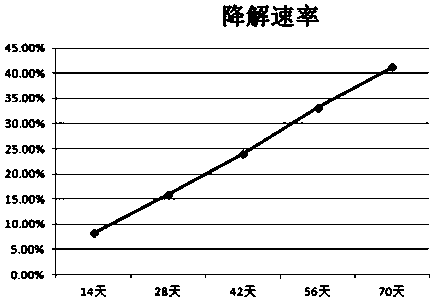
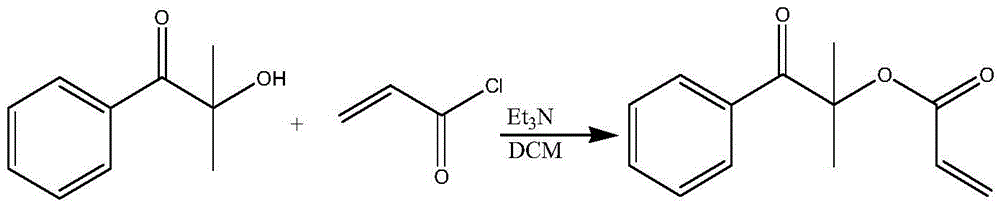
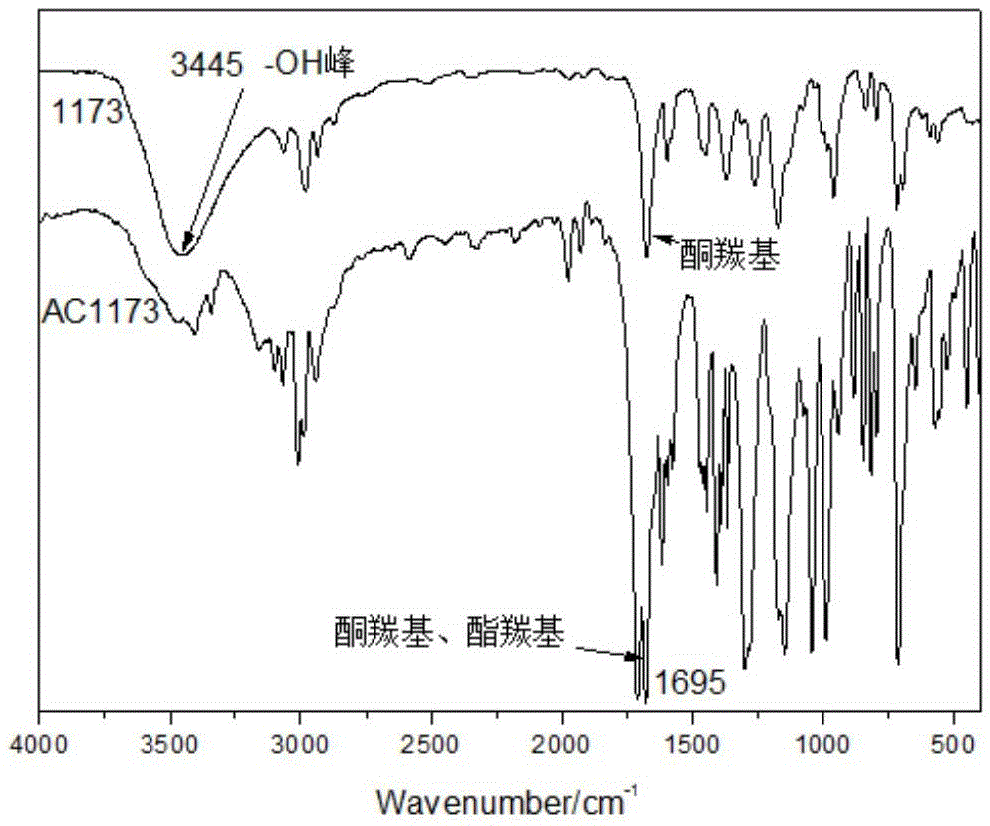
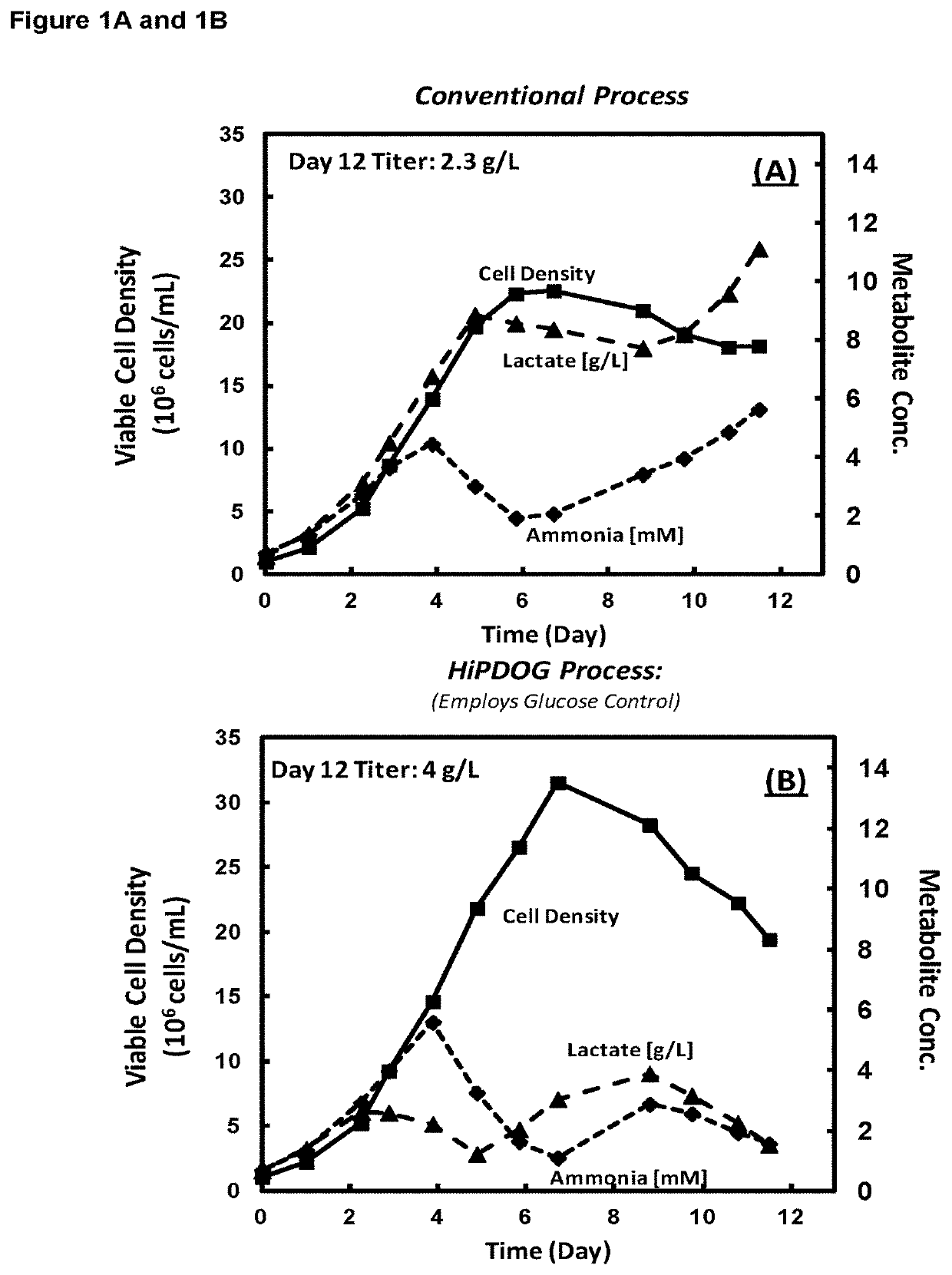
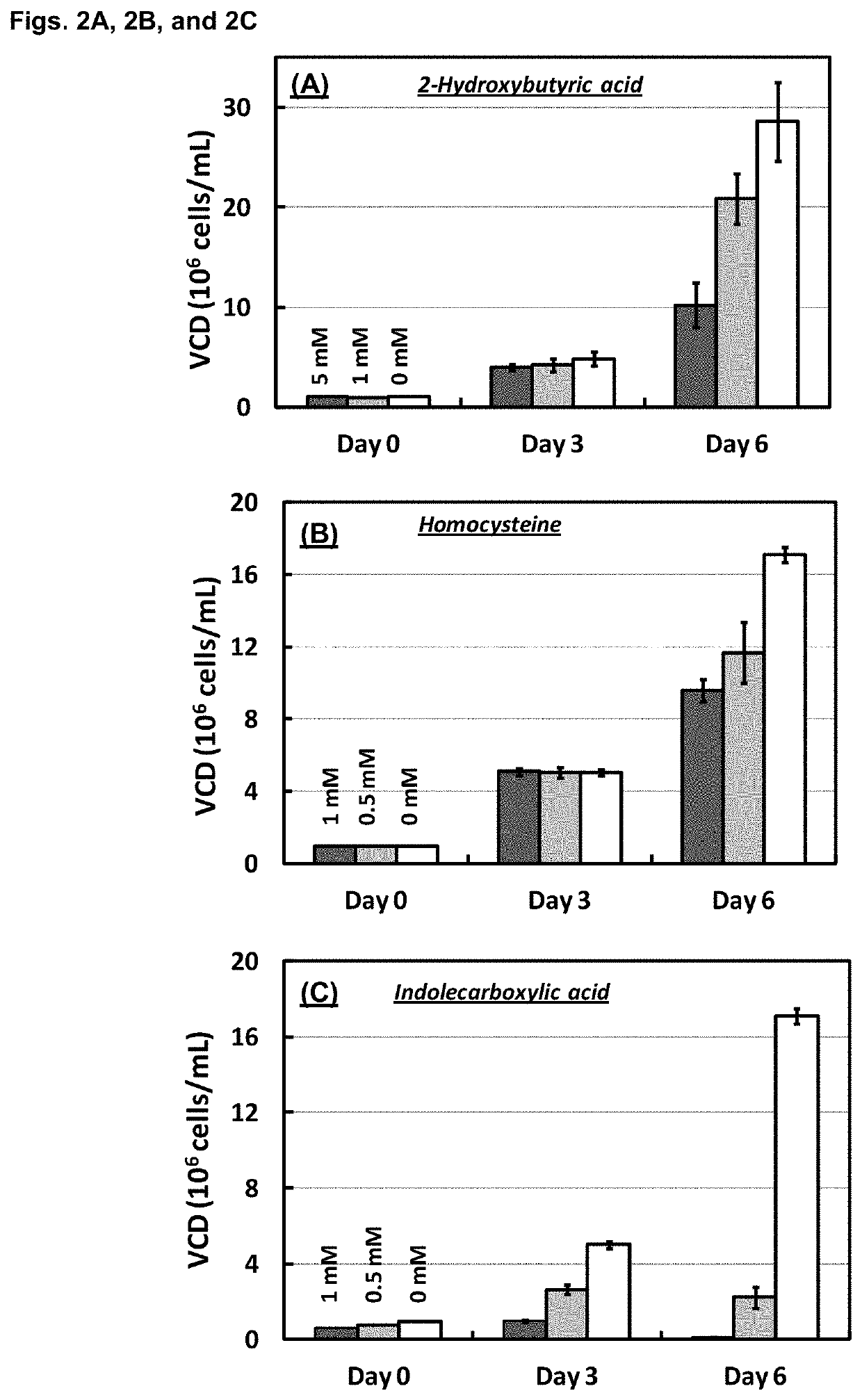
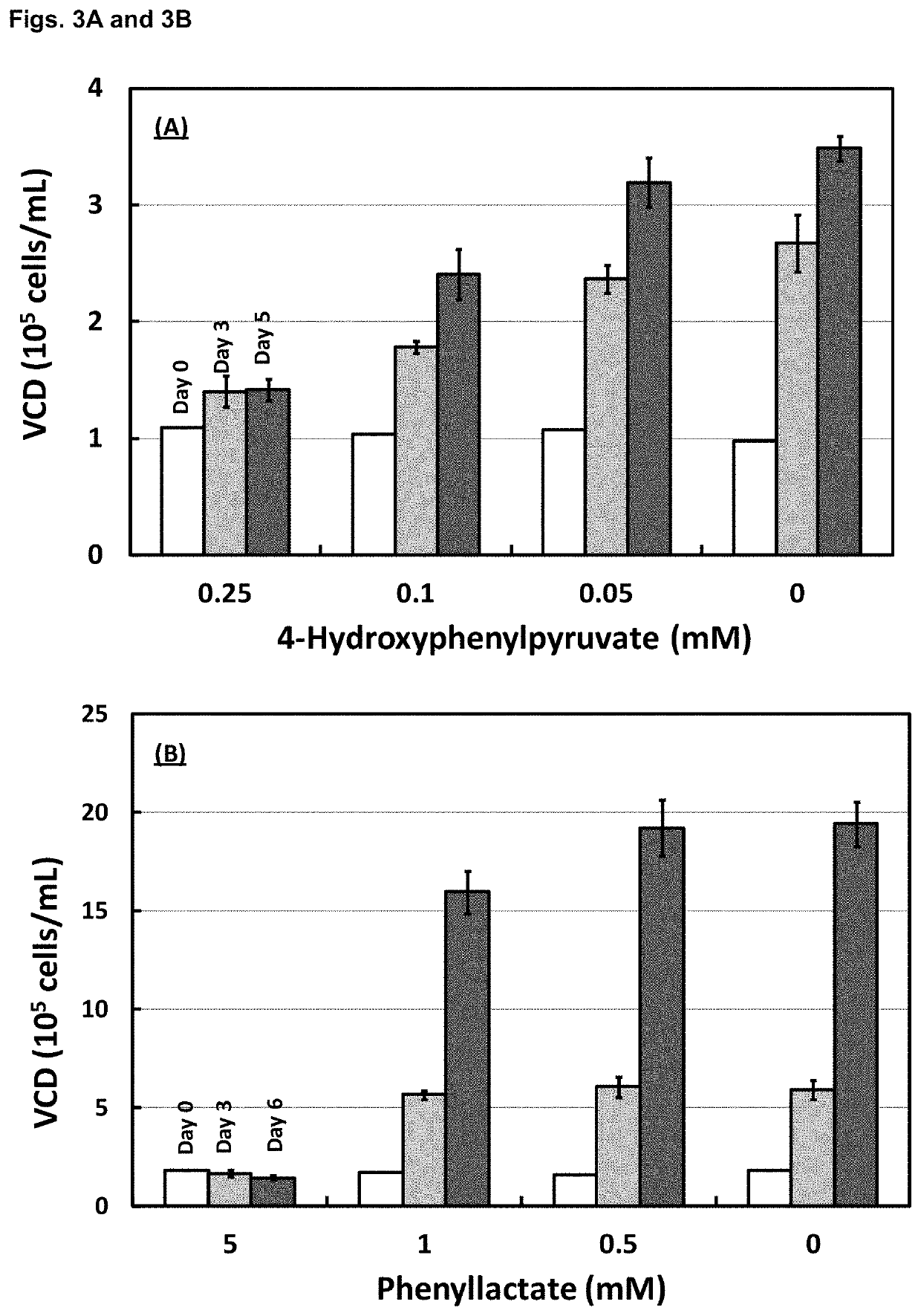
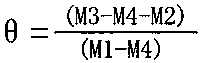Patents
Literature
131 results about "Phenylacetone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Phenylacetone is an organic compound with the chemical formula C₆H₅CH₂COCH₃. It is a colorless oil that is soluble in organic solvents. This substance is used in the manufacture of methamphetamine and amphetamine, where it is commonly known as P2P. Due to the illicit uses in clandestine chemistry, it was declared a schedule II controlled substance in the United States in 1980. In humans, phenylacetone occurs as a metabolite of amphetamine and methamphetamine via FMO3-mediated oxidative deamination.
