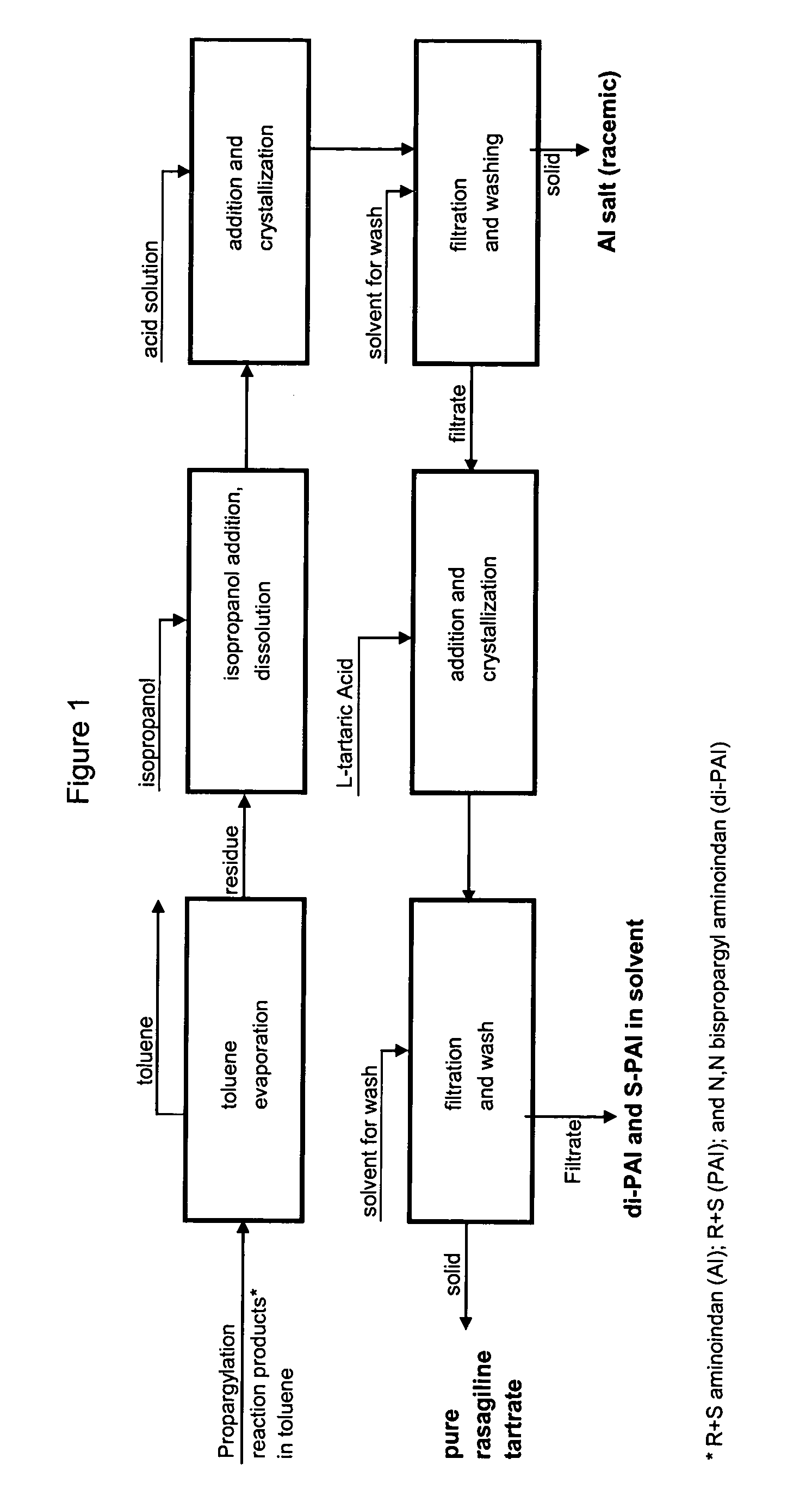
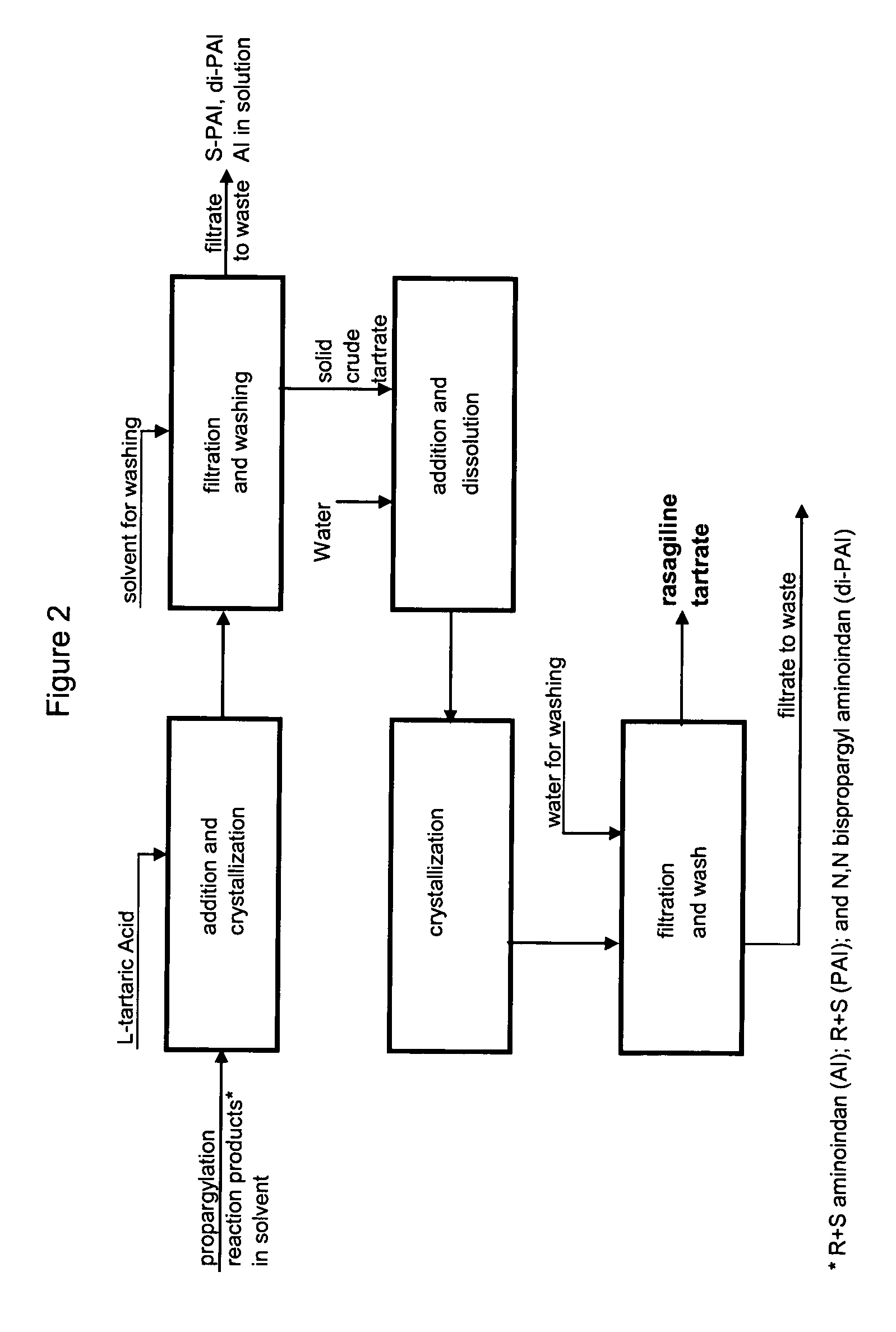
Methods for isolating propargylated aminoindans
A process for isolating from a reaction mixture a salt of a mono-propargylated aminoindan, a process for isolating from a reaction mixture a crystalline diastereomeric salt of a mono-propargylated aminoindan, and a process for isolating from a reaction mixture a salt of enantiomerically pure N-propargyl-1-aminoindan or a salt of enantiomerically pure 6-(N-methyl, N-ethyl-carbamoyloxy)-N′-propargyl-1-aminoindan. The corresponding products are also disclosed.
Owner:TEVA PHARMA IND LTD
Use of R (+) -N-propargyl-1-aminoindan to treat or prevent hearing loss
A method of treating or inhibiting hearing loss in a mammalian subject, comprising administering to the subject an amount of R(+)-N-propargyl-1-aminoindan or pharmaceutically acceptable salt thereof effective to treat or inhibit the hearing loss in the subject.
Owner:TEVA PHARMA IND LTD
Use of rasagilline for the treatment of restless legs syndrome
ActiveUS20070232700A1Effective treatmentRelieve symptomsBiocideNervous disorderN-propargylPediatrics
Disclosed are methods for the treatment of Restless Legs Syndrome comprising administering an amount of R(+)-N-propargyl-1-aminoindan or a pharmaceutically acceptable salt thereof.
Owner:TEVA PHARMA IND LTD
Process for purifying rasagiline base
Disclosed is crystalline R(+)-N-propargyl-1-aminoindan and racemic N-propargyl-1-aminoindan characterized by colorless crystals a pharmaceutical composition comprising the same, and the process for the manufacture and the validation thereof. Also disclosed is pure liquid R(+)-N-propargyl-1-aminoindan and a pharmaceutical composition comprising the same, and the process for the manufacture thereof.
