Aprepitant solid dispersion composition
A technology of solid dispersion and aprepitant, applied in drug combination, drug delivery, organic active ingredients, etc., can solve the problems of slow drug release, affecting drug release, slow dissolution rate, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Embodiment 1: the preparation of aprepitant raw material
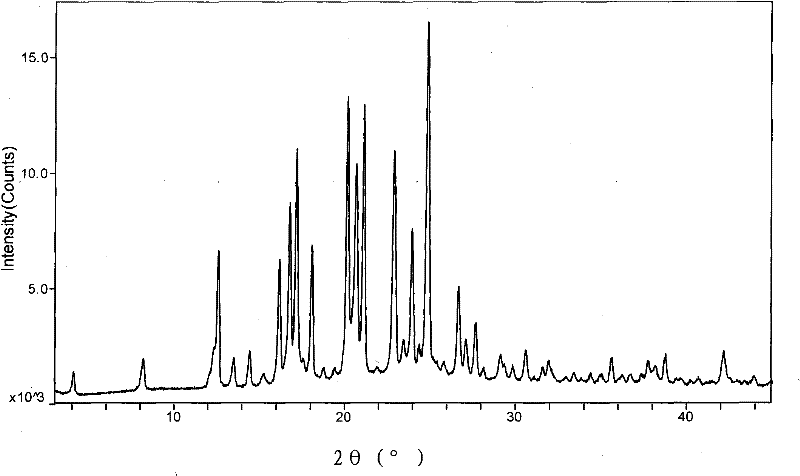
[0053] With reference to the method of Example 75 of Chinese invention patent application CN1142819A, crystalline aprepitant is prepared, and it has the following properties as determined by X-ray diffraction: figure 1 For the XRD pattern shown, the measurement conditions are: Cu (40kV, 40mA), 3.0°~45.0°(2θ) scanning, and the prominent X-ray diffraction peaks are as follows:
[0054]
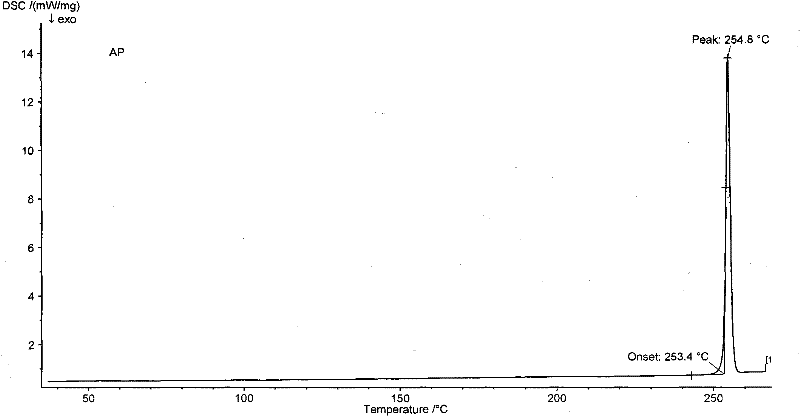
[0055] It also has figure 2 DSC spectrum shown.
Embodiment 2
[0057]Take 48 grams of aprepitant prepared in Example 1 and pass through a 100-mesh sieve for later use, and copovidone (Copovidone, N-vinylpyrrolidone / vinyl acetate copolymer) VA64 pass through a 60-mesh sieve for later use. Divide aprepitant equally into 3 parts, 12 grams each, and mix them with copovidone VA64 at a mass ratio of 1:5, 1:1 and 1:0.5, and then divide the powder mixture into equal parts each containing There are 12 parts of a mixture of 4 grams of aprepitant, and the mixtures containing different mass ratios are heated at 100°C, 150°C, 200°C, and 250°C to prepare solid dispersion compositions. The specific operation is to feed the powder mixture into a twin-screw extruder, pulverize, mix, and melt in the extruder, extrude a strip-shaped translucent melt, and place the extrudate in a tray to cool and solidify at room temperature. Grinding and passing through a 60-mesh sieve to obtain a solid dispersion composition powder, and measuring its stability and dissolut...
Embodiment 3
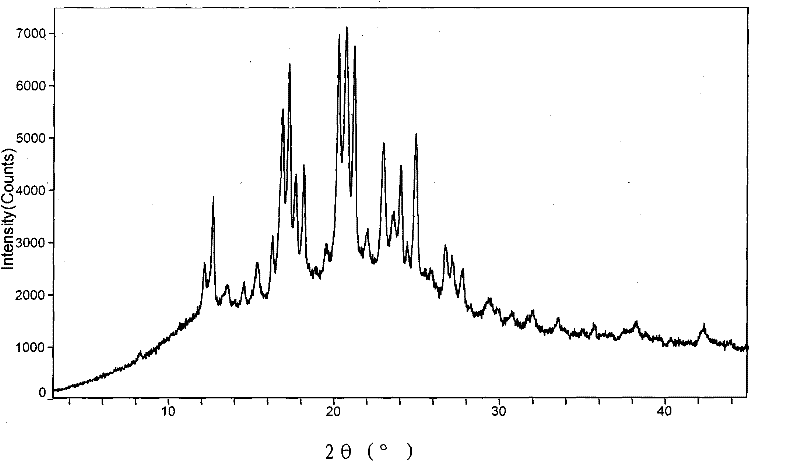
[0067] The aprepitant of 4 grams of embodiment 1 is mixed with 4 grams of copovidone (Copovidone, N-vinylpyrrolidone / vinyl acetate copolymer) VA64 respectively after passing through 100 mesh and 60 mesh sieves, then this powder mixture is fed into In the twin-screw extruder, pulverize, mix and melt in the extruder, extrude a strip-shaped translucent melt, put the extrudate in a pan, cool and solidify at room temperature, pulverize and grind through a 60-mesh sieve to obtain a solid dispersion composition powder, its XRD pattern is as attached image 3 As shown, the measurement conditions are: Cu (40kV, 40mA), 3.0°~45.0°(2θ) scanning, the prominent X-ray diffraction peaks are as follows:
[0068]
[0069]
[0070] It also has Figure 4 DSC spectrum shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com