Valsartan amlodipine tablet and preparation method thereof
A technology of valsartan amlodipine tablets and amlodipine besylate, which is applied in the field of medicine, can solve the problems of low in vitro dissolution rate, influence of drug absorption, and low bioavailability, so as to increase residence time and improve bioavailability Degree, increase the effect of solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1, preparation valsartan amlodipine sheet
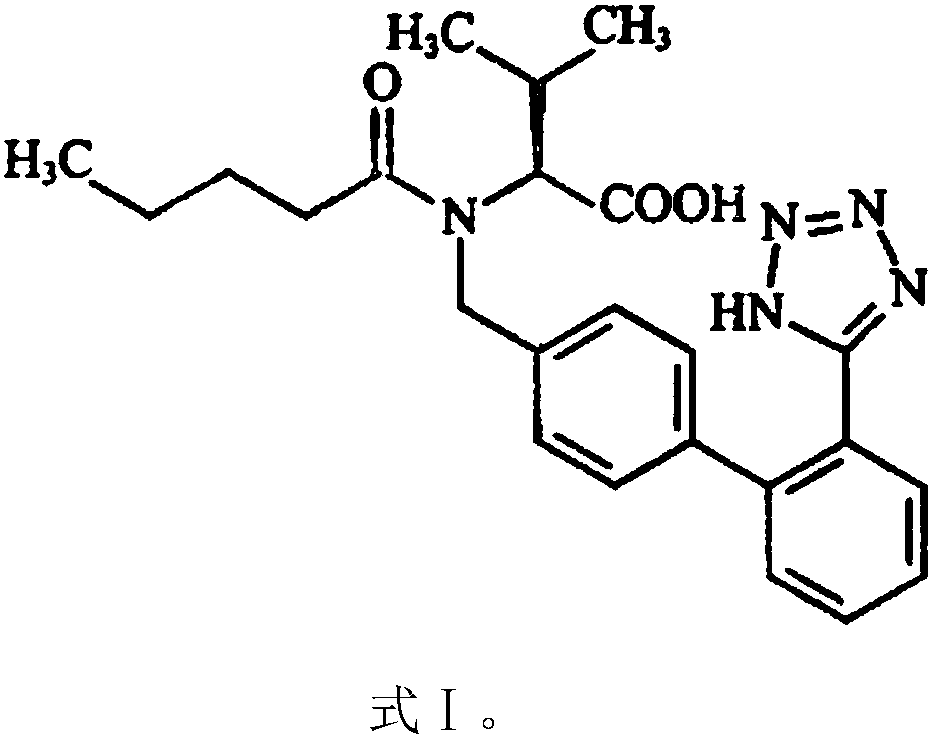
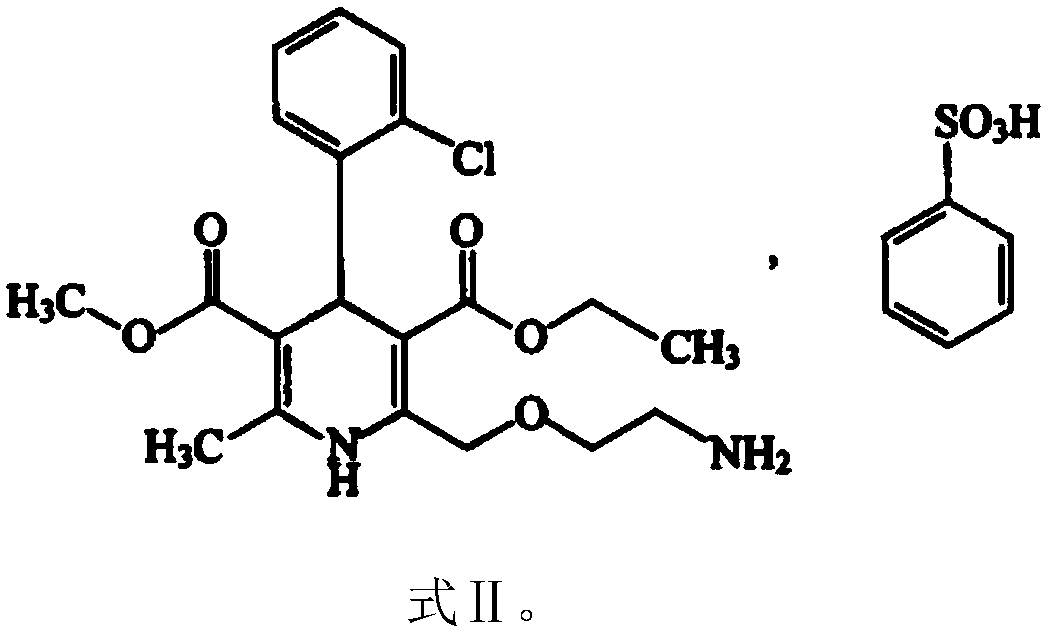
[0045] The present embodiment provides the preparation of 100,000 valsartan and amlodipine tablets (each containing valsartan 80mg, amlodipine 5mg, 6.935mg amlodipine besylate is equivalent to 5mg amlodipine (amlodipine and benzene Amlodipine sulfonate conversion factor is 0.721)) prescription and preparation method, specifically as follows:
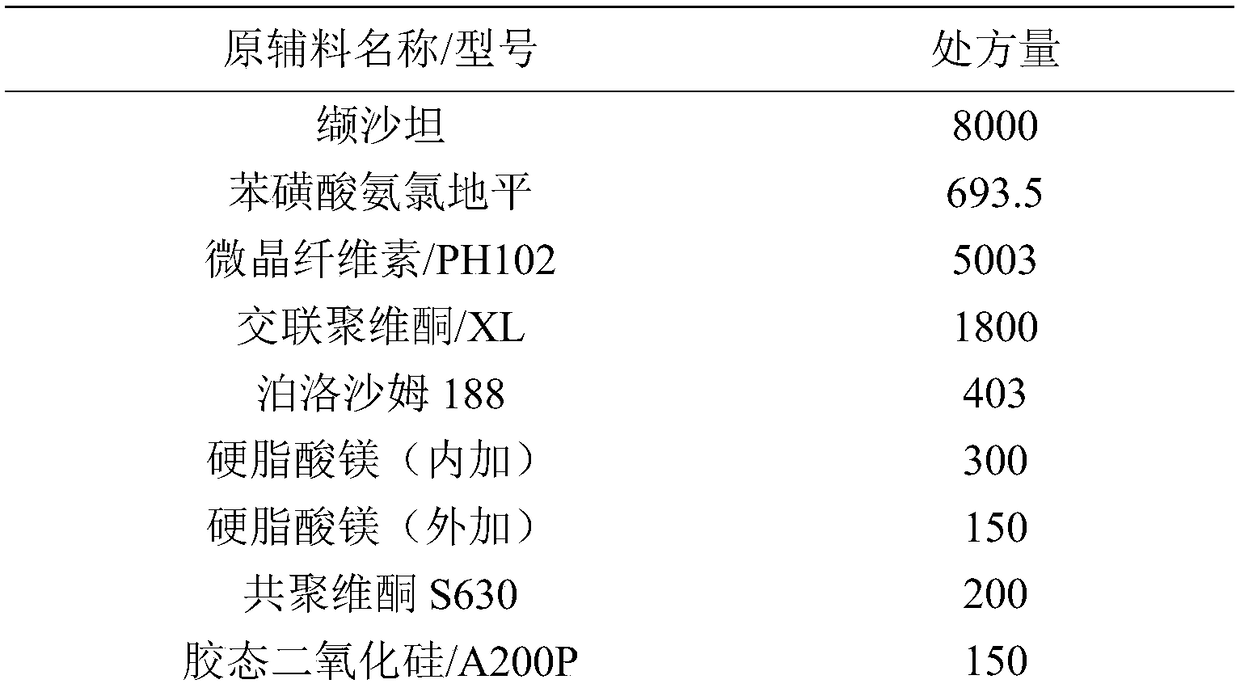
[0046] Prescription composition:
[0047]
[0048]
[0049] Control the ambient humidity <RH25%.
[0050] The preparation method comprises the following steps:
[0051] 1) Pretreatment of raw and auxiliary materials: sieve crospovidone 40 mesh, magnesium stearate (additional) sieve 80 mesh, and set aside; take the prescription amount of valsartan, crospovidone, magnesium stearate ( internally added), amlodipine besylate, colloidal silicon dioxide, and microcrystalline cellulose were mixed in a G10 wet mixing granulator for 2 minutes (stirring 3r / s, shearing 3r / s), and t...
Embodiment 2
[0060] Embodiment 2, preparation valsartan amlodipine sheet
[0061] The present embodiment provides the preparation of 100,000 valsartan and amlodipine tablets (each containing valsartan 80mg, amlodipine 5mg, 6.935mg amlodipine besylate is equivalent to 5mg amlodipine (amlodipine and benzene Amlodipine sulfonate conversion factor is 0.721)) prescription and preparation method, specifically as follows:
[0062] Prescription composition:
[0063]
[0064] The preparation method is basically the same as Example 1 of the present invention.
Embodiment 3
[0065] Embodiment 3, preparation valsartan amlodipine sheet
[0066] The present embodiment provides the preparation of 100,000 valsartan and amlodipine tablets (each containing valsartan 80mg, amlodipine 5mg, 6.935mg amlodipine besylate is equivalent to 5mg amlodipine (amlodipine and benzene Amlodipine sulfonate conversion factor is 0.721)) prescription and preparation method, specifically as follows:
[0067] Prescription composition:
[0068]
[0069] The preparation method is basically the same as Example 1 of the present invention.
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com