Dexamethasone sodium phosphate powder injection and production method thereof
A technology of dexamethasone sodium phosphate and powder injection, which is applied in directions such as antidote, anti-inflammatory agent, powder delivery, etc., can solve the problems of difficulty in ensuring the stability of filling volume, adverse effects on medication safety, and low dosage of propylene glycol, etc. Achieve the effect of reducing loss, reducing energy consumption and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
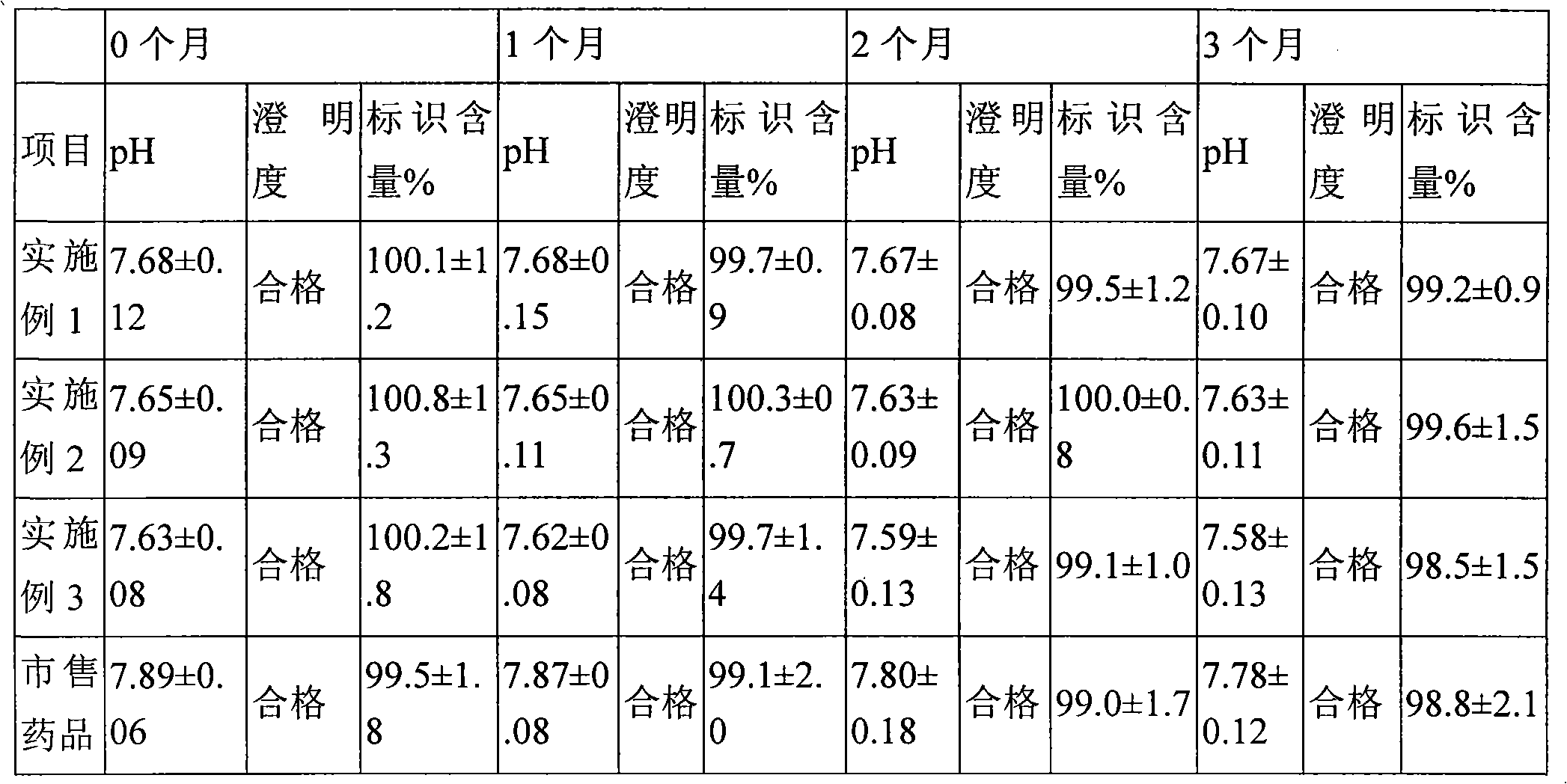
Embodiment 1
[0030] Dexamethasone Sodium Phosphate 50g
[0031]Methanol to 1000ml
[0032] (1) Dissolve the prescribed amount of dexamethasone sodium phosphate in the prescribed amount of methanol, and stir until completely dissolved to obtain the medicinal solution (1).
[0033] (2) The liquid medicine (1) is filtered with a G6 vertically fused glass funnel, and the filtrate is ultrafiltered with a polyimide (PI) ultrafiltration membrane with a molecular weight cut-off of 6000, and filled into a 1ml vial with a micro-filler , each bottle is filled with 0.1ml, a total of 10,000 bottles are filled, and each bottle contains 5 mg of dexamethasone sodium phosphate.
[0034] (3) Put the vials filled in step (2) into a vacuum drying oven, slowly vacuum-dry them at room temperature (20°C), keep the internal pressure of the drying oven at 30kpa, and dry at a temperature of 35°C until methanol is almost After evaporating to dryness, raise the drying temperature to 50°C, apply vacuum to keep the p...
Embodiment 2
[0036] Dexamethasone Sodium Phosphate 20g
[0037] Methanol to 1000ml
[0038] (1) Dissolve the prescribed amount of dexamethasone sodium phosphate in the prescribed amount of methanol, and stir until completely dissolved to obtain the medicinal solution (1).
[0039] (2) The liquid medicine (1) is filtered with a G6 vertically fused glass funnel, and the filtrate is ultrafiltered with a polyimide (PI) ultrafiltration membrane with a molecular weight cut-off of 6000, and filled into a 1ml vial with a micro-filler , each bottle is filled with 0.1ml, a total of 10,000 bottles are filled, and each bottle contains 2mg of dexamethasone sodium phosphate.
[0040] (3) Put the vials filled in step (2) into a vacuum drying oven, slowly vacuum-dry them at room temperature (20°C), keep the internal pressure of the drying oven at 25kpa, and dry at a temperature of 30°C until methanol is almost After evaporating to dryness, raise the drying temperature to 50°C, apply vacuum to keep the p...
Embodiment 3
[0042] Dexamethasone Sodium Phosphate 10g
[0043] 95% ethanol to 1000ml
[0044] (1) Dissolve the prescribed amount of dexamethasone sodium phosphate in the prescribed amount of 95% ethanol, and stir until completely dissolved to obtain the medicinal solution (1).
[0045] (2) The liquid medicine (1) is filtered with a G6 vertically fused glass funnel, and the filtrate is ultrafiltered with a polyimide (PI) ultrafiltration membrane with a molecular weight cut-off of 6000, and filled into a 1ml vial with a micro-filler , each bottle is filled with 0.1ml, a total of 10,000 bottles are filled, and each bottle contains 1mg of dexamethasone sodium phosphate.
[0046] (3) Put the vials filled in step (2) into a vacuum drying oven, slowly vacuum-dry them at room temperature (20°C), keep the internal pressure of the drying oven at 25kpa, and dry at a temperature of 30°C until ethanol is almost After evaporating to dryness, vacuumize to keep the pressure in the drying oven at 5 kap,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com