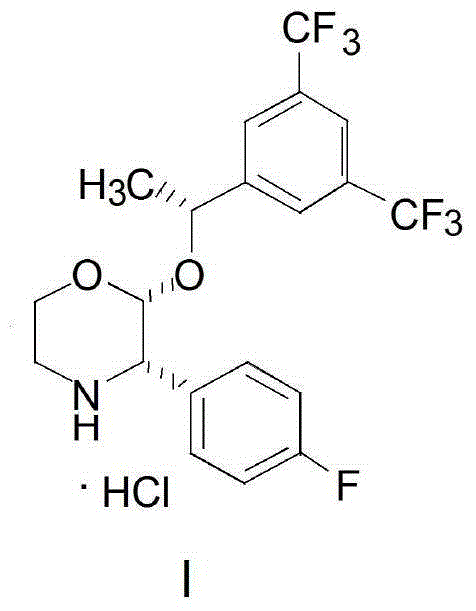
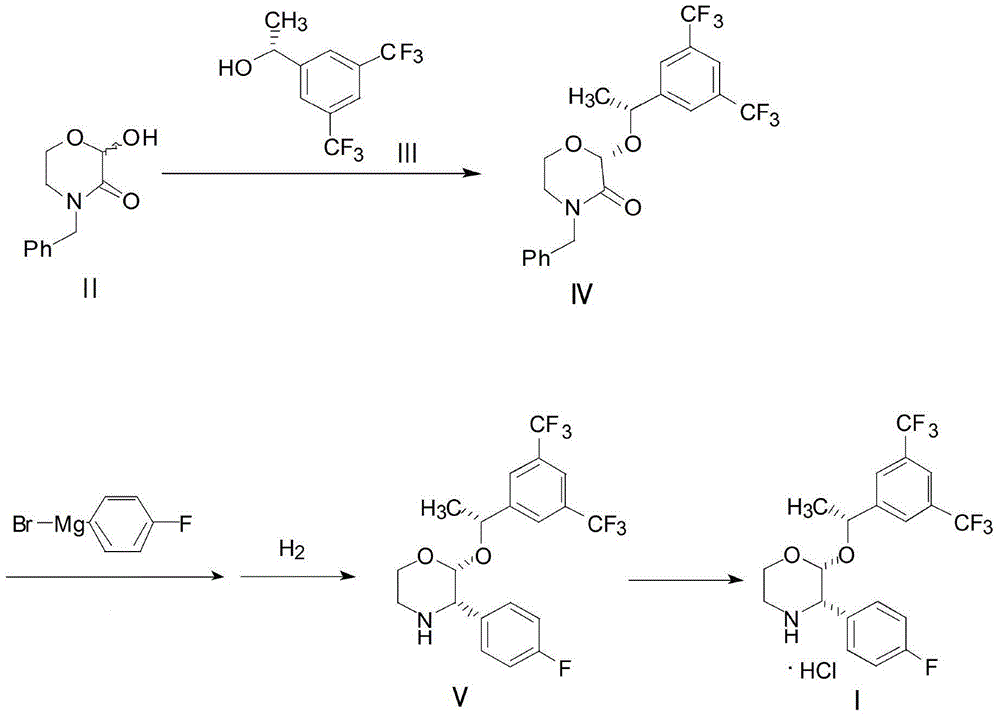
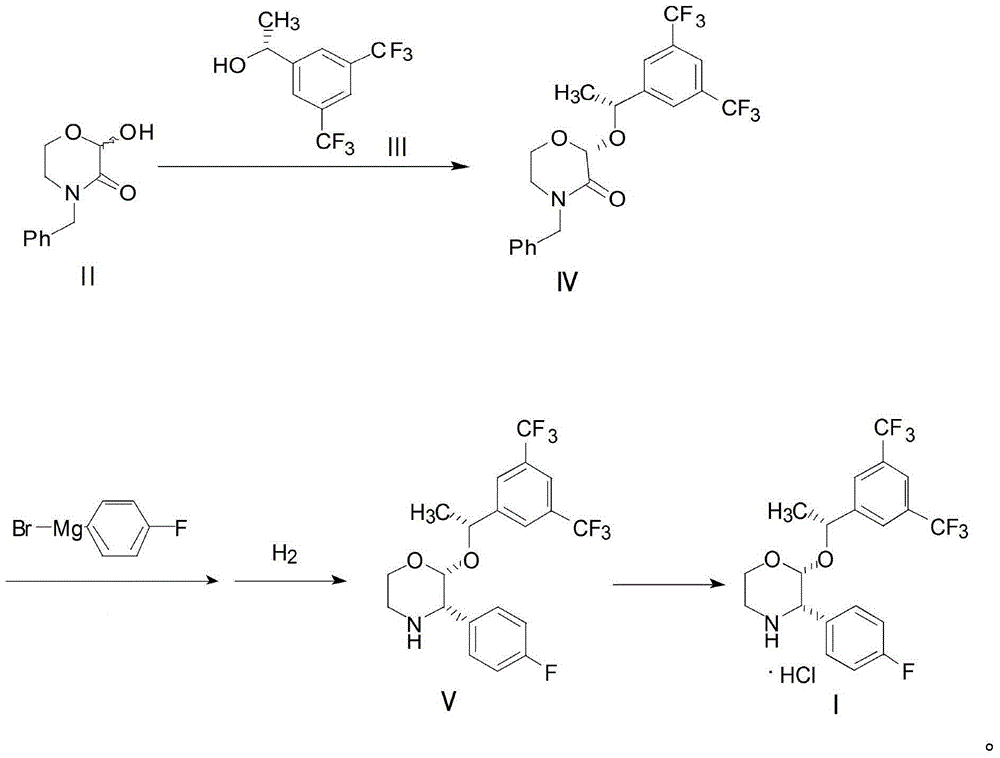
Preparation method of aprepitant intermediate
A technology for aprepitant and intermediates, applied in the field of medicinal chemistry, can solve the problems of increased difficulty in the purification of final products, inability to meet industrialized production, and harsh reaction conditions, and achieve great practical application value, easy large-scale preparation, and overall The effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Add 25g of 4-benzyl-2-hydroxy-morpholin-3-one II, 50g of trifluoroacetic anhydride and 80g of anhydrous acetonitrile into the reaction kettle, stir at 5°C for 1 hour, add (R)-1-[3 , 5-bis(trifluoromethyl)phenyl]ethanol III 60g and boron trifluoride diethyl ether 17g were stirred and condensed at 25°C for 4 hours, and the reaction solution was post-treated and crystallized to obtain compound IV. Add 2g of magnesium, 40g of tetrahydrofuran and 16g of p-fluorobromobenzene in the reaction kettle, heat to initiate the reaction until the magnesium disappears, and then obtain the Grignard reagent and cool it for use; compound IV is dissolved in 100g of tetrahydrofuran to form a solution, and the above-mentioned Grignard reagent is added dropwise until the reaction is complete 16 g of methanol was added to quench the reaction, 0.5 g of palladium carbon was added to the above reaction solution, and hydrogen gas was introduced to carry out the hydrogenation reaction until the reac...
Embodiment 2