Owner:TEVA PHARMA IND LTD
Rasagiline formulations of improved content uniformity
Disclosed are pharmaceutical preparations of R(+)-N-propargyl-1-aminoindan salts having enhanced content uniformity, processes for preparation of the compositions, and their uses.
Owner:TEVA PHARMA IND LTD
Crystalline solid rasagiline base
Owner:TEVA PHARMA IND LTD
Crystalline solid rasagiline base
InactiveUS20100145101A1Organic active ingredientsAmino compound purification/separationN-propargyl1-aminoindan
The subject invention provides crystalline R(+)-N-propargyl-1-aminoindan, pharmaceutical compositions and methods of manufacture thereof.
Owner:TEVA PHARMA IND LTD
Use of rasagiline for the treatment of progressive supranuclear palsy
A method for the treatment of Progressive Supranuclear Palsy. Such method includes administering to a subject an amount of R(+)-N-propargyl-1-aminoindan or a pharmaceutically acceptable salt thereof.
Owner:TEVA PHARMA IND LTD
Process for purifying rasagiline base
Disclosed is crystalline R(+)-N-propargyl-1-aminoindan and racemic N-propargyl-1-aminoindan characterized by colorless crystals a pharmaceutical composition comprising the same, and the process for the manufacture and the validation thereof. Also disclosed is pure liquid R(+)-N-propargyl-1-aminoindan and a pharmaceutical composition comprising the same, and the process for the manufacture thereof.
Owner:TEVA PHARMA IND LTD
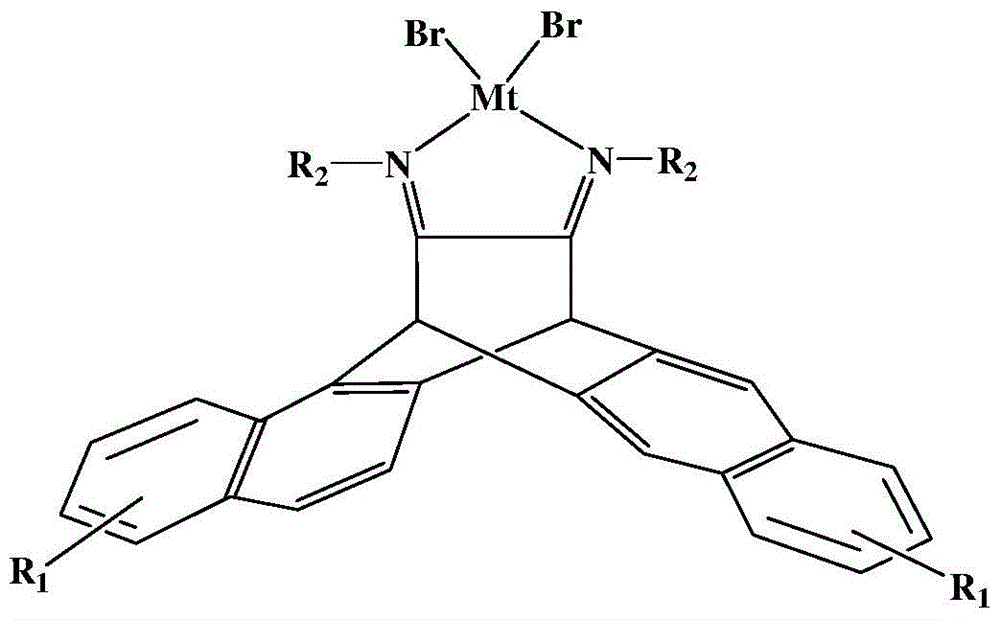
N,N-single ligand metal catalyst with three-dimensional structure and preparation method thereof
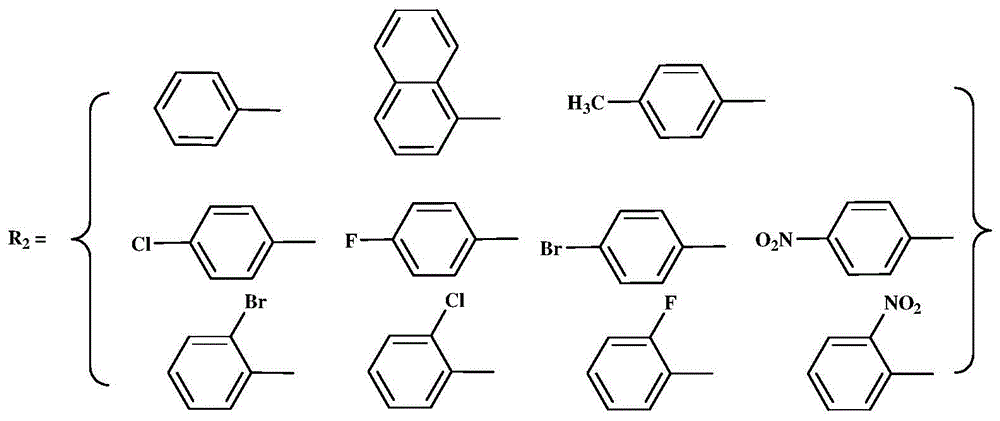
InactiveCN105936659AEasy to processGood light transmissionNickel organic compoundsDimethylaniline N-oxideP-Nitroaniline
The invention provides an N,N-single ligand metal catalyst with three-dimensional structure and a preparation method thereof. The catalyst has a structural general formula as below. Mt represents nickel, palladium, cobalt, iron or copper metal atom; R1 represents bromine, chlorine, iodine, phenyl group, propyl group, butyl group, phenyl group, naphthyl group, methoxy group or ethoxy group; when R2 is aromatic amine, it represents aniline, naphthylamine, p-methylaniline, p-chloroaniline, p-fluoroaniline, p-fluoroaniline, p-bromoaniline, p-nitroaniline, o-bromoaniline, o-chloroaniline, o-nitraniline, 2,6-dichloroaniline, 2,6-difluoroaniline, 2,6-dibromoaniline, 2,6-dinitroaniline, 2,6-dimethylaniline, 2,6-diisopropyl aniline, 2,4-difluoroaniline, 2,4-dibromoaniline, 2,4-dichloroaniline, 