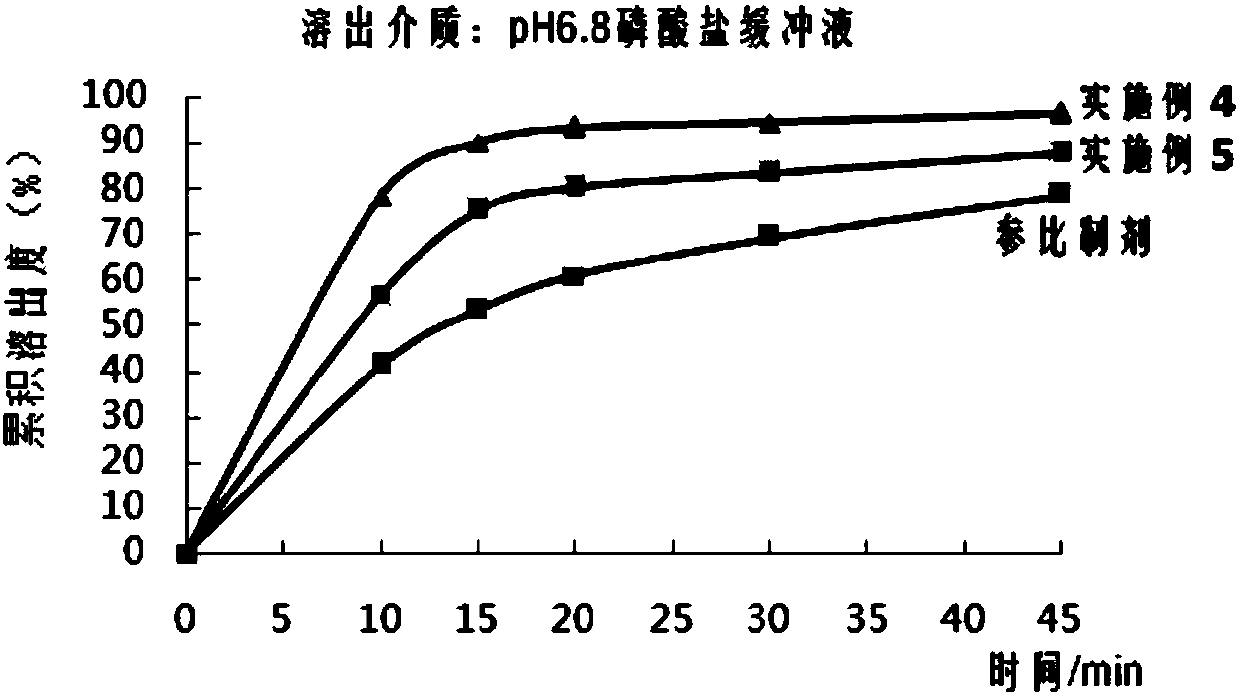
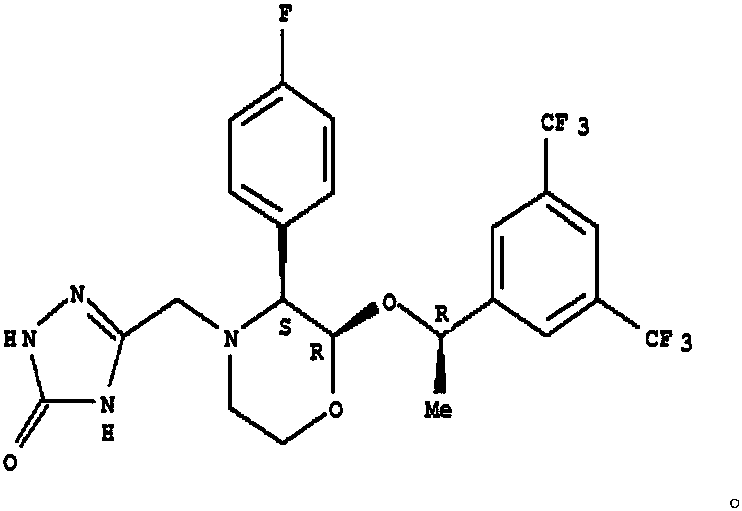
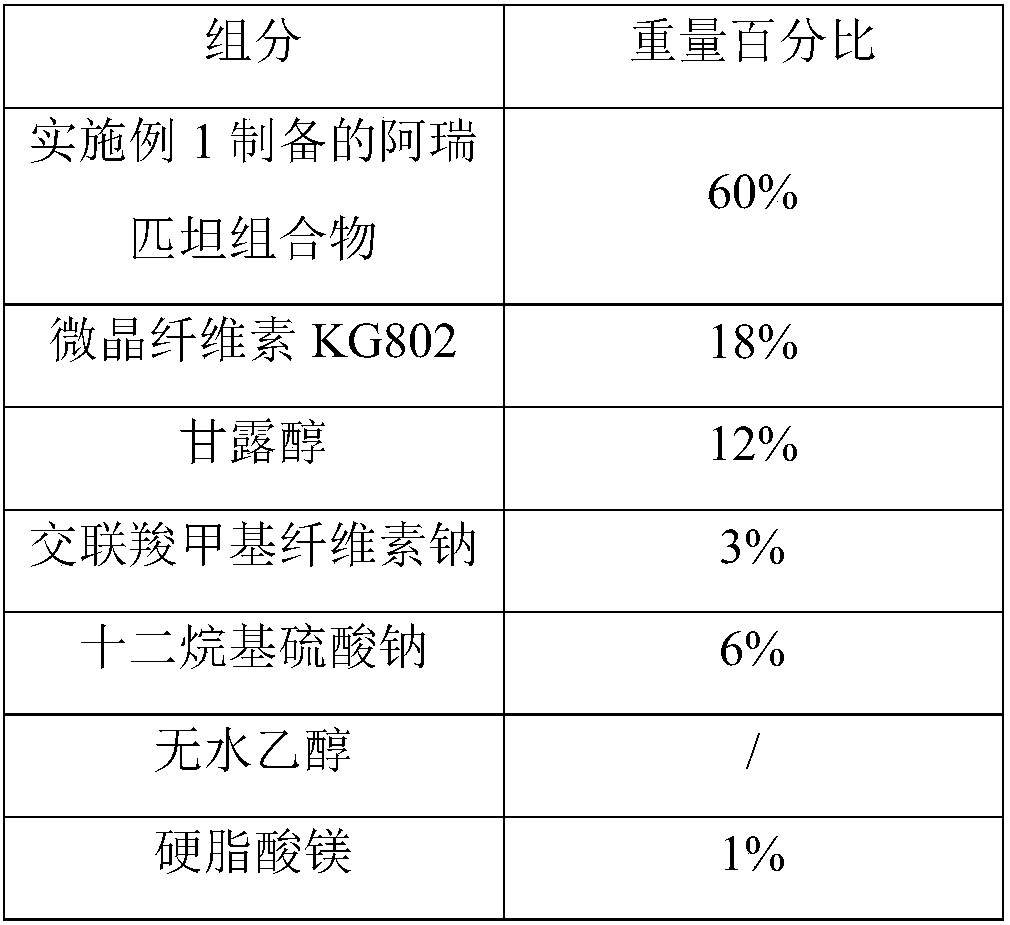
Preparation method of high stability aprepitant composition
A high-stability technology of aprepitant, applied in the field of medicine, can solve the problems of increasing impurity content of aprepitant, affecting drug quality, and decreasing stability, so as to ensure physical stability, improve solubility, and reasonable cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1. Preparation of the composition of the present invention
[0026] Pass 5.0g aprepitant and 0.5g hydroxypropyl cellulose SL type 3 times through a 60-mesh sieve, mix well, place in a refrigerated grinder to keep warm (-150℃) and grind for 1 hour to obtain aprepitant Tan composition.
Embodiment 2
[0027] Example 2. Preparation of the composition of the present invention
[0028] Pass 5.0g of aprepitant and 5.0g of hydroxypropylcellulose SL type 3 times through a 60-mesh screen, mix well, and place in a refrigerated grinder to keep warm (-170℃) and grind for 3 hours to obtain aprepitant. Tan composition.
Embodiment 3
[0029] Example 3. Preparation of the composition of the present invention
[0030] Pass 5.0g of aprepitant and 2.5g of hydroxypropyl cellulose SL type 3 times through a 60-mesh sieve. After mixing, place it in a refrigerated grinder to keep warm (-150℃) and grind for 1 hour to obtain aprepitant. Tan composition.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com