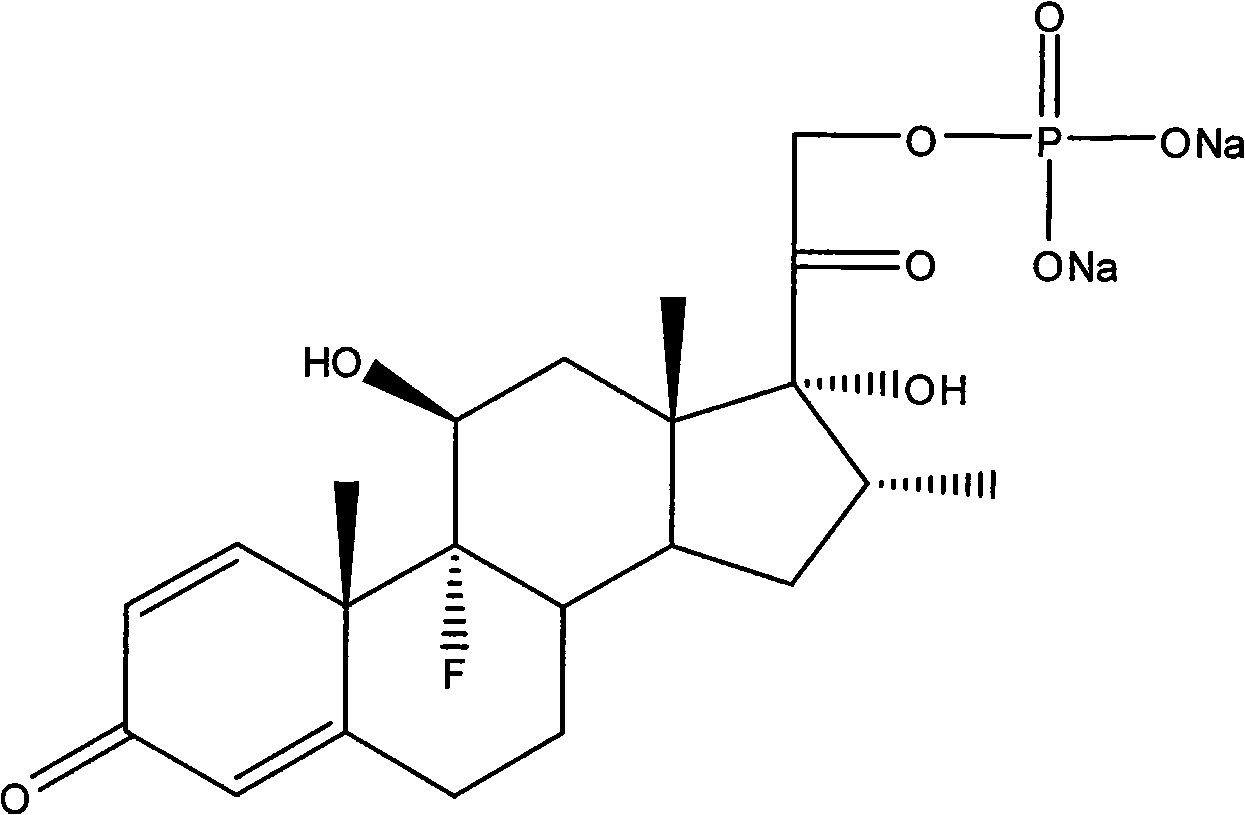
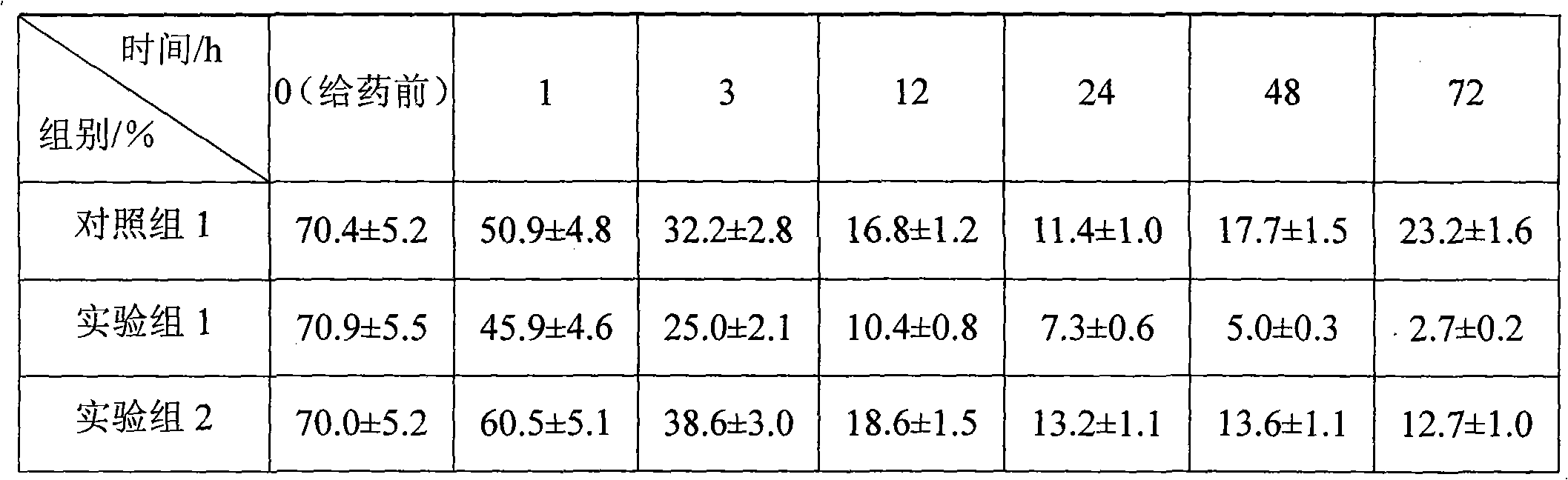
Dexamethasone sodium phosphate lipidosome injection
A technology of dexamethasone sodium phosphate and dexamethasone sodium phosphate, which is applied in the field of injection, can solve the problems of the influence of epidermal sebum structure, the large fluctuation of blood drug concentration, the short action clearance time, etc. Faster, less dosage effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
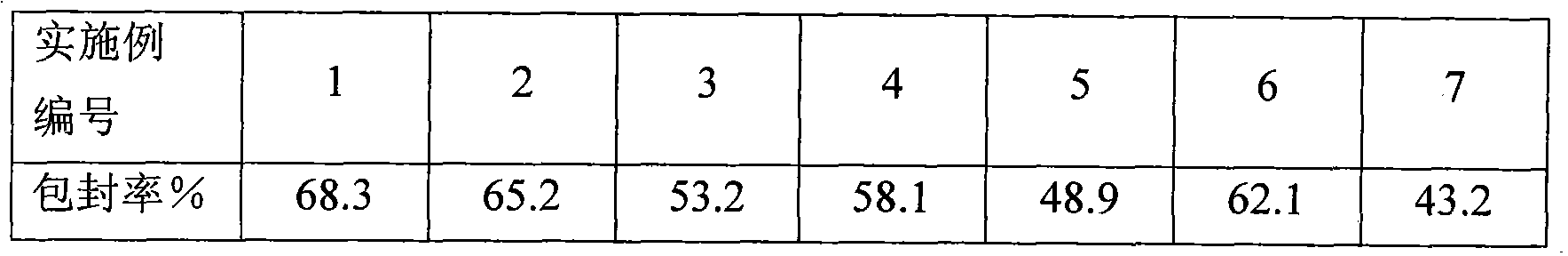
Examples
Embodiment 1
[0033] The formula of liposome solution:
[0034] Dexamethasone Sodium Phosphate 1g Soy Lecithin 20g Cholesterol 2g α-Tocopherol 1g
[0035] Water for injection to 1000ml (adjusted to pH=7.0, 0.2mol / L phosphate buffer)
[0036] Sodium chloride 40g is added in the above-mentioned water for injection and mixed to obtain water for injection (1)
[0037] Preparation:
[0038]Phospholipids, cholesterol, α-tocopherol that take prescription quantity are dissolved in dichloromethane / methanol (V: V=1: 1), will add and melt into the water for injection (1) 200ml of prescription quantity dexamethasone sodium phosphate, use Ultrasonic emulsification for 30 minutes, the resulting emulsion was