Afatinib dimaleate tablet and preparation method thereof
A technology of tinib tablet and maleic acid, which is applied in the field of afatinib maleate tablet and its preparation, can solve the problems of poor powder fluidity, lobes, large differences in tablet weight, etc., and achieve adjustment of bulk density, improvement of Fluidity, effect of improving solubility performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
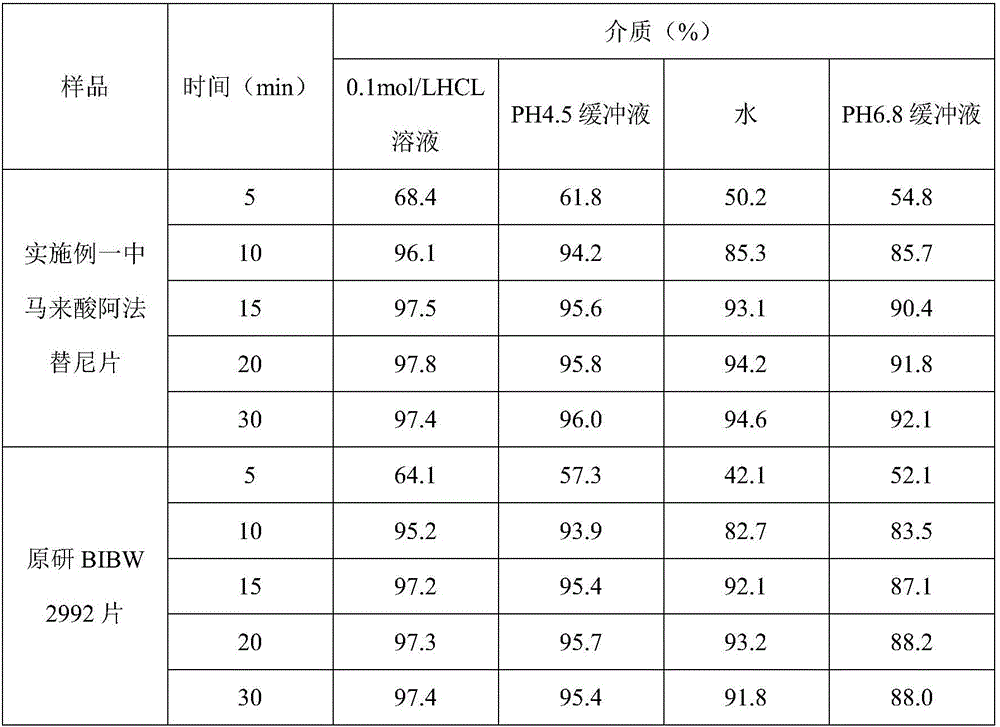
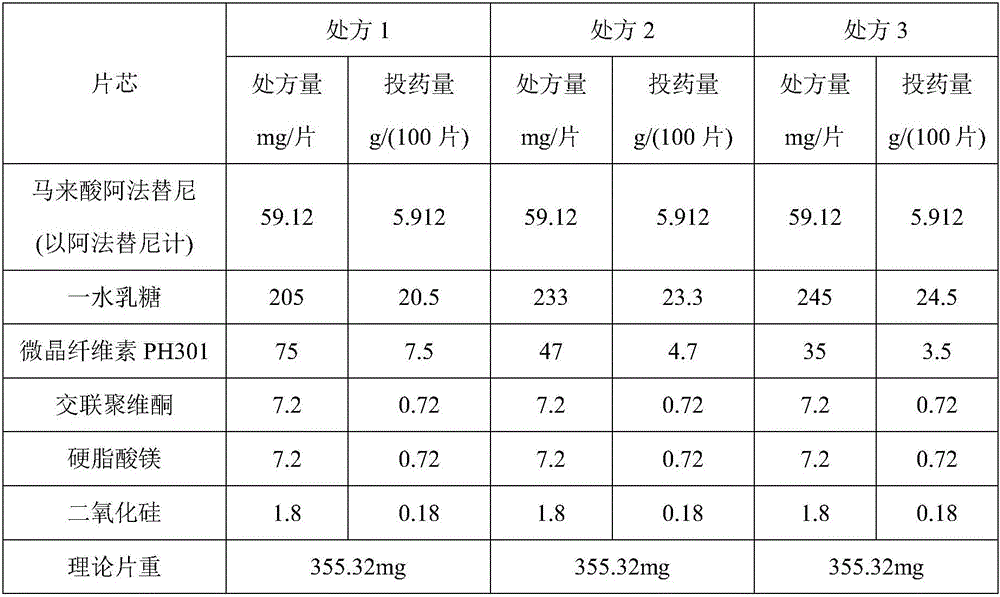
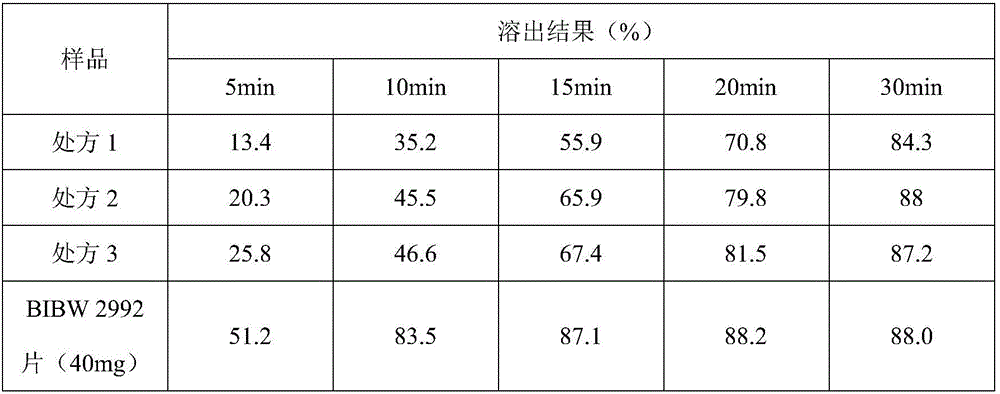
[0020] The afatinib maleate tablet in the present embodiment is made up of a tablet core and a film coating wrapped outside the tablet core, and the tablet core is made of raw materials of the following quality (1000 tablets, afatinib 40mg / tablet) : Afatinib maleate (calculated as afatinib) 59.12g, lactose monohydrate 230.0g, microcrystalline cellulose 42.0g PH301, crospovidone 18.0g, silicon dioxide 2.0g, stearic acid Magnesium 6.0g; film coating adopts the following quality raw materials (1000 tablets): 10.0g, water 152.0g.
[0021] Preparation method: Accurately take the raw and auxiliary materials according to the parts by mass, first mix afatinib maleate, lactose monohydrate, microcrystalline cellulose PH301, crospovidone, and silicon dioxide for 20 minutes, then add stearic acid Magnesium, mixed for 10 minutes, prepared granules by roller compaction, adding magnesium stearate to the prepared granules, mixed for 20 minutes, compressed into tablets to obtain tablet cores. ...
Embodiment 2