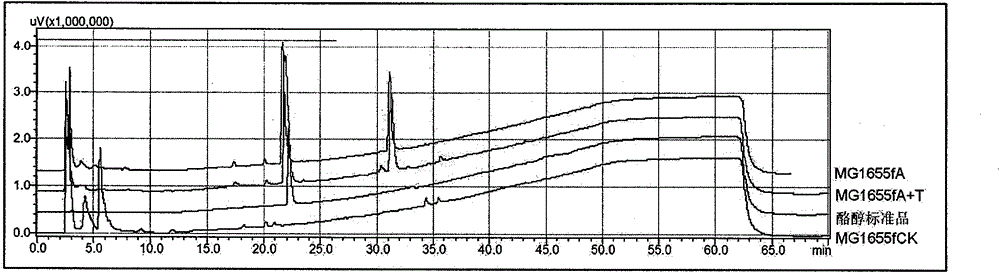
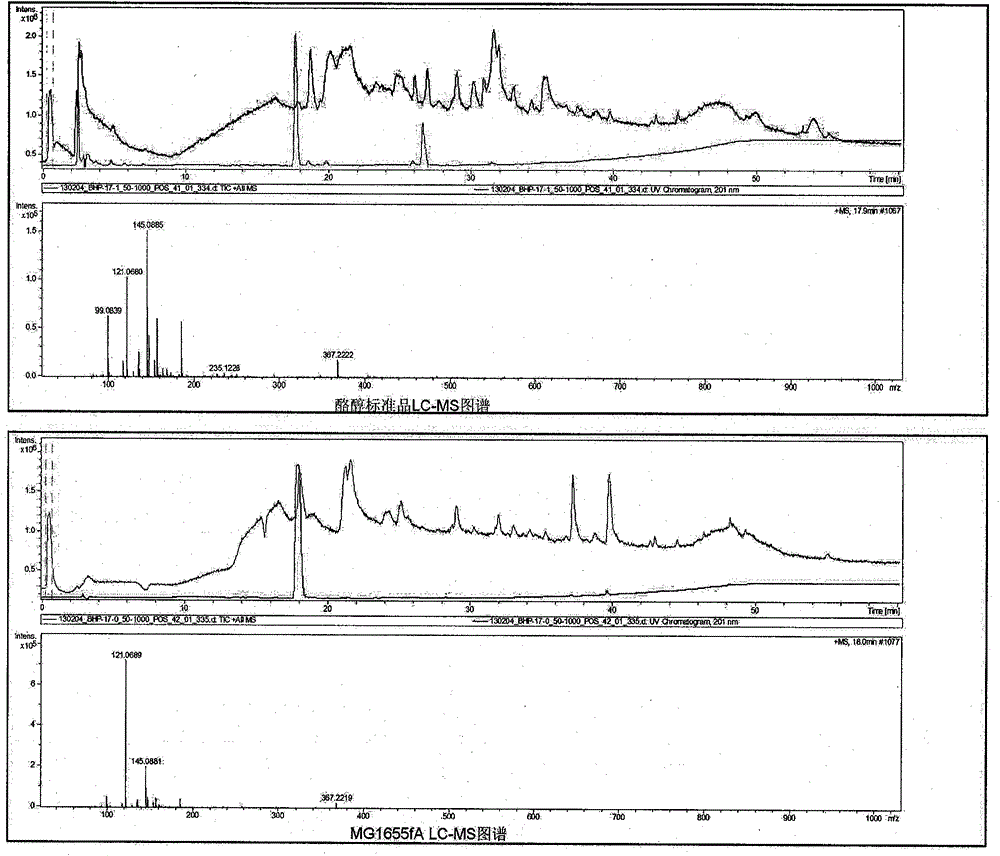
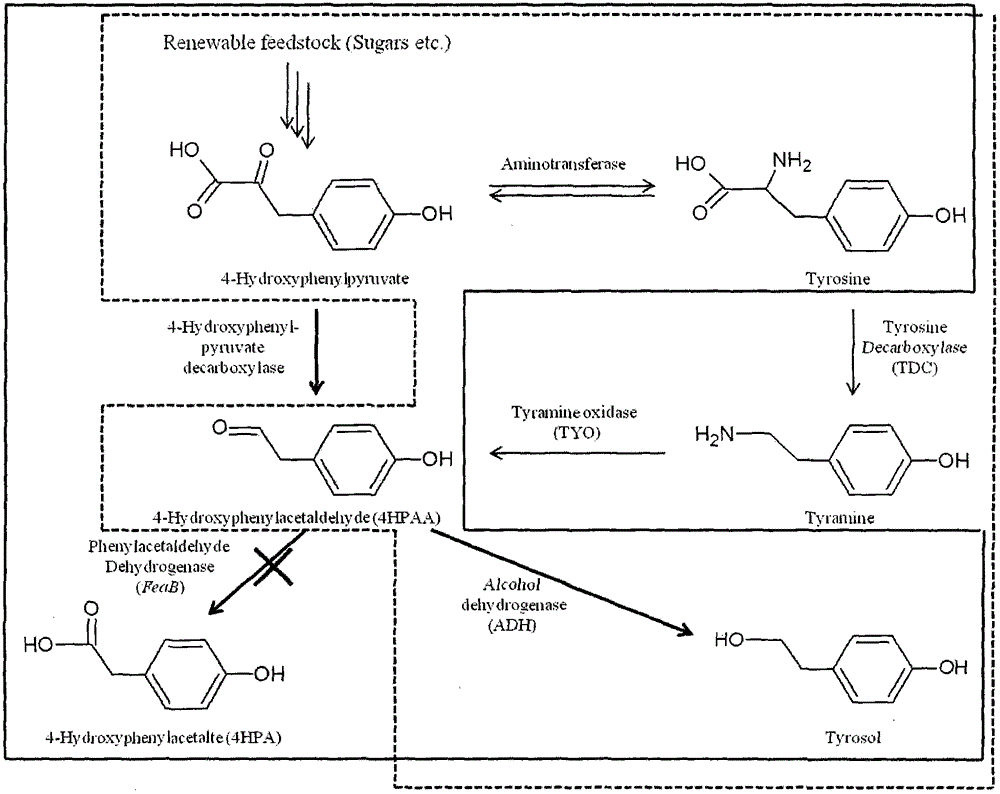
Method for biosynthesis of tyrosol in Escherichia coli and application of tyrosol
ActiveCN104099379ABacteriaMicroorganism based processesEscherichia coliPhenylacetaldehyde dehydrogenase
The invention relates to a method for biosynthesis of tyrosol in Escherichia coli and application of tyrosol, which belongs to the field of bioengineering technology. According to the method for biosynthesis of tyrosol in Escherichia coli, tyrosine or glucose is used as a substrate, (4-hydroxyphenyl)pyruvic acid is produced under the catalysis of ammonialyase coded by Escherichia coli; (4-hydroxyphenyl)acetaldehyde is produced under the action of the microzyme 4-hydroxyphenylpyruvate decarboxylase; (4-hydroxyphenyl)acetaldehyde is catalyzed by alcohol dehydrogenase so as to produce tyrosol; and a phenylacetaldehyde dehydrogenase gene of Escherichia coli is knocked out at the same time so as to block the transformation channel for (4-hydroxyphenyl)acetaldehyde into (4-hydroxyphenyl)acetic acid and promote accumulation of (4-hydroxyphenyl)acetaldehyde and transformation of (4-hydroxyphenyl)acetaldehyde into tyrosol. The invention provides a novel production approach for tyrosol; and the method lays a foundation for large-scale industrial production of tyrosol and has important economic values and social benefits.
Owner:TIANJIN INST OF IND BIOTECH CHINESE ACADEMY OF SCI
