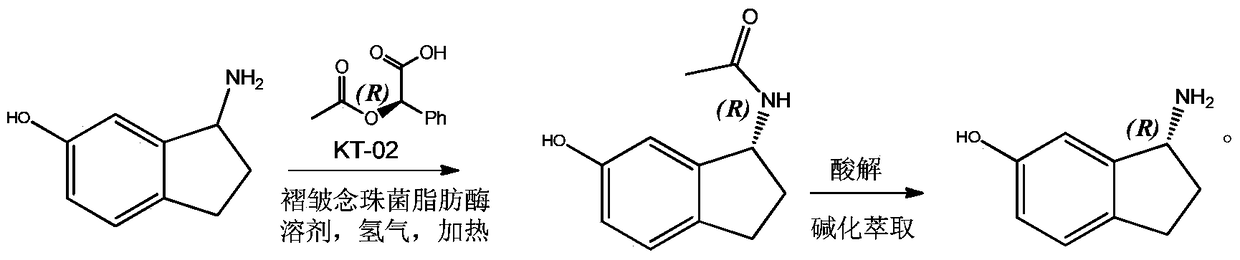
Preparation of r-6-Hydroxy-1-aminoindan by Dynamic Kinetic Resolution
A dynamic kinetics, R-6- technology, which is applied in the field of dynamic kinetics splitting and preparing R-6-hydroxy-1-aminoindane, can solve the problem of splitting and preparing R-6-hydroxy-1-aminoindene, etc. problem, to achieve the effect of complete utilization of raw materials, high optical purity and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0007] 1. Resolution of 6-hydroxy-1-aminoindan
[0008] In a 1000ML autoclave, add 500ML toluene, 74.5G 6-hydroxy-1-aminoindan, 115.9g D-(-)-O-acetylmandelic acid, 6g Candida plicata lipase and 10g KT-02, seal the high pressure In the autoclave, replace the air in the autoclave with nitrogen, then pass hydrogen into the autoclave to a pressure of 1.0MP, start stirring, and heat up to 50°C for reaction; after 19 hours, take a sample for detection, 6-hydroxy-1-aminoindane Completely converted to the acetyl compound of R-6-hydroxyl-1-aminoindane; after the reaction, the solution was concentrated and subjected to column chromatography to obtain the acetyl compound 90.8 of pure R-6-hydroxyl-1-aminoindane g, the yield is 95.1%.
[0009] 2. Acid hydrolysis to obtain R-6-hydroxy-1-aminoindan
[0010] Take 95.5 g of the acetyl compound of R-6-hydroxy-1-aminoindane obtained by repeating the previous step several times and add it to 1000 ml of ethanol and concentrated hydrochloric acid...
Embodiment 2
[0014] 1. Resolution of 6-hydroxy-1-aminoindan
[0015] In a 1000ML autoclave, add 500ML toluene, 74.5G6-hydroxy-1-aminoindan, 144.9g D-(-)-O-acetylmandelic acid, 7g Candida plicata lipase and 13g KT-02 in sequence, seal Autoclave, replace the air in the autoclave with nitrogen, then pass hydrogen into the autoclave to a pressure of 1.5MP, start stirring, and raise the temperature to 70°C for reaction; after 13 hours, sample detection, 6-hydroxy-1-amino Indane is completely converted into the acetyl compound of R-6-hydroxyl-1-aminoindane; after the reaction, the solution is concentrated and subjected to column chromatography to obtain the acetyl compound of R-6-hydroxyl-1-aminoindane 90.4g, the yield is 94.7%.
[0016] 2. Acid hydrolysis to obtain R-6-hydroxy-1-aminoindan salt
[0017] Take 95.5 g of the acetyl compound of R-6-hydroxy-1-aminoindane obtained by repeating the previous step several times and add it to a solution mixed with 1000 ml of ethanol and concentrated su...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
| enantiomeric excess | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com