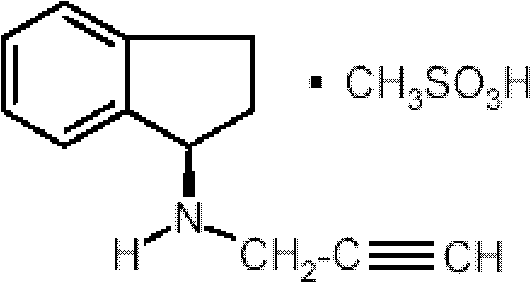
Pharmaceutical composition containing 1h-inden-1-amine, 2,3-dihydro-n-2-propynyl-, (1r)-, methanesulfonate
The technology of a composition and mesylate, applied in the fields of depression and hyperactivity syndrome in children, can solve the problems of unsuitability for consumption, lack of stable impurities in pharmaceutical compositions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] making process:
[0032] 1. Sift mannitol, cornstarch and pregelatinized starch and mix in a suitable mixer;
[0033] 2. Dissolve rasagiline in purified water;
[0034] 3. Granulate mannitol, corn starch, pregelatinized starch with rasagiline solution and dry the granules in a suitable drier;
[0035] 4. Sieve the dried granules;
[0036] 5. Sieve colloidal silicon dioxide, talcum powder and stearic acid;
[0037] 6. Mix the sieved granules with colloidal silicon dioxide and lubricate with talc and stearic acid;
