Ramelteon sublingual tablet and preparation method thereof
A technology for ramelteon and sublingual tablets, applied in the field of ramelteon sublingual tablets and its preparation, can solve the problems of low drug efficacy, low oral absolute bioavailability, poor content uniformity of low-dose drugs, etc. Achieve high bioavailability, avoid hepatic first-pass effect, and have good taste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
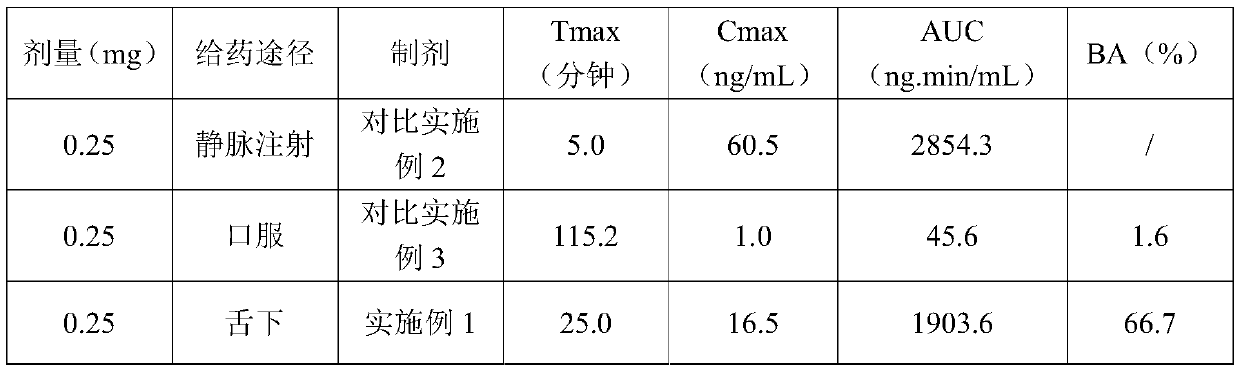
[0013] Embodiment 1. Ramelteon tablet prescription composition: specification 0.25mg, prescription quantity is 10000 tablets.
[0014] Ramelteon 2.5g Mannitol 956g Crospovidone 40g Magnesium stearate 1g mint flavor 0.5g total 1000g
[0015] Preparation method: Pass ramelteon through a 80-mesh sieve, mannitol, crospovidone and mint essence pass through a 60-mesh sieve; weigh each material in the prescription amount, and set aside; put ramelteon and 1 / 4 prescription amount of manna Alcohol was placed in a ball mill and mixed for 2 hours; the ramelteon-mannitol mixture, the remaining prescription amount of mannitol, crospovidone and peppermint essence were placed in a mixer and mixed evenly; then magnesium stearate was added and mixed evenly; Tablets were compressed on a tablet machine with a 7mm die.
[0016] Comparative Example 1. Ramelteon Tablets Prescription Composition: Specification 0.25 mg, prescription quantity 10,000 tablets....
Embodiment 2
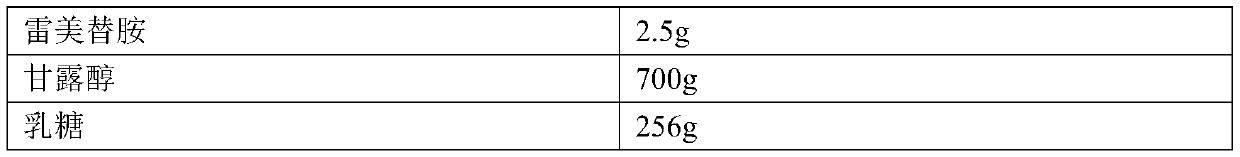
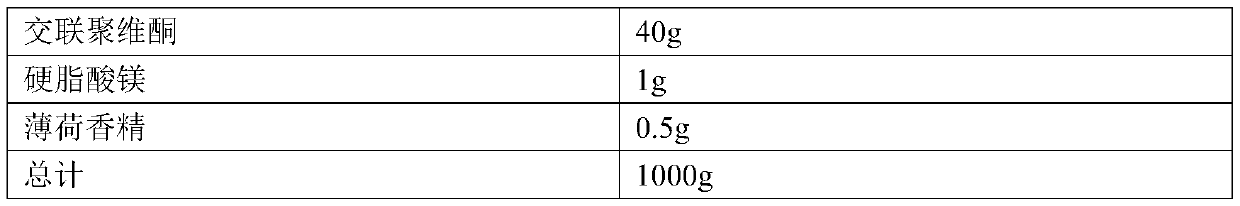
[0025] Example 2. The composition of the prescription of ramelteon tablets: the specification is 0.25 mg, and the prescription quantity is 10,000 tablets.
[0026]
[0027]
[0028] Preparation method: pass ramelteon through a 80-mesh sieve, mannitol, lactose, crospovidone and mint essence pass through a 60-mesh sieve; weigh each material in the prescription amount, and set aside; put ramelteon, mannitol and lactose Grind and mix in a ball mill for 2 hours; put ramelteon-mannitol-lactose mixture, crospovidone and peppermint flavor in a mixer and mix evenly; then add magnesium stearate and mix evenly; Die for tableting.
Embodiment 3
[0029] Example 3. The composition of the prescription of ramelteon tablets: the specification is 0.25 mg, and the prescription quantity is 10,000 tablets.
[0030] Ramelteon 2.5g xylitol 456g sucrose 500g Low-substituted hydroxypropyl cellulose 40g silica 1g Sodium stearyl fumarate 1g mint flavor 0.5g total 1000g
[0031] Preparation method: pass ramelteon through an 80-mesh sieve, sucrose, xylitol, low-substituted hydroxypropyl cellulose and mint flavor through a 60-mesh sieve; weigh each material in the prescription amount, and set aside; put ramelteon, xylose Alcohol and sucrose were ground and mixed in a ball mill for 2 hours; the ramelteon-xylitol-sucrose mixture, low-substituted hydroxypropyl cellulose and mint essence were placed in a mixer and mixed evenly; then silicon dioxide and hard sodium fumarate were added , mix evenly; press on a tablet machine with a 7mm die.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com