Preparation method of hexadecadrol sodium phosphate freeze-dried powder injection
A technology of dexamethasone sodium phosphate and freeze-dried powder injection, which is applied in the field of medicine, can solve problems such as unstable product quality, high production cost, and poor stability, and achieve the effects of increasing single batch output, saving energy consumption, and ensuring quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
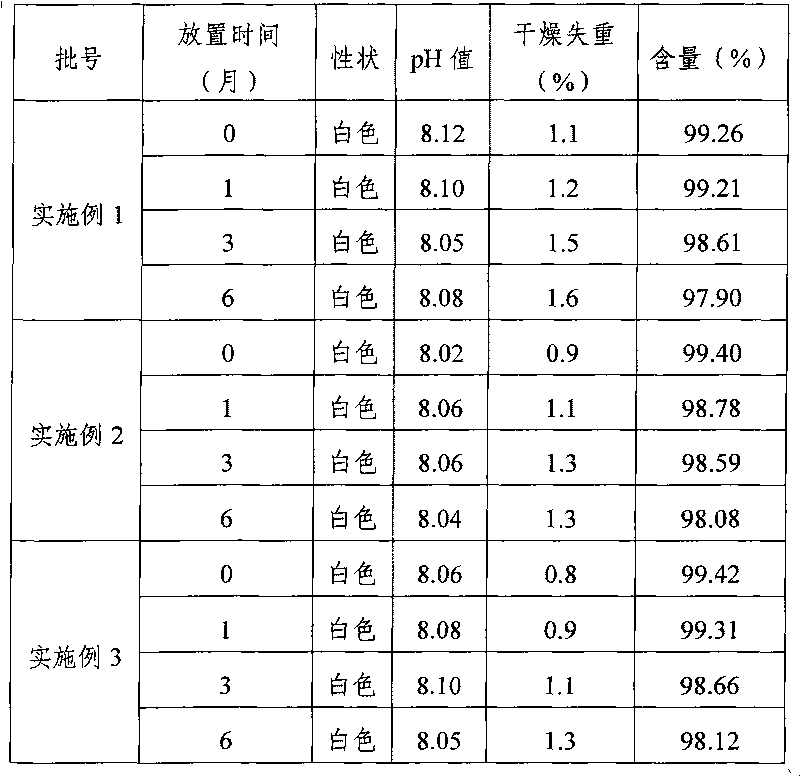
Embodiment 1
[0026] Dexamethasone Sodium Phosphate 20g
[0027] Mannitol 800g
[0028] Add water for injection to 10L
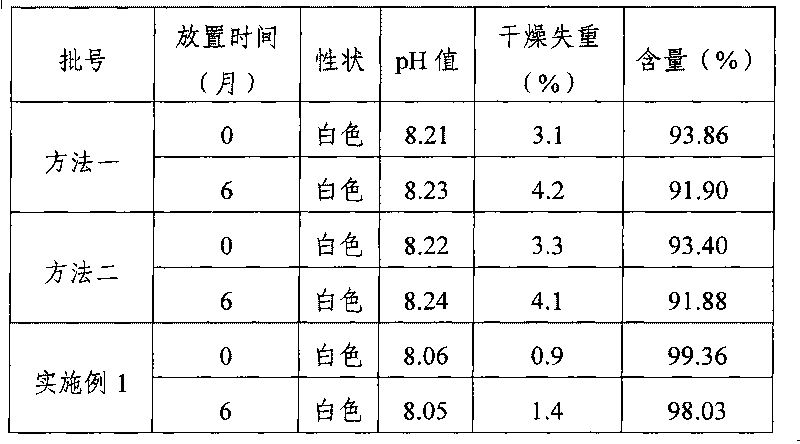
[0029] (1) Preparation of medicinal solution: Weigh 800g of mannitol quantitatively, add 6L of water for injection, stir until completely dissolved, add 2.0g / L of activated carbon according to the full volume, boil for 30min, filter to remove carbon; add 2L of water for injection, 20g of ground Sodium dexamethasone phosphate was dissolved completely, and the pH value of the medicinal solution was measured to be 8.12, and water for injection was added to 10 L, filtered through a 0.22 μm microporous membrane filter to a storage tank, prepared into a medicinal solution, and set aside;
[0030] (2) Freeze-drying: Pre-lower the temperature of the condenser of the freeze-drying machine (FCM50D type) to below -40°C, divide the above-mentioned medicinal solution into 2mL vials of 1mL (2mg specification) per bottle, and put them into freeze-drying For the plate layer in the mach...
Embodiment 2
[0036] Dexamethasone Sodium Phosphate 50g
[0037] Mannitol 200g
[0038] Add water for injection to 10L
[0039] (1) Preparation of medicinal solution: Weigh 200g of mannitol quantitatively, add 3L of water for injection, stir until completely dissolved, add 1.0g / L of activated carbon according to the full volume, boil for 15min, filter to remove carbon; add 5L of water for injection, 50g of ground Sodium dexamethasone phosphate was dissolved completely, and the pH value of the medicinal solution was measured to be 8.02, and water for injection was added to 10 L, filtered through a 0.22 μm microporous membrane filter to a storage tank, prepared into a medicinal solution, and set aside;
[0040] (2) Freeze-drying: Pre-lower the temperature of the condenser of the freeze-drying machine (FCM50D type) to below -40°C, divide the above-mentioned medicinal solution into 2mL vials of 1mL (5mg specification) per bottle, and put them into freeze-drying For the plate layer in the mach...
Embodiment 3
[0046] Dexamethasone Sodium Phosphate 20g
[0047] Mannitol 600g
[0048] Add water for injection to 10L
[0049] (1) Preparation of medicinal solution: Weigh 600g of mannitol quantitatively, add 5L of water for injection, stir until completely dissolved, add 2.0g / L of activated carbon according to the full volume, boil for 20min, filter to remove carbon; add 3L of water for injection, 50g of ground Sodium dexamethasone phosphate was dissolved completely, and the pH value of the medicinal solution was measured to be 8.06, and water for injection was added to 10 L, filtered through a 0.22 μm microporous membrane filter to a storage tank, prepared into a medicinal solution, and set aside;
[0050] (2) Freeze-drying: Pre-lower the temperature of the condenser of the freeze-drying machine (FCM50D type) to below -40°C, divide the above-mentioned medicinal solution into 2mL vials of 1mL (2mg specification) per bottle, and put them into freeze-drying For the plate layer in the mach...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com