[0038] 7. Compress the lubricated mixture into tablets.
Embodiment 2
[0041] making process:
[0042] 1. Rasagiline, mannitol, cornstarch and pregelatinized starch and part of the colloidal silicon dioxide are sieved and mixed in a suitable mixer;
[0043] 2. Granulate the dry mixture with purified water and dry the granules in a suitable drier;
[0044] 3. Sieve the dried particles;
[0045] 4. Sieve the remaining colloidal silicon dioxide, talcum powder and stearic acid;
[0046] 5. Mix the sieved granules with colloidal silicon dioxide and lubricate with talc and stearic acid;
[0047] 6. Compress the lubricated mixture into tablets.
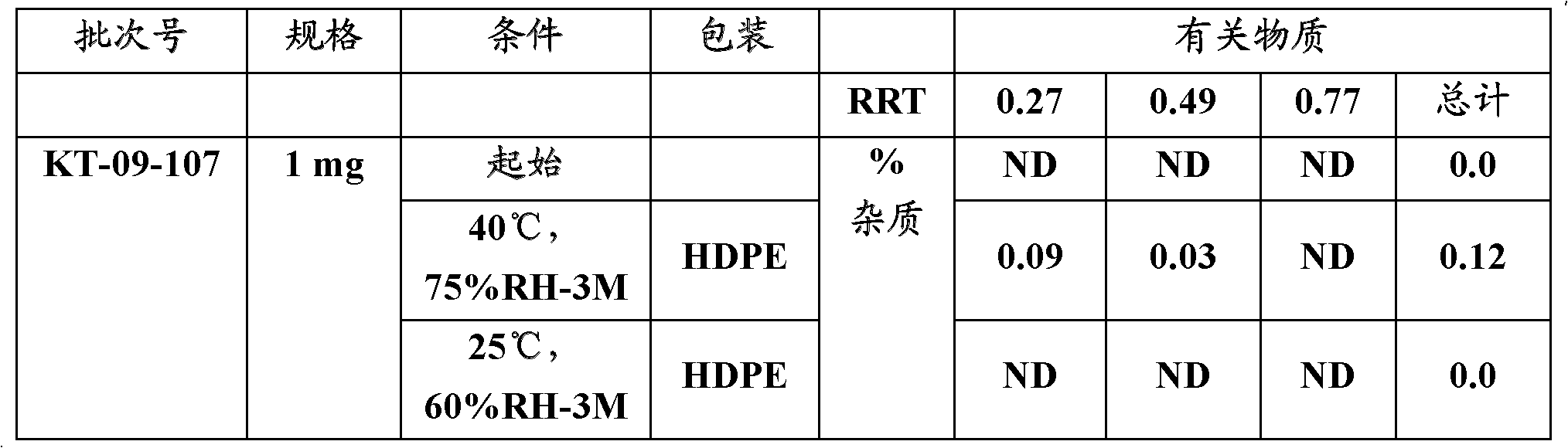
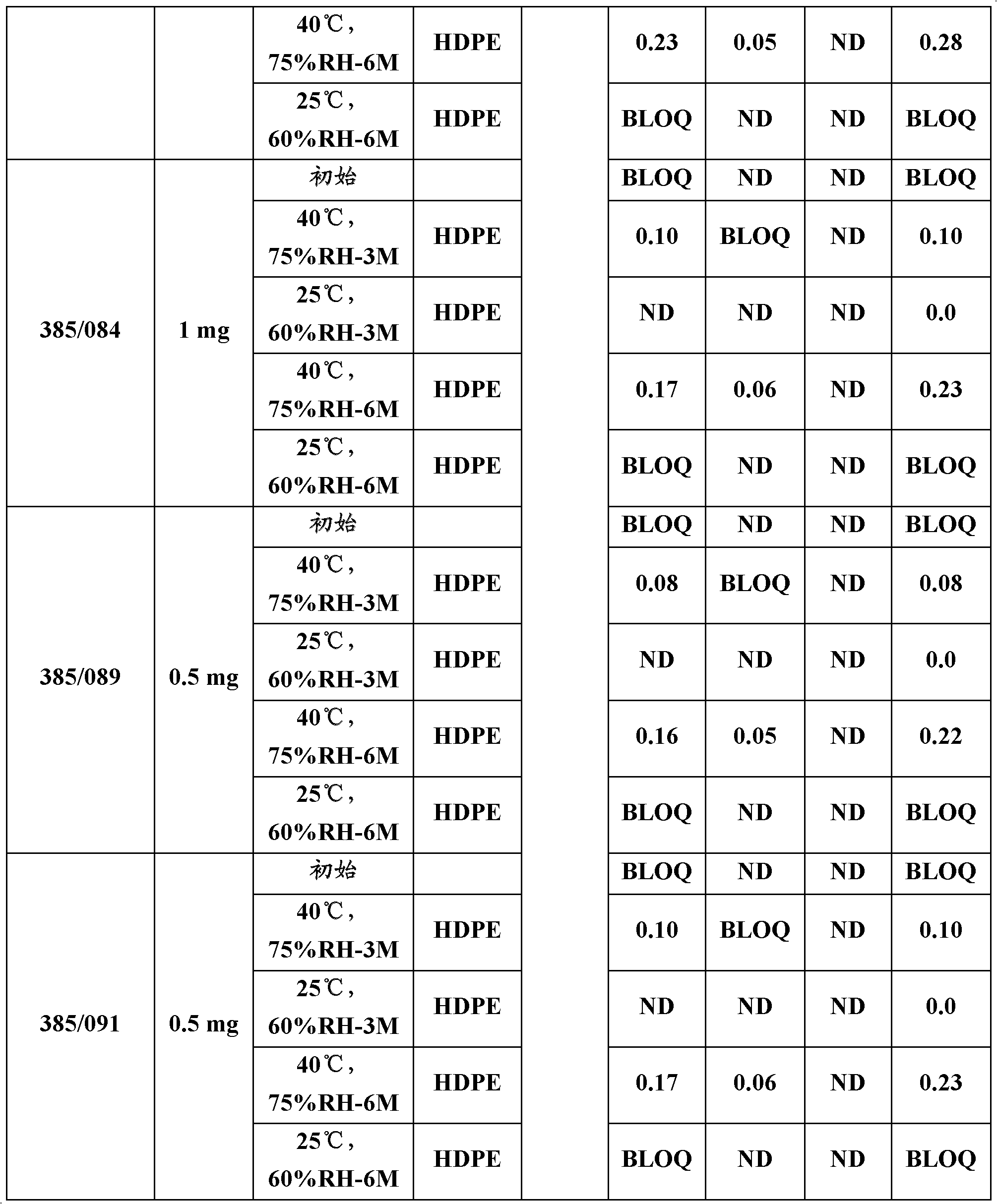
[0048] Table 1 below gives the stability data for the different batches of rasagiline that were sometimes examined.
[0049] Table 1: Stability data of formulations of the present invention
[0050]
[0051]
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com