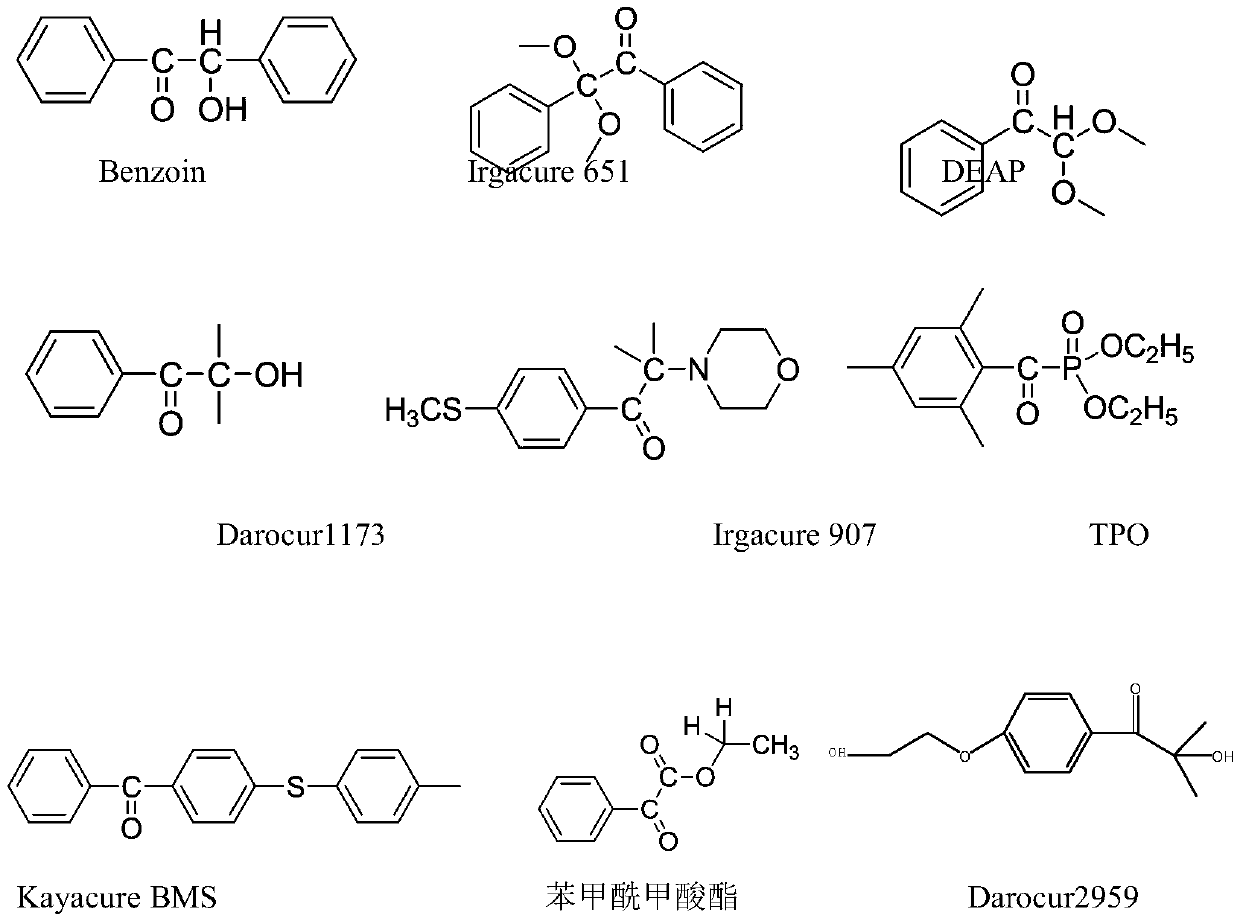
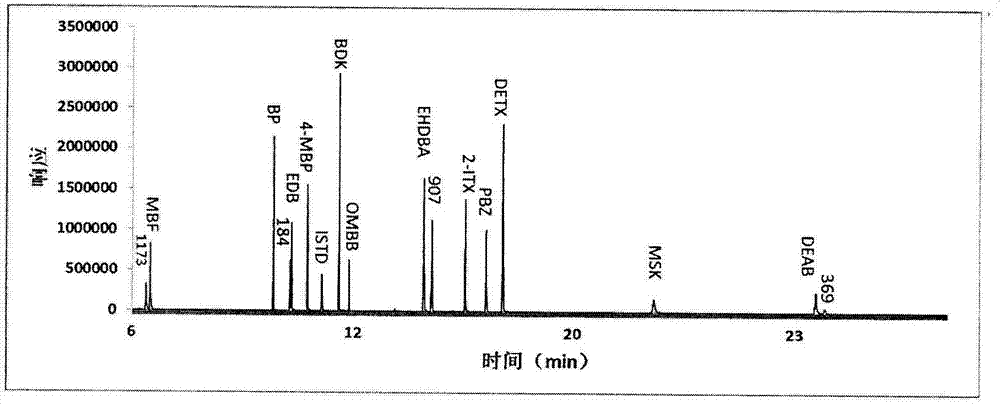
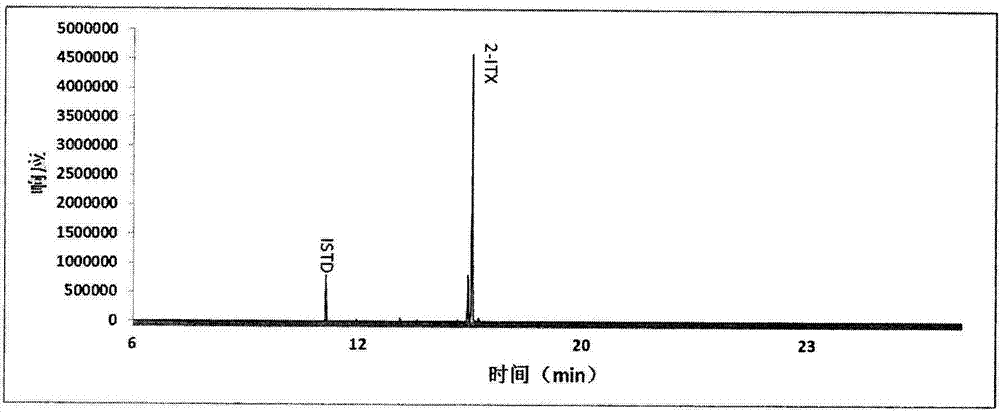
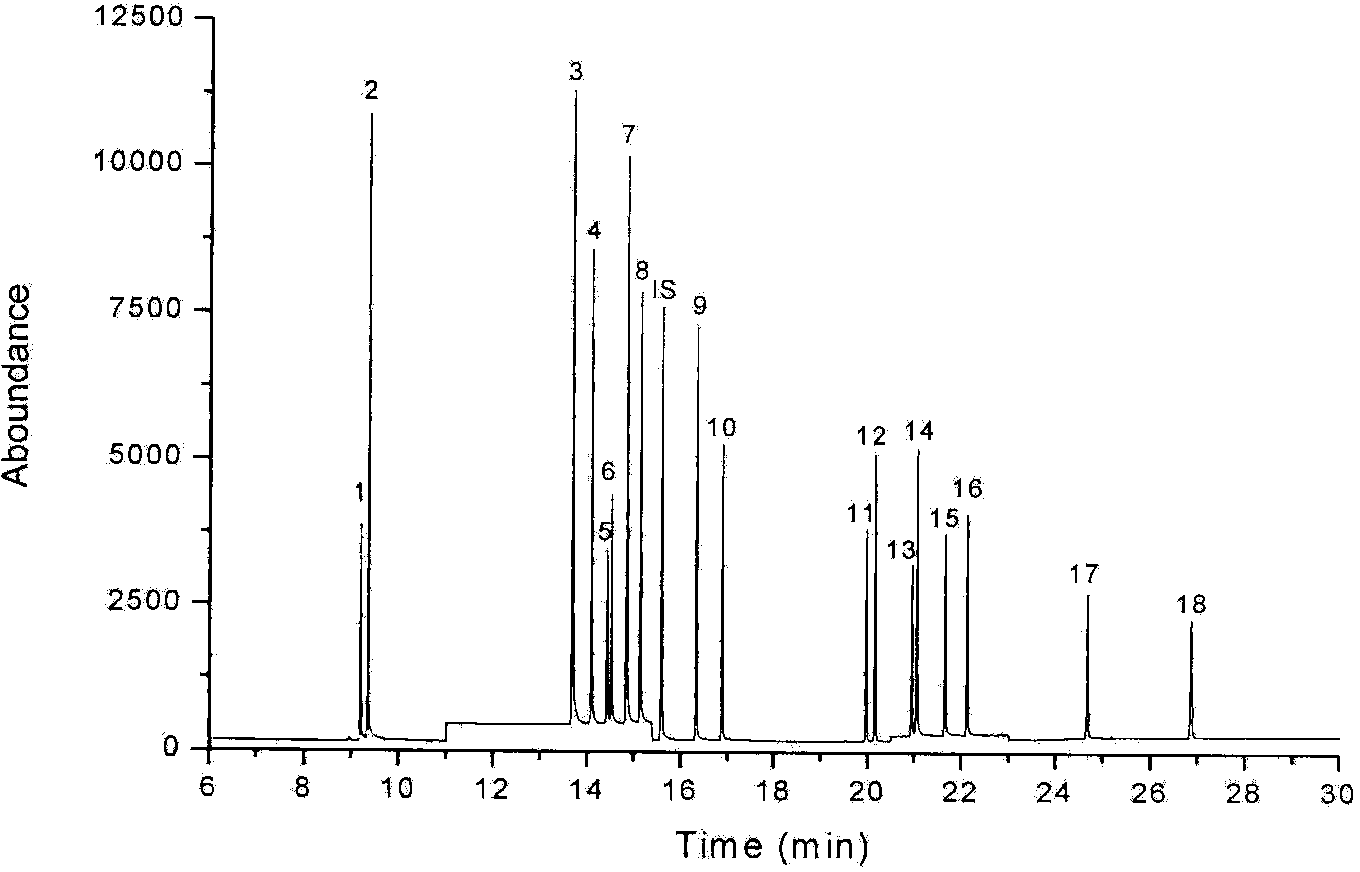
Detection method of determining 16 types of photoinitiators in paper printing and packaging material
ActiveCN102818874AEnable simultaneous analysisRealize determinationComponent separationLiquid liquid partitionPrinting ink
The invention relates to a detection method of determining 16 types of photoinitiators in a paper printing and packaging material, which is used for determining residual amounts of the 16 types of photoinitiators of 2-hodroxy-2-methyl-1-phenylacetone, mehtyl phenylglyoxylate, benzophenone and the like and is characterized by comprising the following concrete steps of: a, sample pretreatment; b, preparation of a standard work solution; c, instrumental analysis; and d, result calculation. Compared with the prior art, through sample pretreatment and optimization of instrumental analysis conditions, the detection method has the advantages that efficiency is high, the 16 kinds of photoinitiators can be simultaneously analyzed and determined within 25m; and printing ink impurities in a sample extraction solution can be better removed in a liquid-liquid distribution impurity removal mode adopted in the sample pretreatment process, so that cost is low, consumption is little, efficiency is high, and sensitivity is high; and in addition, the detection method also has the advantages of high accuracy and good repeatability.