2,4,6-trichloroaniline, 2,4,6-tribromo aniline, 2,4,6-trifluoroaniline, 2,4,6-trinitroaniline, 6-hydroxynaphthylamine, 1-aminoindan, fluoreneamine or 4-nitro naphthylamine; and when R2 is aliphatic amine, it represents ethylamine, propylamine, butylamine, heptylamine, isopropylamine, tert-butylamine or cyclohexylamine. The preparation method is simple, less in by-products, and environmentally friendly.
Owner:YICHUN UNIVERSITY +1
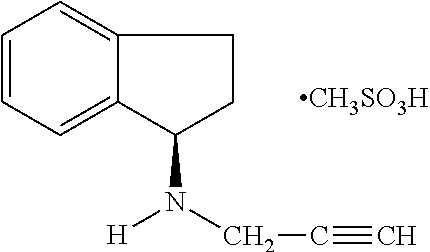
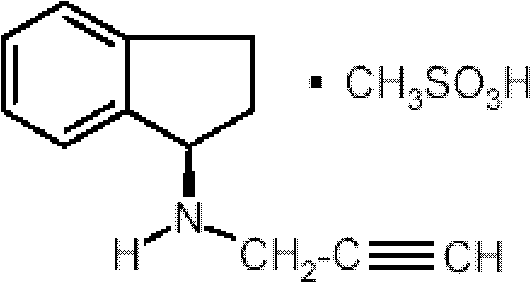
Pharmaceutical composition containing 1h-inden-1-amine, 2,3-dihydro-n-2-propynyl-(1R)-, methanesulfonate
A pharmaceutical composition comprising as an active ingredient a therapeutically effective amount of R(+)-N-propargyl-1-aminoindan mesylate thereof, and less than 50% by weight of hexahydric sugar alcohols.
Owner:SANDOZ AG
Compositions and methods for treatment of cardiovascular disorders and diseases
Propargylamine, propargylamine derivatives including N-propargyl-1-aminoindan and analogs thereof, and pharmaceutically acceptable salts thereof, are useful for prevention or treatment of cardiovascular disorders and diseases.
Owner:TECHNION RES & DEV FOUND LTD +1
Method for preparing S-1-aminoindane
InactiveCN105061219AReasonable choice of reaction processThe split method is simpleAmino compound purification/separationSolubilityAlcohol
The invention discloses a method for preparing S-1-aminoindane through separation. D-mandelic acid is taken as a chirality resolving agent, a mixed solution of alcohol and water is taken as a solvent, under the condition of heating reflux, 1-aminoindane is dropwise added and racemized, the 1-aminoindane is reacted with the D-mandelic acid to form diastereomeric salt, and the D-mandelate of the S-1-aminoindane is obtained through crystallization and separation according to different solubility of the diastereomeric salt; the S-1-aminoindane is obtained by conducting purification and alkalization on the D-mandelate; all the solutions containing the D-mandelate are mixed, the alcohol is removed through steaming, acidification is conducted on the solution, and the D-mandelate can be recycled. The method for preparing the S-1-aminoindane through the separation has the advantages of being mild in condition, easy to operate, high in product yield, high in optical purity, capable of recycling and reusing the resolving agent and the like, and the method is extremely suitable for industrial production of the S-1-aminoindane.
