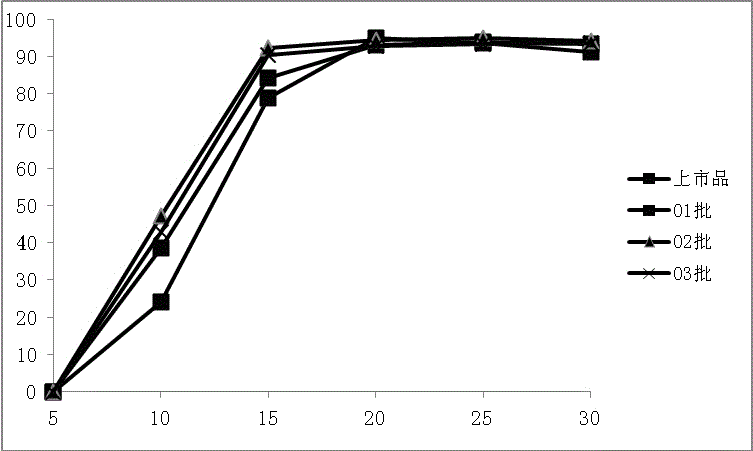
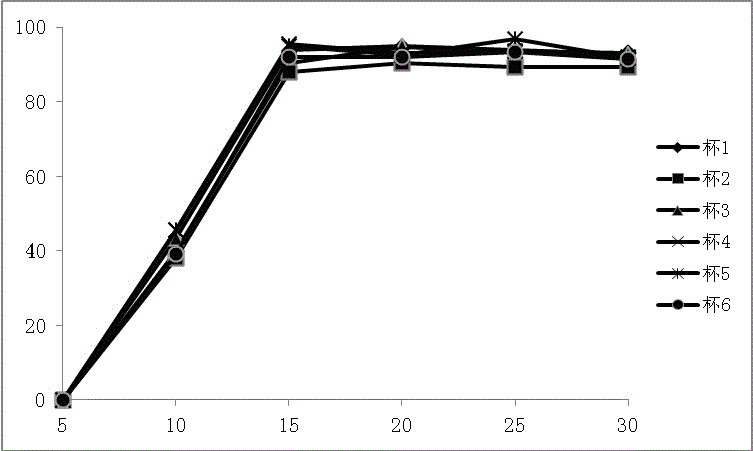
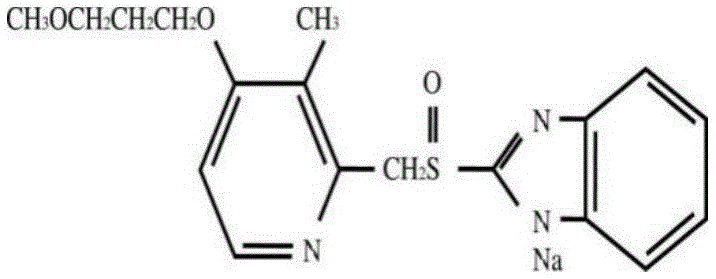
Rebeprazole sodium enteric-coated tablet and preparation process thereof
A technology of rabeprazole sodium and enteric-coated tablets, which is applied in the field of medicine, can solve the problem that the efficacy of rabeprazole sodium enteric-coated tablets is not solved, the rabeprazole sodium enteric-coated tablets have good bioavailability, and the proton pump Inhibitor instability and other problems, to achieve the effect of controllable product quality, easy to scale industrial production, and improve bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Tablet core prescription: 100 parts by weight of rabeprazole sodium, 100 parts by weight of heavy magnesium carbonate, 200 parts by weight of lactose, 200 parts by weight of mannitol, 140 parts by weight of low-substituted hydroxypropyl cellulose, cross-linked 15 parts by weight of povidone, 8 parts by weight of talcum powder, and 237 parts by weight of absolute ethanol.
[0043] Isolation layer prescription: 20 parts by weight of ethyl cellulose, 180 parts by weight of absolute ethanol.
[0044] Enteric layer prescription: 90 parts by weight of cellulose acetate phthalate, 30 parts by weight of talcum powder, and 1080 parts by weight of purified water.
[0045] (1) Preparation of tablet core
[0046] The main ingredient is mixed with heavy sodium carbonate, lactose, mannitol, and low-substituted hydroxypropyl cellulose through a 100-mesh sieve, and 5% (g / ml) crospovidone absolute ethanol solution is used as a binder , quickly add to the well-mixed medicinal powder an...
Embodiment 2
[0078] Tablet core prescription: 100 parts by weight of rabeprazole sodium, 120 parts by weight of anhydrous sodium sulfite, 205 parts by weight of microcrystalline cellulose, 200 parts by weight of lactose, 160 parts by weight of sodium lauryl sulfate (inner 80 parts by weight) parts by weight, 80 parts by weight), hypromellose 10 parts by weight, magnesium stearate 5 parts by weight, purified water 200 parts by weight.
[0079] The isolation layer prescription: 5 parts by weight of anhydrous sodium sulfite, 5 parts by weight of ethyl cellulose, and 190 parts by weight of absolute ethanol.
[0080] Enteric layer prescription: 80 parts by weight of 93F19255 type coating agent, 720 parts by weight of purified water.
[0081] (1) Preparation of tablet core
[0082] The main drug is mixed with anhydrous sodium sulfite, microcrystalline cellulose, lactose, and sodium lauryl sulfate through a 100-mesh sieve, and the hypromellose aqueous solution is used as a binder, quickly added ...
Embodiment 3
[0088] Tablet core prescription: 100 parts by weight of rabeprazole sodium, 150 parts by weight of magnesium oxide, 140 parts by weight of microcrystalline cellulose, 260 parts by weight of pregelatinized starch, 140 parts by weight of polyvinylpyrrolidone (within 70 parts by weight parts, 70 parts by weight), hypromellose 6 parts by weight, magnesium stearate 4 parts by weight, purified water 200 parts by weight.
[0089] The prescription of the isolation layer: 20 parts by weight of magnesium oxide, 20 parts by weight of ethyl cellulose, and 360 parts by weight of absolute ethanol.
[0090] Enteric layer prescription: 127.5 parts by weight of hypromellose phthalate, 42.5 parts by weight of magnesium stearate, and 1530 parts by weight of purified water.
[0091] (1) Preparation of tablet core
[0092] The main ingredient is mixed with magnesium oxide, microcrystalline cellulose, pregelatinized starch, and polyvinylpyrrolidone through a 100-mesh sieve, and the hypromellose aq...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com