[0034] Add 20.7g of 4-benzyl-2-hydroxy-morpholin-3-one II, 40g of trifluoroacetic anhydride and 70g of anhydrous acetonitrile into the reaction kettle, stir at -10°C for 2 hours, add (R)-1 -[3,5-bis(trifluoromethyl)phenyl]ethanol III 25.8g and (R)-2-methyl-CBS-oxazoboridine 2.7g, stirred and condensed at 35°C for 6 hours, and the reaction After liquid treatment and crystallization, compound IV was obtained. Add 1.6g of magnesium, 30g of tetrahydrofuran and 26g of p-fluorobromobenzene into the reaction kettle, heat to initiate the reaction until the magnesium disappears, and then the Grignard reagent is obtained and cooled for use; compound IV is dissolved in 90g of tetrahydrofuran to form a solution, and the above-mentioned Grignard reagent is added dropwise until the reaction Completely, 15 g of methanol was added to quench the reaction, 0.9 g of Raney nickel was added to the above reaction solution, and hydrogen gas was introduced to carry out the hydrogenation reaction unti...
Embodiment 3
[0036] Add 20.7g of 4-benzyl-2-hydroxy-morpholin-3-one II, 50g of trifluoroacetic anhydride and 100g of anhydrous acetonitrile into the reaction kettle, stir at 10°C for 2 hours, add (R)-1- [3,5-bis(trifluoromethyl)phenyl]ethanol III 51.6g and (R)-2-methyl-CBS-oxazoboridine 5.4g were stirred and condensed at 15°C for 3 hours, and the reaction solution After workup and crystallization, compound Ⅳ was obtained. Add 2.6g of magnesium, 50g of tetrahydrofuran and 32g of p-fluorobromobenzene into the reaction kettle, heat to initiate the reaction until the magnesium disappears, and then the Grignard reagent is obtained and cooled for use; Compound IV is dissolved in 110g of tetrahydrofuran to form a solution, and the above-mentioned Grignard reagent is added dropwise until the reaction Complete, add 20g of methanol to quench the reaction, add 3g of sodium triacetate borohydride to the above reaction solution to carry out hydrogenation reaction until the reaction is complete, filter ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com