Owner:吴玲
Method for preventing or attenuating anthracycline-induced cardiotoxicity
InactiveUS20080090915A1Improve survivalReduce weightBiocideOrganic active ingredientsUltrasound attenuationN-propargyl
Propargylamine, propargylamine derivatives including N-propargyl-1-aminoindan, enantiomers and analogs thereof, and pharmaceutically acceptable salts thereof, are useful for prevention or attenuation of anthracycline-induced cardiotoxicity.
Owner:TECHNION RES & DEV FOUND LTD +1
Methods for treatment of cardiovascular disorders and diseases
ActiveUS20060287401A1Prevents hypertrophic increaseBiocideAmine active ingredientsN-propargyl1-aminoindan
Propargylamine, propargylamine derivatives including N-propargyl-1-aminoindan, enantiomers and analogs thereof, and pharmaceutically acceptable salts thereof, are useful for prevention or treatment of cardiovascular disorders, diseases and conditions.
Owner:TECHNION RES & DEV FOUND LTD +1
Process for the preparation of enantiomerically pure amines
ActiveUS20130303741A1High yieldEfficient and economicalAmino compound purification/separationNervous disorderMedicinal chemistry1-aminoindan
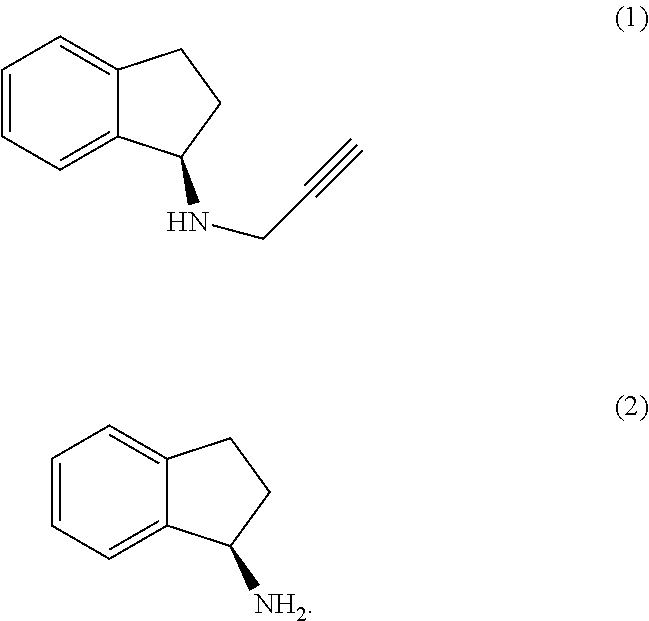
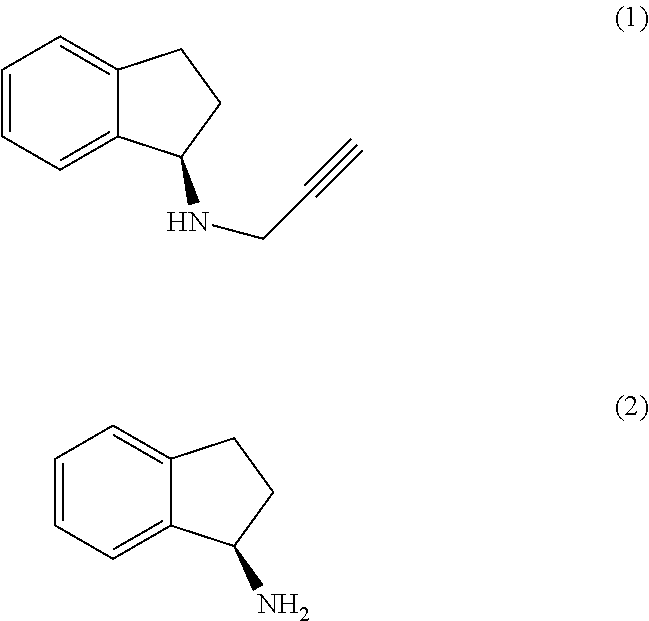
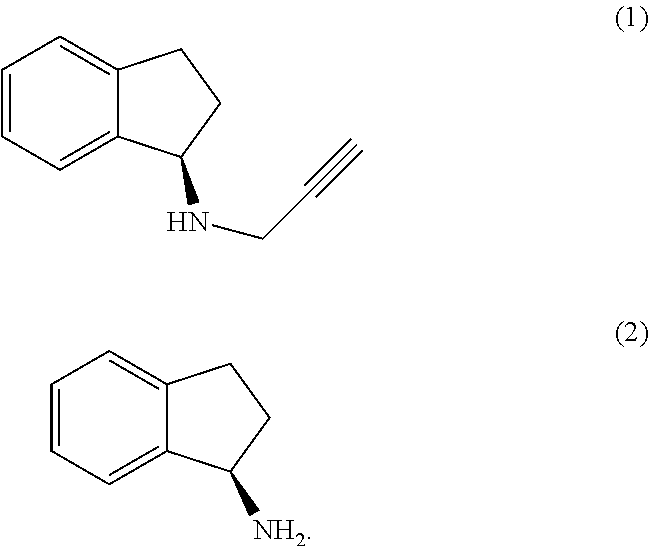
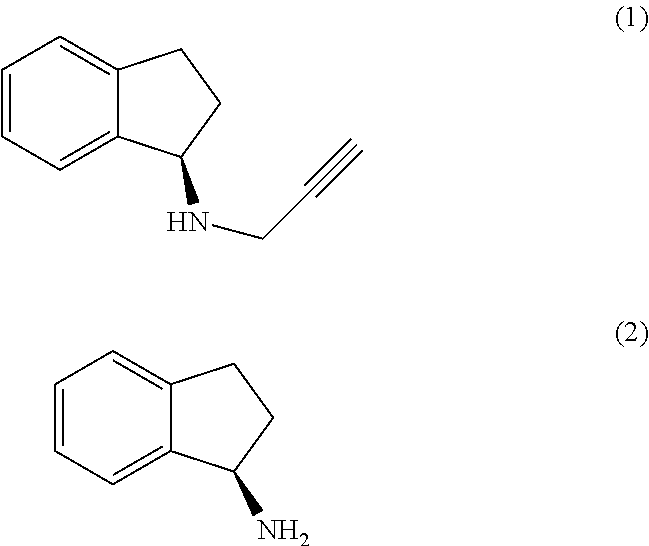
The present invention relates to an improved process for the preparation of (R)-1-aminoindan (2), rasagiline (1) and pharmaceutically acceptable salts of rasagiline.
Owner:GENERICS UK LTD
Process for the preparation of enantiomerically pure amines
InactiveUS8569545B2High yieldEfficient and economicalOrganic active ingredientsBiocideMedicinal chemistry1-aminoindan
