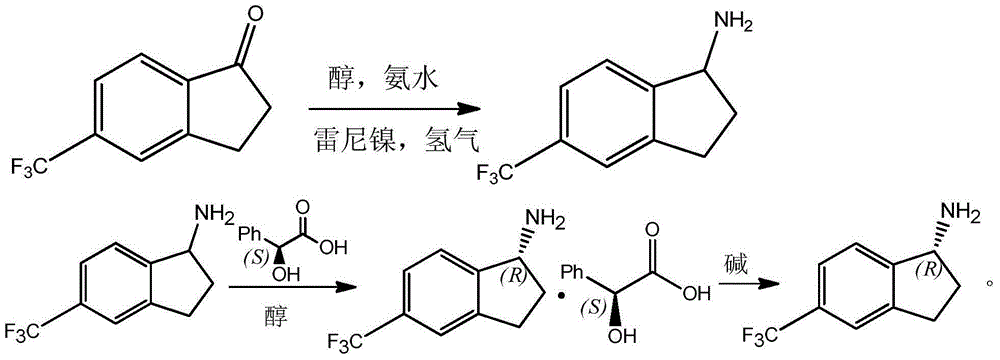
Preparation method for R-5-trifluoromethyl-1-aminoindane
A technology of trifluoromethyl and amino indane is applied in the field of preparation of optically pure chiral compounds, and achieves the effects of high optical purity, high resolution efficiency, and easy separation, recovery and use.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0008] (1) Preparation of 5-trifluoromethyl-1-aminoindan
[0009] In a 1000ml autoclave, add 600ml methanol, 100g (0.5mol) 5-trifluoromethyl-1-indanone, and 10g catalyst Raney nickel in sequence; seal the autoclave, first replace the air in the autoclave with nitrogen three times, and then use After replacing the nitrogen in the autoclave with hydrogen for two times, 51g (3.0mol) of liquid ammonia was introduced into the autoclave. After the liquid ammonia was added, hydrogen was introduced until the pressure was 3.0MPa, stirring was started, and the temperature was raised to 60°C for reaction. When there is no more decrease, stop the reaction, filter and concentrate the reaction solution to obtain 5-trifluoromethyl-1-aminoindan.
[0010] (2) Resolution of 5-trifluoromethyl-1-aminoindane
[0011] In a 1000ml round bottom flask, add 22.8G (0.15mol) of L-mandelic acid and 400ml of methanol, stir magnetically, add 20.1G (0.1mol) of 5-trifluoromethyl-1-aminoindan at 55°C, and ref...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com