Owner:CHINA NAT TOBACCO QUALITY SUPERVISION & TEST CENT
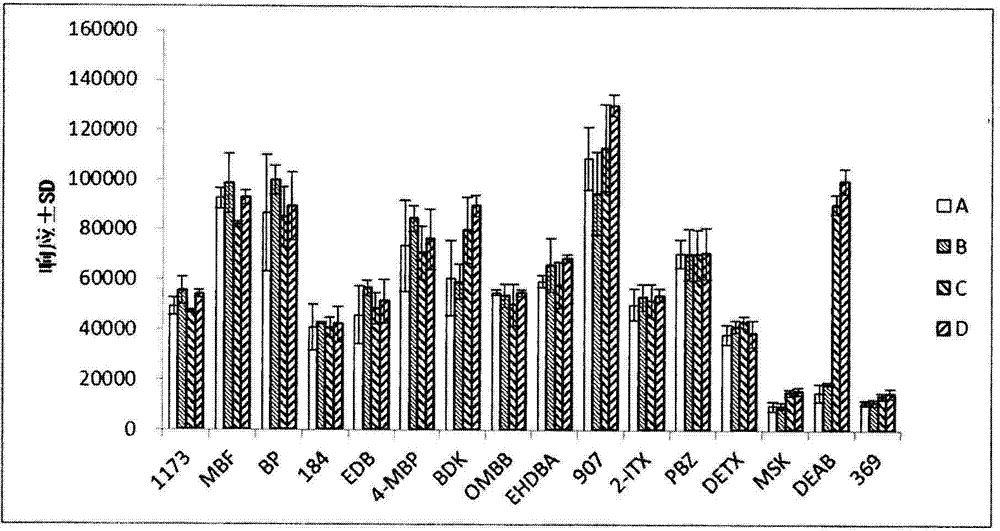
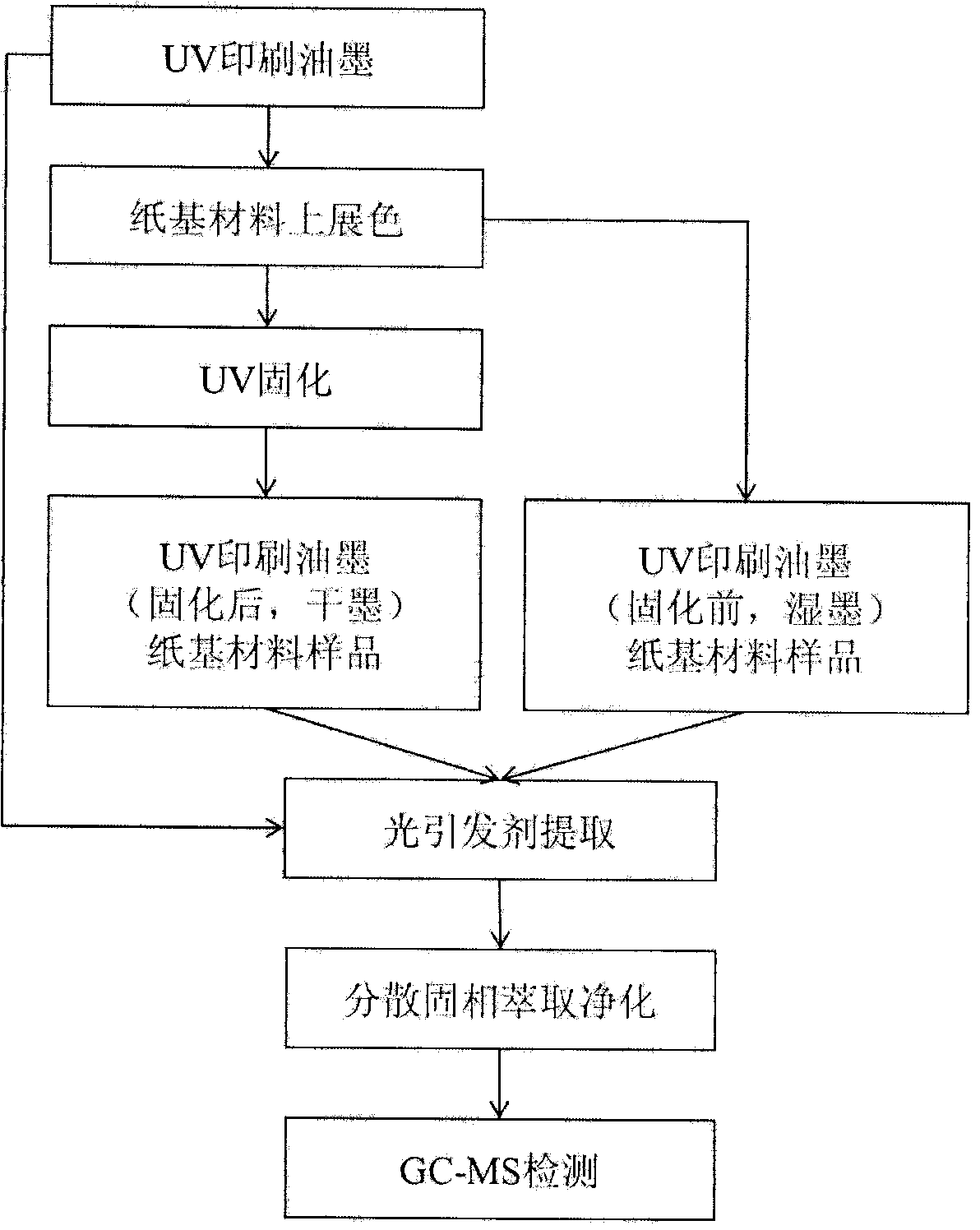
Aanalysis method of residual quantities of eighteen photoinitiators in ultra violet (UV) printing ink
ActiveCN103399105AEfficient removalExtend detection objectComponent separationPrinting inkSolid phase extraction
An analysis method of residual quantities of eighteen photoinitiators in ultra violet (UV) printing ink aims to determine the residual quantities of the eighteen photoinitiators including 2-hydroxy-2-methyl-1-phenylpropanone, methyl benzoylformate, diphenyl ketone and the like. The analysis method is characterized by mainly comprising the following steps: 1) preparation of a test sample, 2) extraction of a sample, 3) dispersive solid phase extraction purification, 4) preparation of standard working solutions, 5) instrument analysis and 6) result calculating. According to the analysis method, through sample pretreatment and optimization and validation by instrument analysis, a sample pretreatment technology of dispersive solid phase extraction purification is applied to the determination of the residual quantities of the photoinitiators in the UV printing ink sample, and compared with a liquid-liquid extraction (LLE) technology and a solid phase extraction (SPE) technology adopted by related methods in the prior art, the analysis method has the characteristics of being rapid, simple, cheap, effective, reliable and safe. So far, the analysis method covers most kinds of residual photoinitiators than analysis methods at home and abroad.
Owner:CHINA NAT TOBACCO QUALITY SUPERVISION & TEST CENT
High-fire-retardant ultraviolet curing coating
InactiveCN105038569AImprove wear resistanceStrong adhesionFireproof paintsPolyurea/polyurethane coatingsPolyethylene glycolDefoaming Agents
The invention discloses a high-fire-retardant ultraviolet curing coating. The high-fire-retardant ultraviolet curing coating comprises the following raw materials: urethane acrylate, epoxy acrylate, epoxidized soybean oil acrylate, phosphorus-containing acrylate, N-vinyl pyrrolidone, polyethylene glycol acrylate, tri(propylene glycol) diacrylate, pentaerythritol triacrylate, trimethylolpropane triacrylate, benzophenone, 2-methyl-2-hydroxyl-1-phenylacetone, coumarone, nano-silica, nano aluminum hydroxide, ammonium polyphosphate, silica powder, expanded graphite, a flattening agent, a defoaming agent, a wetting agent, a dispersant and a coupling agent. The high-fire-retardant ultraviolet curing coating disclosed by the invention is good in flame retardance property, high in adhesion force, excellent in comprehensive performance and long in service life.
Owner:WUHU SHUANGBAO BUILDING MATERIAL
Ultraviolet curing coating formula for eliminating formaldehyde in air and preparation technology thereof
ActiveCN103305115AWork quicklyStrong adhesionPolyurea/polyurethane coatingsUltravioletHexanediol diacrylate
The invention relates to the technical field of ultraviolet curing coatings, and particularly relates to an ultraviolet curing coating formula for eliminating formaldehyde in air and a preparation technology thereof. The coating consists of the following components in percentage by weight: 20-40% of urethane acrylate, 10-30% of 1,6 hexanediol diacrylate, 1-10% of trimethylolpropane triacrylate, 1-5% of 2-phenoxy ethyl acrylate, 1-5% of dispersing agent, 2-6% of superfine silicon dioxide, 1-4% of superfine aluminum oxide powder, 1-12% of matte powder, 1-4% of wax powder, 4-6% of 2-hydroxyl-2-methyl-1-phenyl-1-acetone, 0.1-2% of surface aid and 1-15% of formaldehyde removal aid. The preparation technology of the coating comprises the steps of sequentially feeding the raw materials and stirring for uniformly mixing. Through the formula and the technology provided by the invention, the prepared coating is applicable to the fast operation of a factory production line, and also has the protection functions such as good adhesion, scratch resistance and the like.
Owner:韶关市和荣化工有限公司
Weatherproof bronzing UV gloss oil
ActiveCN101284968AMeet bronzing requirementsLiquid surface applicatorsCoatingsAcrylic resinPhenylacetone
The invention relates to all-weather UV gloss oil capable of bronzing. Raw material consists of the following compositions in percentage by weight: 40 to 60 percent of amino-acrylics, 1 to 2 percent of 1-hydroxy cyclohexyl phenylacetone, 30 to 40 percent of 2-hydroxy acrylics, 1 to 2 percent of A-hydroxy isobutyrophenone, 8 to 18 percent of epoxy acrylic resin. The UV gloss oil has the advantage of long-term effect, is not limited by the light-curing condition in use and can be gilded after being solidified and placed for a long time.