The present invention relates to an improved process for the preparation of (R)-1-aminoindan (2), rasagiline (1) and pharmaceutically acceptable salts of rasagiline.
Owner:GENERICS UK LTD
Method for preparing (S)-6-hydroxy-1-aminoindane through dynamic kinetic resolution
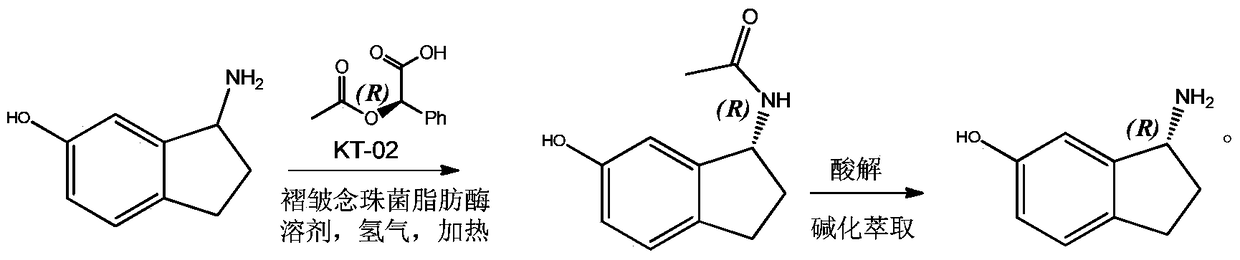
InactiveCN105017035AEasy to operateHigh optical purityOrganic compound preparationOptically-active compound separationAlkali freeHydrogen
The invention relates to a method for preparing (S)-6-hydroxy-1-aminoindane through dynamic kinetic resolution. According to the method, 6-hydroxy-1-aminoindane serves as the raw materials, fold candida lipase serves as a resolution catalyst, L-(+)-O-acetyl-Alpha-hydroxyphenylacetic acid serves as an acyl donor, KT-02 serves as a racemization catalyst, reaction is performed in a hydrogen environment, and the 6-hydroxy-1-aminoindane can be converted into an acetyl compound of the (S)-6-hydroxy-1-aminoindane. After the compound is purified, acid hydrolysis and alkali free operation are performed on the compound under the protection of nitrogen, then the (S)-6-hydroxy-1-aminoindane is obtained, and an ee value of the final product is above 99 percent. According to the method, the advantages that operation is easy, the racemization catalyst is low in price and easy to obtain, the raw materials can be completely utilized and the optical purity of the product is high are achieved, and great instruction and application value is achieved in the production preparation of the (S)-6-hydroxy-1-aminoindane.