evaporated at 37°C under reduced pressure to remove the solvent, and when the emulsion formed a viscous gel-like liquid, add the remaining water for injection (1), continue to evaporate the solvent under reduced pressure at 37°C, and ultrasonically Emulsify, make up the evaporated water for injecti...
Embodiment 2
[0040] The liposome solution formula is:
[0041] Dexamethasone Sodium Phosphate 0.5g Hydrogenated Soy Lecithin 10g Cholesterol 1.5g α-Tocopherol 0.5g
[0042] Water for injection to 1000ml (adjusted to pH=7.2, 0.2mol / L phosphate buffer)
[0043] Sodium chloride 70g is added in the above-mentioned water for injection and mixed to obtain water for injection (1)
[0044] Prepare 0.05% dexamethasone sodium phosphate liposome injection as in Example 1.
Embodiment 3
[0046] Liposome Solution Formula:
[0047] Dexamethasone Sodium Phosphate 0.25g Egg Yolk Lecithin 1.25g Cholesterol 0.2g
[0048] Water for injection to 1000ml (adjusted to pH=7.2, 0.2mol / L phosphate buffer)
[0049] Add 1g of EDTA and 10ml of propylene glycol to the above water for injection and mix well to obtain water for injection (1)
[0050] Prepare 0.05% dexamethasone sodium phosphate liposome injection as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com