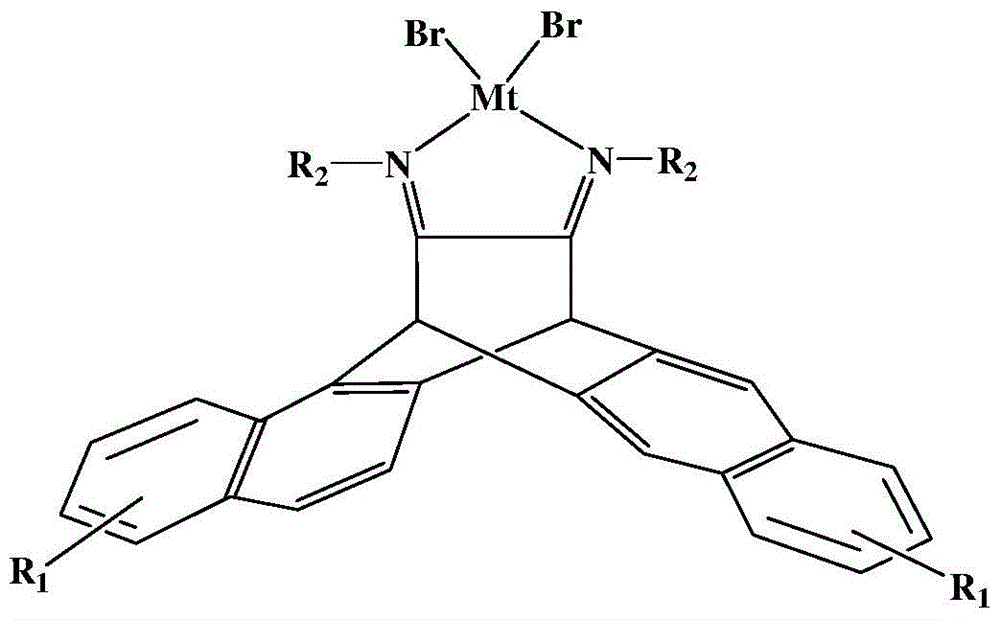
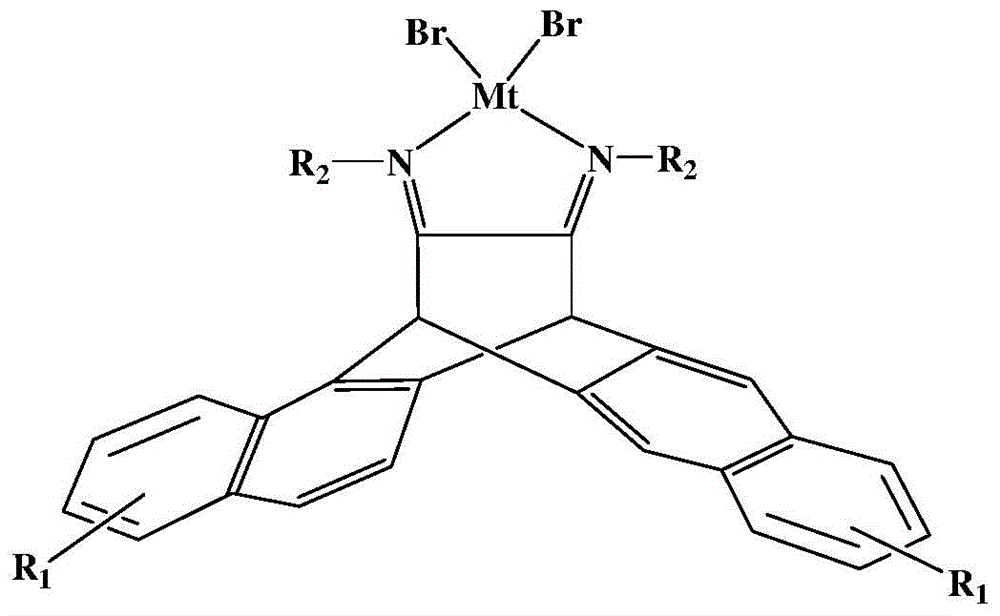
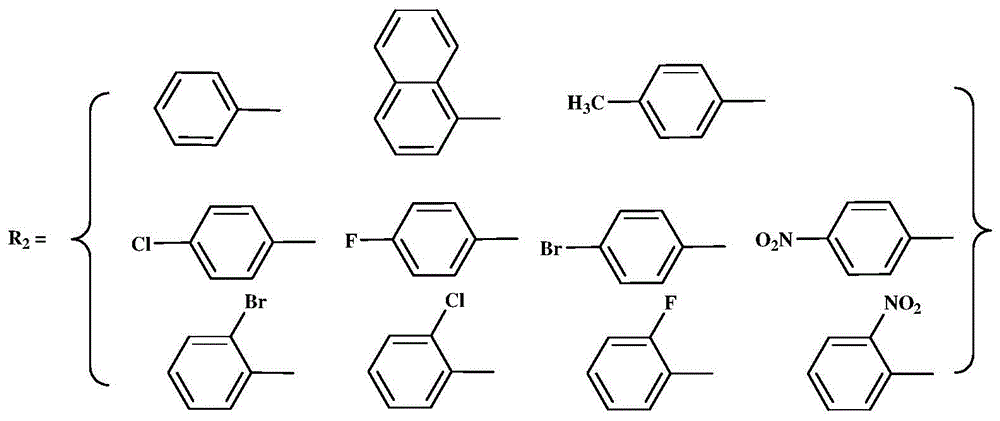
N,N-single ligand metal catalyst with three-dimensional structure and preparation method thereof
A technology of metal catalysts and three-dimensional structures, applied in chemical instruments and methods, organic chemistry, nickel-organic compounds, etc., can solve the problems of few studies on three-dimensional three-dimensional skeleton structures, and achieve good processing performance, low dielectric constant, and good thermal conductivity. The effect of stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] (1)R 2 When it is an aromatic amine aniline, the preparation of the three-dimensional structure α-diimine ligand:
[0047]Add 10mmol of 6,13-dihydro-6,13-ethylene to a 500mL reaction flask equipped with a reflux condenser and a water separator
[0048] Pentacene-15,16-dione, 30 mmol of aniline, a catalytic amount of p-toluenesulfonic acid and 150 mL of toluene were used as solvents. Reflux reaction at 130°C for 24 hours. During the reaction, the generated water was removed in the form of water-toluene azeotrope through a water separator. The remaining solvent was sucked away under vacuum, and the precipitated solid was purified on a silica gel column with sherwood oil / ethyl acetate (v / v=20 / 1) as an eluent. Yellow crystals were precipitated in the mixed solvent;
[0049] (2) When R2 is aromatic amine aniline, the preparation of three-dimensional structure α-diimine nickel (II) catalyst:
[0050] Under nitrogen protection, in a 100mL polymerization bottle, add 0.2mmo...
Embodiment 2
[0056] (1) When R2 is an aromatic amine aniline, the preparation of the α-diimine ligand of the stereostructure:
[0057] With embodiment 1.