[0023] The afatinib maleate tablet in the present embodiment is made up of a tablet core and a film coating wrapped outside the tablet core, and the tablet core is made of raw materials of the following quality (1000 tablets, afatinib 40mg / tablet) : Afatinib maleate (calculated as afatinib) 59.12g, lactose monohydrate 235.0g, microcrystalline cellulose PH103 45.0g, crospovidone 20.0g, silicon dioxide 2.2g, stearic acid Magnesium 7.0g; film coating adopts the following quality raw materials (1000 tablets): 12.0g, water 156.0g.
[0024] Preparation method: Accurately take the raw and auxiliary materials according to the parts by mass, first mix afatinib maleate, lactose monohydrate, microcrystalline cellulose PH301, crospovidone, and silicon dioxide for 20 minutes, then add stearic acid Magnesium, mixed for 10 minutes, prepared granules by roller compaction, adding magnesium stearate to the prepared granules, mixed for 20 minutes, compressed into tablets to obtain tablet cores. ...
Embodiment 3
[0026] The afatinib maleate tablet in the present embodiment is made up of tablet core and the film coating that is wrapped in outside the tablet core, and tablet core is made of the raw material of following quality (1000 tablets, afatinib 30mg / tablet) : Afatinib maleate (calculated as afatinib) 44.34g, lactose monohydrate 172.5g, microcrystalline cellulose PH-F20 31.5g, crospovidone 13.5g, silicon dioxide 1.5g, hard Magnesium fatty acid 4.5g; film coating adopts the following quality raw materials (1000 tablets): 7.5g, water 114.0g.
[0027]Preparation method: Accurately take the raw and auxiliary materials according to the parts by mass, first mix afatinib maleate, lactose monohydrate, microcrystalline cellulose PH301, crospovidone, and silicon dioxide for 20 minutes, then add stearic acid Magnesium, mixed for 10 minutes, prepared granules by roller compaction, adding magnesium stearate to the prepared granules, mixed for 20 minutes, compressed into tablets to obtain table...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com