Owner:HEBEI ANTAI PLASTIC PACKAGING PROD LTD
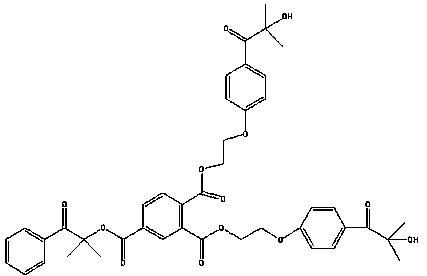
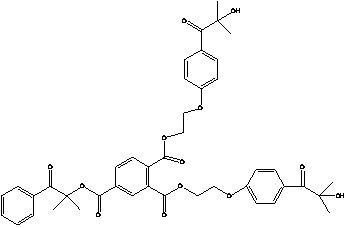
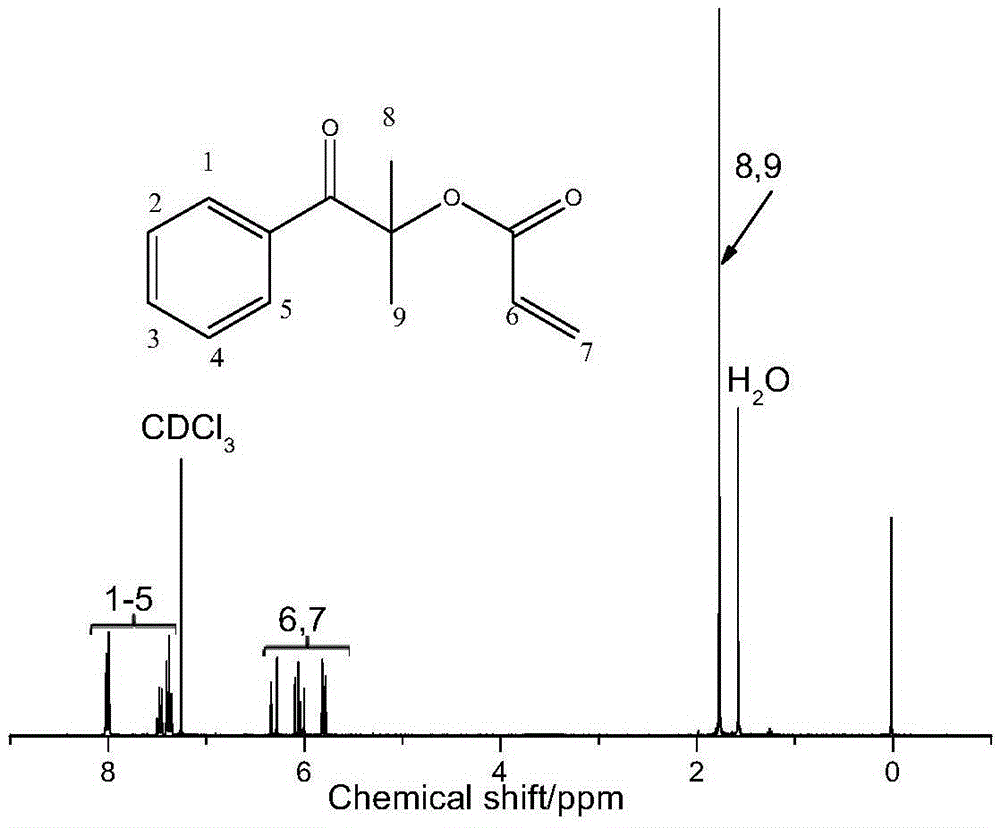
Method for synthesizing 2-methyl-1-[4-(methylthio)phenyl]-2-(4-morpholinyl)-1-propanon
ActiveCN101659644AReduce manufacturing costEasy to operateOrganic chemistrySolution systemPhotoinitiator
The invention relates to a method for synthesizing a high-efficiency photoinitiator 2-methyl-1-[4-(methylthio)phenyl]-2-(4-morpholinyl)-1-propanon. In the method, 2-methyl-1-[4-(chloro)phenyl]-2-(4-morpholinyl)-1-propanon serving as a raw material is reacted with sodium methyl mercaptide in an organic water two phase solution system taking quaternary ammonium salt as a catalyst to generate the target product.
Owner:INSIGHT HIGH TECH (BEIJING) CO LTD
Unsymmetrical hydrogen migration synthesizing method for (R, R)-formoterol
InactiveCN101468954AIn line with the concept of green chemistryLow costOrganic compound preparationCarboxylic acid amides preparationSynthesis methodsPhotochemistry
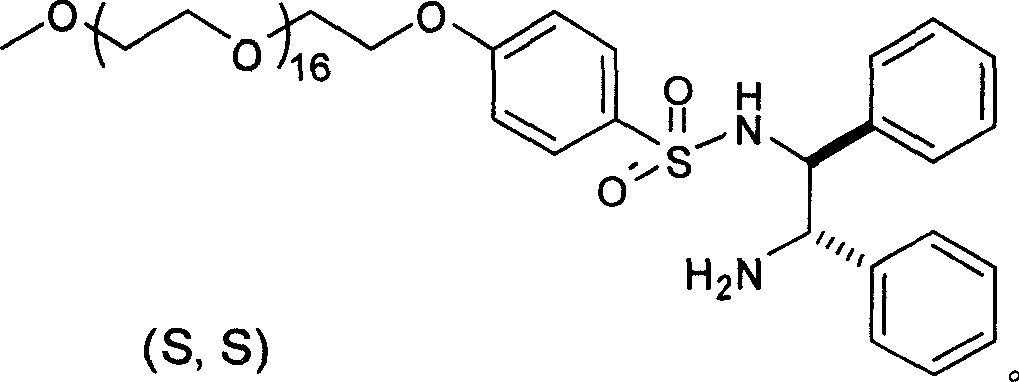
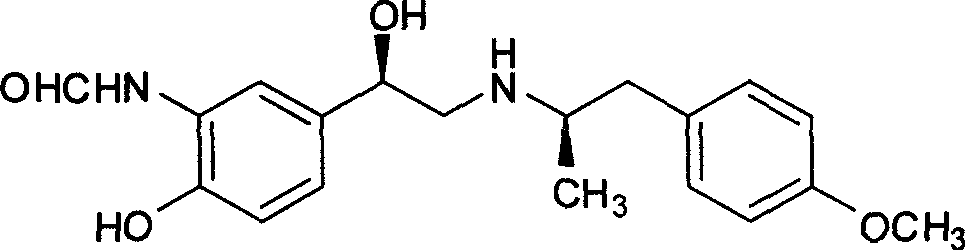
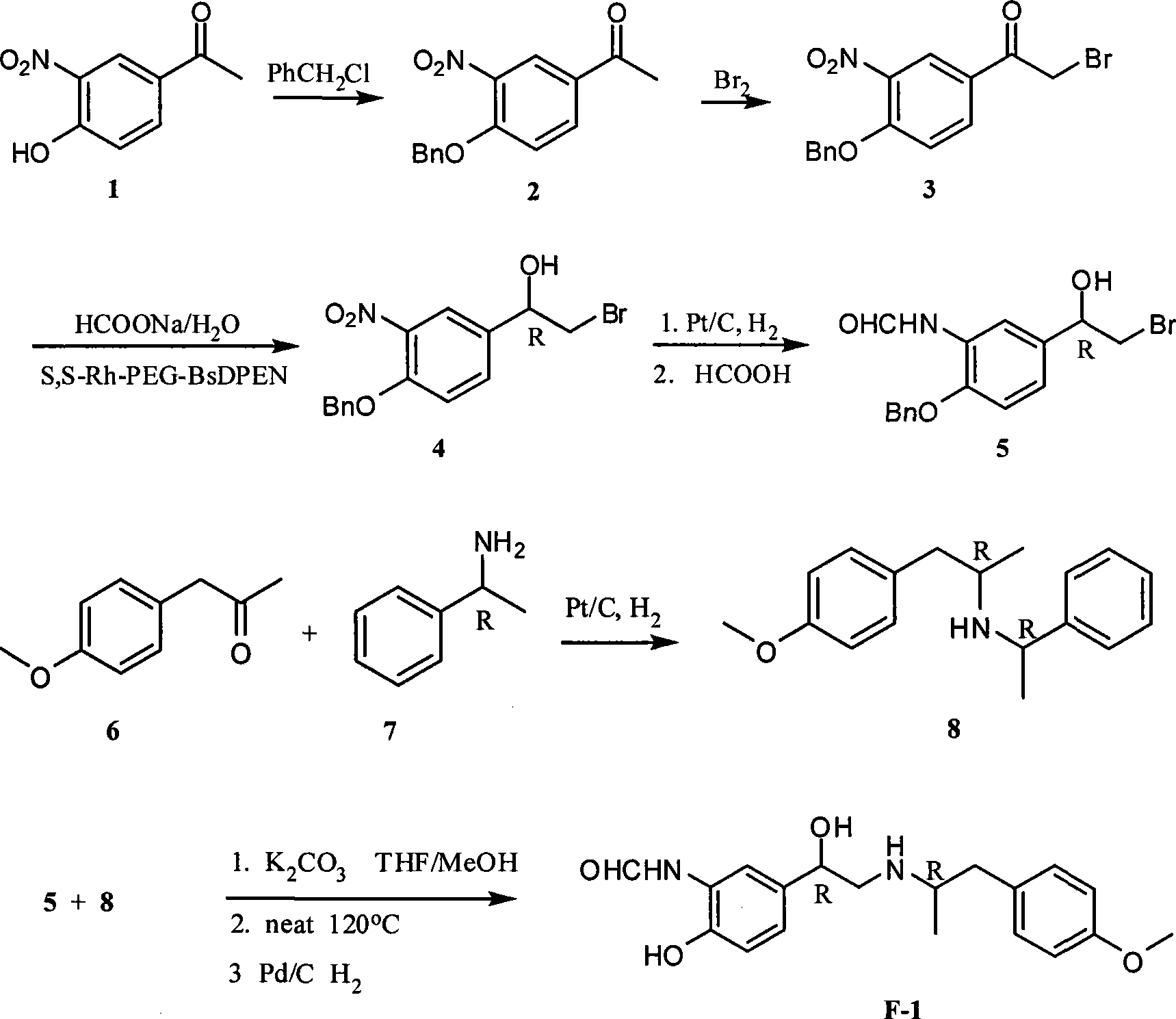
The invention relates to an asymmetric hydrogen transfer synthesis method for (R,R)-formoterol, and relates to a novel method for synthesizing an optical pure beta 2-adrenoreceptor excitant, namely formoterol. The method comprises: firstly, taking 4-hydroxyl-3 nitroacetophenone as a raw material, using benzyl groups to protect phenolic hydroxyl groups, and obtaining alpha-bromo keto after bromination; secondly, taking (S,S)-Rh-PEG-BsDPEN as a catalyst and formic acid and derivatives of the formic acid as hydrogen sources, and synthesizing chiral alcohol intermediate by an asymmetric hydrogen transfer method; thirdly, using (R)-alpha-methyl phenylethylamine and methoxyl phenylacetone to generate imine compounds, and obtaining chiral amine intermediate through hydrogenation reduction under the catalysis of Pt / C; and fourthly, reacting and coupling the chiral alcohol intermediate and the chiral amine intermediate, removing protective groups, and obtaining the (R,R)-formoterol. The invention uses the asymmetric hydrogen transfer method and a chiral auxiliary reagent to synthesize the (R,R)-formoterol, and has high yield and good ee value. Compared with a method for synthesizing chiral formoterol through chemical splitting, the method has the advantages of high total yield, mild reaction conditions, low cost and so on, and is favorable for industrial production.
