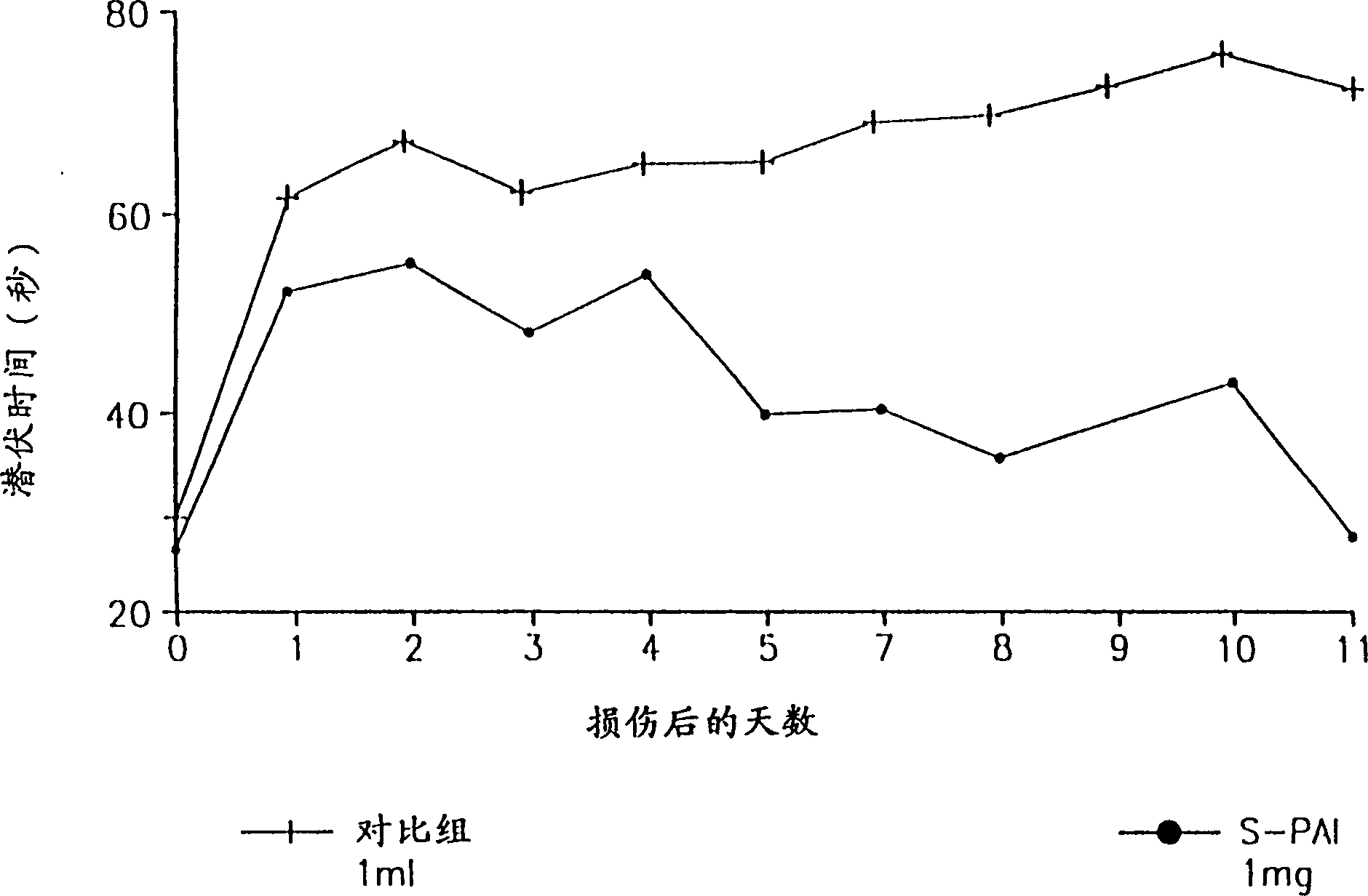
Pharmaceutical compositions comprising S-(-)-N-Propargyl-1-amino indan
A technology of aminoindan and propargyl, which is applied in the field of pharmaceutical compositions containing S-(-)-N-propargyl-1-aminoindan, and can solve the problem of reduced selectivity of MAO subtypes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Di-(S-(-)-N-propargyl-1-aminoindan) D-tartrate
[0038] a) racemic N-propargyl-1-aminoindan
[0039] To a mixture of racemic 1-aminoindan (64 g), 15% aqueous sodium hydroxide solution (141 g), water (107 mL) and toluene (192 mL) was added propargyl benzenesulfonate (94.3 mL) over 20 minutes at room temperature. g). The resulting mixture was heated to 45°C for 4 hours, at which point pH > 12 was confirmed (45% sodium hydroxide was added if necessary), and the phases were separated. Water (64 mL) was added to the organic phase and the pH was adjusted to 2 with 33% aqueous sulfuric acid. The aqueous phase was separated, diluted with water and mixed with toluene. The pH was adjusted to 6 with 25% aqueous sodium hydroxide solution and the phases were separated. The aqueous phase was extracted again with toluene to ensure pH=6. The combined organic layers were concentrated in vacuo to give 51 g of crude racemic N-propargyl-1-aminoindan as a yellow oil.
[0040] b)...
Embodiment 2
[0043] S-(-)-N-propargyl-1-aminoindan methanesulfonate
[0044] Di-(S-(-)-N-propargyl-1-aminoindan)D-tartrate (15 g) from Example 1 and methanesulfonic acid (6 g) were dissolved in isopropanol (150 mL) The solution was heated to reflux for 30 minutes. The reaction mixture was allowed to cool to room temperature and the resulting precipitate was isolated by suction filtration to give the title compound (11.1 g), m.p. 157°C, [α] D -22°. About C 13 h 17 NSO 3 Anal. Calcd.: C, 58.43; H, 6.37; N, 5.24; S, 11.98; Found: C, 58.70; H, 6.39; N, 5.20;
Embodiment 3
[0046] S-(-)-N-propargyl-1-aminoindan methanesulfonate
[0047] To a solution of sodium hydroxide (4.8 g) in water (80 mL) was added bis-(S-(-)-N-propargyl-1-aminoindan) D-tartrate from Example 1 and toluene ( 80mL). After stirring for 30 minutes, the mixture was filtered through celite with suction and the organic layer was separated and washed with water. The organic phase was concentrated in vacuo, diluted with isopropanol and concentrated. The residue was dissolved in isopropanol (125 mL) and treated with methanesulfonic acid (11.5 g). The resulting mixture was heated to reflux for 30 minutes, filtered (celite) and allowed to cool to room temperature. The resulting precipitate was collected by filtration and washed with isopropanol to give the title compound having the same physical and chemical properties as the product of Example 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com