Owner:SHANDONG PROVINCIAL HOSPITAL
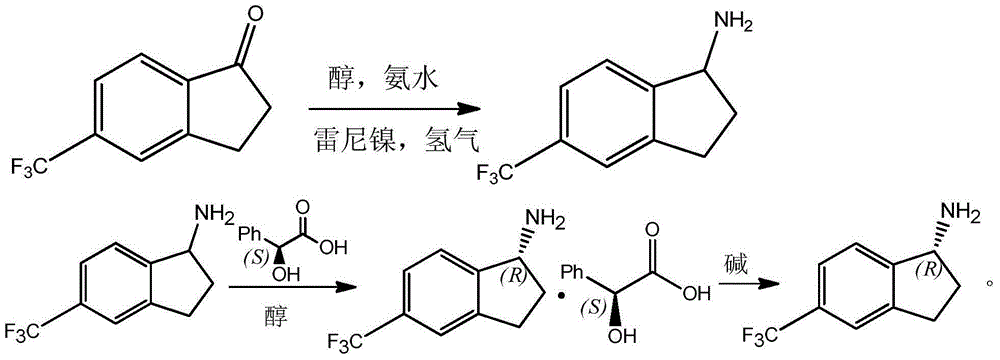
Preparation method for R-5-trifluoromethyl-1-aminoindane
InactiveCN105175270AHigh split efficiencyChemically stableAmino compound purification/separationPreparation by reductive alkylationSolventMandelic acid
The invention discloses a method for preparing R-5-trifluoromethyl-1-aminoindane from 5-trifluoromethyl-1-indanone. The method comprises the following concrete steps: catalyzing the 5-trifluoromethyl-1-indanone and carrying out reductive amination reaction in an alcohol solvent with the 5-trifluoromethyl-1-indanone as a raw material and raney nickel as a catalyst in the presences of ammonia gas and hydrogen so as to generate 5-trifluoromethyl-1-aminoindane; under the action of a resolving agent L-mandelic acid, allowing the obtained 5-trifluoromethyl-1-aminoindane to generate resolving-agent enantiomer salt of the R-5-trifluoromethyl-1-aminoindane; and carrying out salt recrystallization and alkaline treatment so as to obtain the R-5-trifluoromethyl-1-aminoindane, wherein the resolving agent L-mandelic acid contained in reaction residual liquid can be recovered through acidization treatment. The technical method provided by the invention has the advantages of simple operation, high resolution efficiency, easily recycled and reused resolving agent and resolving solvent, less environmental pollution and applicability to industrial production.
Owner:吴玲
New salt forms of an aminoindan derivative
The present invention relates generally to novel salt forms of R-(+)-N-propargyl-1-aminoindan (i.e. rasagiline base), to a compound of formula Ia, to processes for their preparation and isolation, and to pharmaceutical compositions comprising the same.
Owner:MEDICHEM
Use of rasagilline for the treatment of restless legs syndrome
Methods for the treatment of Restless Legs Syndrome using R(+)-N-propargyl-1-aminoindan or a pharmaceutically acceptable salt thereof.
Owner:TEVA PHARMA IND LTD
Method for preparing R-6-hydroxy-1-aminoindane through dynamic kinetic resolution
ActiveCN105087742AGuaranteed racemization abilityEnsure safetyFermentationCandida rugosaMandelic acid
The invention relates to a method for preparing R-6-hydroxy-1-aminoindane through dynamic kinetic resolution. The method is characterized by using candida rugosa lipase as a biological resolution catalyst, D-(-)-O-acetyl mandelic acid as an acyl donor and a non-crystal nickel catalyst KT-02 as a racemization catalyst to carry out one-pot dynamic kinetic resolution reaction in an autoclave in the presence of hydrogen to convert 6-hydroxy-1-aminoindane to an R-6-hydroxy-1-aminoindane acyl compound and carrying out acid hydrolysis, alkali freeing, and the like on the acyl compound to obtain R-6-hydroxy-1-aminoindane with ee (enantiomeric excess) value more than 99%. The method has the characteristics of simplicity in operation, cheap and accessible racemization catalyst, complete utilization of the raw materials, high optical purity of the product, and the like, and has great guidance and application values in production and preparation of R-6-hydroxy-1-aminoindane.