Owner:SUN YAT SEN UNIV
Soluble hydrogel microsphere and preparation method and application thereof in single cell detection
ActiveCN109851711AIncrease profitAvoid cloggingMicrobiological testing/measurementOn/in organic carrierReverse transcriptaseSingle cell suspension
The invention discloses a soluble hydrogel microsphere and a preparation method and an application thereof in single cell detection. The preparation method comprises following steps: evenly mixing acrylamide, N,N-bis(acrylamide)amide, acetic acid, and 2-hydroxyl-2-methyl-1-phenyl-1-acetone, adding sodium hydroxide to obtain a prepolymer solution; adopting a single emulsion device, taking the prepolymer solution as an inner phase and an oil phase substance as an outer phase to obtain micro droplets, and carrying out online polymerization to obtain the microsphere. The single cell detection method comprises following steps: marking a bar code on the soluble hydrogel microsphere; injecting the soluble hydrogel microsphere, single cell suspension, and lysate of reverse transcriptase into a droplet micro reactor to form oil-in-water droplets, breaking the droplets, dissolving the soluble hydrogel microsphere, carrying out specific catalytic reverse transcriptional reactions and cDNA amplification; and differentiating single cells through the bar codes. The provided single cell detection method can improve the utilization rate of reverse transcriptase and solves the problem of channel obstruction.
Owner:SUZHOU GENO TRUTH BIOTECHNOLOGY CO LTD
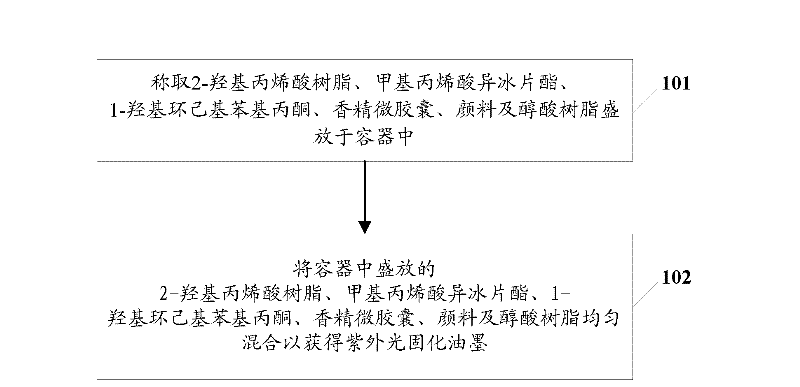
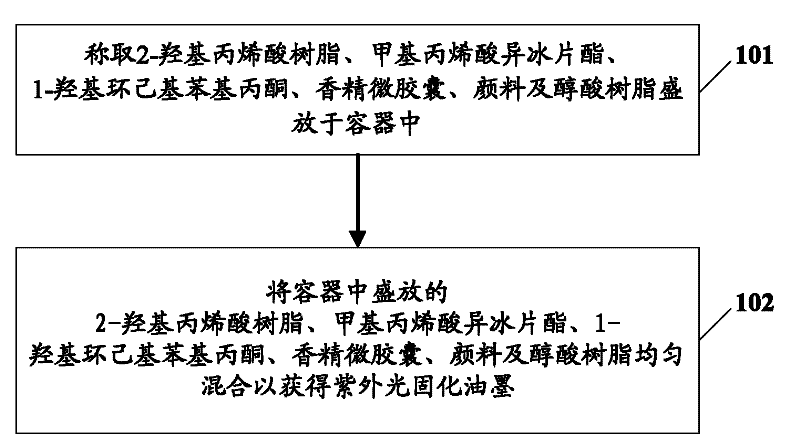
Ultraviolet light curing ink and preparation method thereof
The embodiment of the invention provides ultraviolet light curing ink and a preparation method of the ultraviolet light curing ink, wherein the ultraviolet light curing ink comprises the following ingredients: methacrylic acid isobornyl thiocyanoacetate, 2-hydroxy acrylic resin, 1-hydroxy cyclohexyl phenylacetone, essence microcapsules, pigments and alkyd resin. The ultraviolet light curing ink provided by the embodiment of the invention is favorable for improving the ink toughness, and further, the product quality is improved.
Owner:SHENZHEN BEAUTY STAR
Energy transmission optical fiber
ActiveCN102721998AReduce manufacturing costImprove adhesionCladded optical fibrePolyurea/polyurethane coatingsMethacrylateRefractive index
The invention relates to an energy transmission optical fiber, which comprises an optical fiber and a layer of low-refractive-index coating coated on the surface of the optical fiber. The energy transmission optical fiber is characterized in that low-refractive-index coating comprises the following components in percentage by weight: 10 to 25 percent of fatty group urethane acrylate, 3 to 10 percent of vinyl silicone oil, 8 to 15 percent of perfluoroalkylethyl acrylate, 3 to 10 percent of TMMPS, 40 to 65 percent of trifluoro ethyl methacrylate and 3 to 8 percent of 2-hydroxy-2-methyl-1-phenylacetone. The energy transmission optical fiber has the advantage of reducing the production cost of the energy transmission optical fiber.