[0058] (2) R When being aromatic amine aniline, the preparation of stereostructure α-diimine palladium (II) catalyst:
[0059] (3) Under the protection of nitrogen, in a 100mL polymerization bottle, add 0.2mmol ligand, 0.2mmol cyclooctadiene methyl palladium chloride and 10mL anhydrous dichloromethane respectively, stir at room temperature for 12 hours, then pass through sand while hot Filter the core ball under a nitrogen atmosphere, add 10mL of anhydrous n-hexane to the filtrate, and let it stand for several days. The new stereostructure α-diimine palladium (II) complex of yellow crystals is released from the mixed solution of dichloromethane and n-hexane. precipitation;
[0060] (4) Catalytic polymerization of norbornene
[0061] (5) Heat the reaction bottle equipped with a magnetic stirrer and a branch with a heat gun, and simult...
Embodiment 3
[0066] (1)R 2 When it is the aromatic amine 2,6-diisopropylaniline, the preparation of the three-dimensional structure α-diimine ligand:
[0067] Add 10mmol of 6,13-dihydro-6,13-ethylenepentacene-15,16-dione, 30mmol of 2,6-diiso Propylaniline, a catalytic amount of p-toluenesulfonic acid and 150 mL of toluene were used as solvents. Reflux reaction at 130°C for 24 hours. During the reaction, the generated water was removed in the form of water-toluene azeotrope through a water separator. The remaining solvent was sucked away under vacuum, and the precipitated solid was purified on a silica gel column with sherwood oil / ethyl acetate (v / v=20 / 1) as an eluent. Yellow crystals were precipitated in the mixed solvent;
[0068] (2) R 2 When it is aromatic amine 2,6-diisopropylaniline, the preparation of three-dimensional structure α-diimine nickel (II) catalyst:
[0069] Under nitrogen protection, in a 100mL polymerization bottle, add 0.2mmol of α-diimine ligand, 0.2mmol of ethyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com