Owner:陈永军
Preparation of R-6-methoxy-1-aminoindane through resolution
InactiveCN105063162AEfficient use ofComplete utilization of raw materialsFermentationNickel catalystAcid hydrolysis
The invention discloses a method for preparing R-6-methoxy-1-aminoindane through dynamic kinetic resolution. The method comprises the following steps: using 6-methoxy-1-aminoindane as a raw material, and under co-catalysis of a biological resolution catalyst, namely, candida rugosa lipase, and a racemization catalyst, namely, a non-crystal nickel catalyst KT-02, conducting transesterification reaction on 6-methoxy-1-aminoindane and an acyl donor, namely, (R)-(-)-alpha-acetylmandelic acid, to generate an R-6-methoxy-1-aminoindane acyl compound, and conducting acid hydrolysis and alkali ionization on the acyl compound to obtain R-6-methoxy-1-aminoindane, wherein the optical purity of the finished product is greater than 99%. The method has the characteristics that the operation is simple, the racemization catalyst is cheap and easy to obtain, the raw material utilization is complete, and the optical purity of the product is high. The method has great guidance and application values in preparation of R-6-methoxy-1-aminoindane.
Owner:陈永军
Pharmaceutical composition containing 1h-inden-1-amine, 2,3-dihydro-n-2-propynyl-, (1r)-, methanesulfonate
A pharmaceutical composition comprising as an active ingredient a therapeutically effective amount of R(+)-N-propargyl-1-aminoindan mesylate thereof, and less than 50% by weight of hexahydric sugar alcohols.
Owner:SANDOZ AG
Preparation of r-6-Hydroxy-1-aminoindan by Dynamic Kinetic Resolution
ActiveCN105087742BGuaranteed racemization abilityEnsure safetyFermentationCandida rugosaMandelic acid
The invention relates to a method for preparing R-6-hydroxy-1-aminoindane through dynamic kinetic resolution. The method is characterized by using candida rugosa lipase as a biological resolution catalyst, D-(-)-O-acetyl mandelic acid as an acyl donor and a non-crystal nickel catalyst KT-02 as a racemization catalyst to carry out one-pot dynamic kinetic resolution reaction in an autoclave in the presence of hydrogen to convert 6-hydroxy-1-aminoindane to an R-6-hydroxy-1-aminoindane acyl compound and carrying out acid hydrolysis, alkali freeing, and the like on the acyl compound to obtain R-6-hydroxy-1-aminoindane with ee (enantiomeric excess) value more than 99%. The method has the characteristics of simplicity in operation, cheap and accessible racemization catalyst, complete utilization of the raw materials, high optical purity of the product, and the like, and has great guidance and application values in production and preparation of R-6-hydroxy-1-aminoindane.
Owner:陈永军
Methods for treatment of renal failure
InactiveUS20070082958A1Promising clinical valueLarge responseBiocideUrinary disorderN-propargylFAILURE KIDNEY
Propargylamine, propargylamine derivatives including N-propargyl-1-aminoindan and analogs thereof, and pharmaceutically acceptable salts thereof, are useful for prevention or treatment of renal failure.
Owner:TECHNION RES & DEV FOUND LTD +1
Preparation method for R-6-hydroxy-1-aminoindan
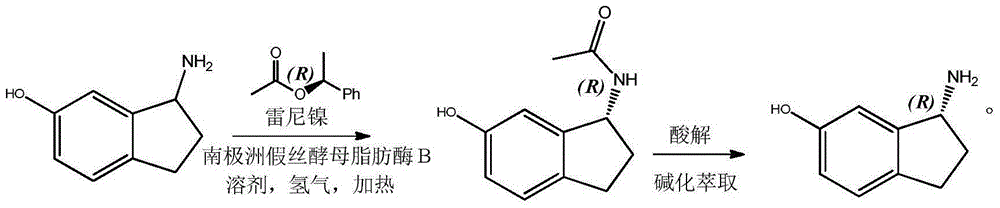
ActiveCN105177103AEasy to operateRaw materials are easy to obtainOrganic compound preparationFermentationKinetic resolutionSolvent
The invention discloses a method for preparing R-6-hydroxy-1-aminoindan through biocatalytic dynamic kinetic resolution. The method comprises the following concrete steps: in an autoclave, allowing Candida antarctica Lipase B to catalyze transesterification reaction of 6-hydroxy-1-aminoindan with R-1-styracitol acetate so as to produce an R-6-hydroxy-1-aminoindan acyl compound and introducing Raney nickel as a racemization catalyst at the same time so as to guarantee complete conversion of 6-hydroxy-1-aminoindan into R-6-hydroxy-1-aminoindan; and hydrolyzing the obtained acyl compound so as to obtain R-6-hydroxy-1-aminoindan with an ee value of more than 99%. The method is simple to operate; raw materials are completely utilized; prepared R-6-hydroxy-1-aminoindan has high optical purity; and the method has great guidance and application values in production and preparation of R-6-hydroxy-1-aminoindan.
Owner:陈永军
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com