Owner:JIANGSU FASTEN PHOTONICS +1
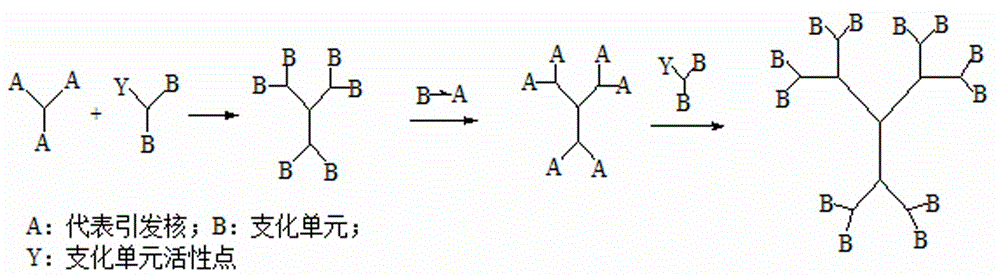
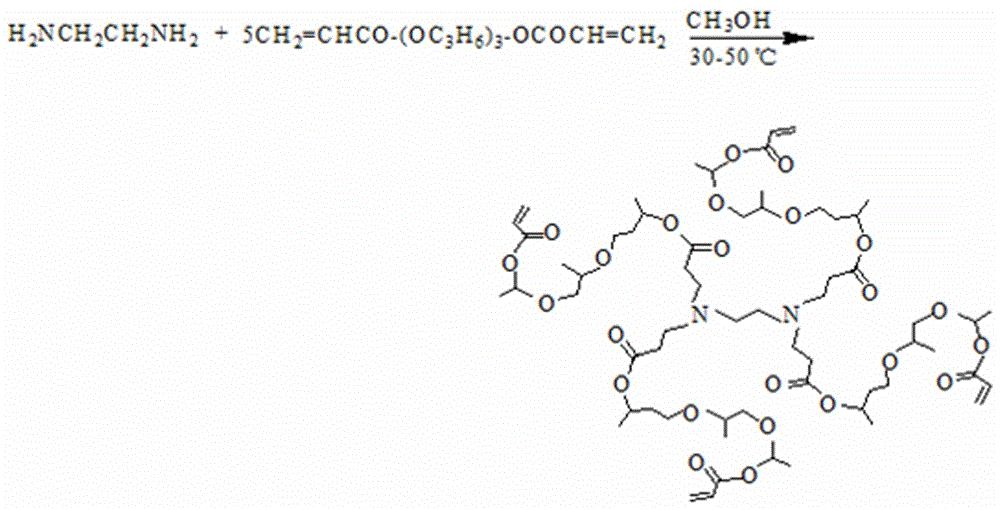
Ultraviolet-quick-curing dendriform resin, and divergent preparation method and application thereof
The invention provides an ultraviolet-quick-curing dendriform resin, and a divergent preparation method and application thereof. Ethylenediamine (EDA), diethylenetriamine (DETA), triethlenetetramine (TETA) and other polyamines are used as initiation cores, tripropylene glycol diacrylate (TGPDA), ethoxylated trimethylolpropane triacrylate (TEPTA) and other polyfunctional group monomers are used as branching units, and a divergent method is utilized to prepare the regular-structure highly-branched high-double-bond-density low-viscosity dendriform series resins containing different acrylate double bonds at terminal groups; benzoin aether and 2-hydroxy-2-methyl-1-phenyl-1-acetone with different photoinitiation activities are used as photoinitiators, are matched and uniformly mixed with the dendriform series resins containing different acrylate double bonds at terminal groups in a dark room, and are cured under the irradiation of an ultraviolet curing lamp to prepare different product series of quick-curing dendriform photosensitive resins. The preparation method can implement large-scale application and production, and has the characteristics of small pollution, high curing speed and high production efficiency.
Owner:HUBEI GREENHOME MATERIALS TECH INC
Trifunctional photoinitiator and preparation method thereof
ActiveCN110724060AImprove light absorption capacityImprove initiation performanceOrganic compound preparationCarboxylic acid esters preparationPolymer scienceEsterification reaction
The invention relates to a trifunctional photoinitiator and a preparation method thereof. The molecular formula of the trifunctional photoinitiator is C43H44O13, and the molecular weight of the trifunctional photoinitiator is 769.82. 2-hydroxyl-2-methyl-phenylacetone (Darocur 1173 or HMPP) and chloridized metaphthalic anhydride which are used as raw materials undergo an esterification reaction tointroduce acid anhydride, and the acid anhydride reacts with two molecules of a photoinitiator [4-(2-hydroxyethoxy)phenyl]-2-methyl-1-acetone (Darocur 2959 or HHMP) to obtain the trifunctional photoinitiator HTH containing one molecule of Darocur 1173 and two molecules of the Darocur 2959 group. The photoinitiator contains three active groups, sp the photoinitiator has the advantages of increasedmolecular weight, an enhanced activity, excellent mobility, excellent volatility and excellent thermal stability.
Owner:NANCHANG HANGKONG UNIVERSITY
Solvent-free type ultraviolet (UV) photocuring texture paint and preparation and use method thereof
InactiveCN105153926AEasy to operateFast curing and dryingPretreated surfacesPolyurea/polyurethane coatingsTripropylene glycolPolymer science
The invention discloses solvent-free type ultraviolet (UV) photocuring texture paint and a preparation and use method thereof. The solvent-free type ultraviolet (UV) photocuring texture paint is prepared from, by mass, 9-11 parts of aromatic polyurethane 3 functional group crylic acid oligomer, 28-33 parts of aliphatic polyurethane 2 functional group crylic acid oligomer, 36-44 parts of bisphenol A epoxy 2 functional group crylic acid oligomer, 7.5-8.5 parts of tripropylene glycol diacrylate (TPGDA) monomers, 6-7 parts of 1,6-high definition discrete architecture (HDDA) monomers, 4-6 parts of pentaerythritol triacrylate (PET3A) monomers, 2-3.5 parts of 2-hydroxy-2-methyl-1-phenyl-1-acetone light initiator (1173), 2-4 parts of reaction type tertiary amine promoter and 0.48-0.58 part of polyether modified polydimethylsiloxane flatting agent. According to the solvent-free type ultraviolet (UV) photocuring texture paint and the preparation and use method thereof, energy saving and environment friendliness can be achieved, operation is easy and convenient, a texture shape is formed, curing and drying are fast, the visual effect is good, and the paint is especially suitable for being used for indoor PVC coated wall slab decoration of building houses through a simple roller painting coating mode.
Owner:HAIYAN SANTIAN TECH CO LTD
Ultraviolet light polymerization water-based paint universal for metal and non-metal and preparation method thereof
InactiveCN103756533AShort curing timeImprove stabilityPolyurea/polyurethane coatingsPolyester coatingsPolymer scienceMeth-
The invention discloses ultraviolet light polymerization water-based paint universal for metal and non-metal and a preparation method of the paint. The ultraviolet light polymerization water-based paint universal for metal and non-metal comprises the following raw materials in parts by mass: 60-70 parts of vinyl ester resin, 38-46 parts of urethane acrylate, 1-2 parts of 2-hydroxy-2-methyl-1-phenylacetone, 0.5-1 part of bimercaptoacetic acid isooctyl di-n-octyltin, 35-40 parts of ethanol and 150-180 parts of water. The paint is short in curing time, that is, the curing time is 3 minutes and the adhesion capability to a substrate is of 1 grade, is good in stability and free of layering after being preserved for 6 months. The paint is simple in preparation method and applicable to popularization and application in a wide range.
Owner:SUZHOU CITY BANGCHENG ELECTRICITY TECH
Antibacterial flame-retardant ultraviolet-curable coating and preparation method thereof
InactiveCN103834292AGood mold resistanceImprove antioxidant capacityAntifouling/underwater paintsPaints with biocidesPolyesterTurpentine
The invention discloses an antibacterial flame-retardant ultraviolet-curable coating. The antibacterial flame-retardant ultraviolet-curable coating is prepared from the following raw materials in parts by weight: 33-36 parts of urethane acrylate, 19-22 parts of polyester acrylate, 12-14 parts of polyethylene glycol acrylate, 6-8 parts of glycidyl acrylate, 1-2 parts of turpentine, 4-5 parts of 2-methyl-2-hydroxy-1-phenylacetone, 5-8 parts of trimethylolpropane triacrylate, 4-7 parts of tripropylene glycol acrylate, 3-5 parts of butanone, 1-2 parts of polyacrylate, 4-6 parts of zinc oxide, 2-3 parts of talcum powder, 1-2 parts of IPBC (Iodo Propynyl Butyl Carbamate) and 4-5 parts of auxiliaries. Due to the added IPBC, the antibacterial flame-retardant ultraviolet-curable coating is safe and non-toxic, and has excellent antifungal and antioxidation effects; due to the added zinc oxide, the antibacterial flame-retardant ultraviolet-curable coating has closed effect in addition to flame retardant and antibacterial effects; furthermore, due to the added auxiliaries, the antibacterial flame-retardant ultraviolet-curable coating is good in leveling property, and an even coating coating can be formed, and the coating layer is high in curing speed, hard, wearproof, flexible and high in adhesion.
Owner:WUHU EDISON AUTOMATION EQUIP
Environment-friendly type UV hand-brush filling and coloring coating and preparation method thereof
ActiveCN108517178ASmall smellImprove adhesionPretreated surfacesPolyurea/polyurethane coatingsPolyesterFunctional monomer
The invention provides an environment-friendly type UV hand-brush filling and coloring coating, which is composed of the following ingredients (by weight0: 15-25 parts of a functional monomer, 13-30 parts of aliphatic polyurethane acrylic resin, 25-32 parts of polyether modified polyester acrylic resin, 15-25 parts of pigments and fillers, 0.5-5 parts of a wetting dispersant, 0.1-1 part of an antifoaming agent, and 4-8 parts of a photoinitiator, wherein the functional monomer is one of polyethylene glycol 400 diacrylate and glycerol methoxyl monoacrylate or a mixture thereof; and the photoinitiator is 2-hydroxy-2-methyl-1-phenyl-1-acetone matched with 2,4,6-trimethylbenzoyl-diphenylphosphine oxide or phenyl bis(2,4,6-trimethylbenzoyl)phosphine oxide. By using low-viscosity UV resin, matching special UV monomer and adding proper amounts of high-slipperiness fillers and the photoinitiator with different wavelengths, a good construction effect and non-irritating effect on human skin are achieved. The coating of the invention has good adhesion to a base material and an upper paint film. After color brushing, the next working procedure can be followed. The construction efficiency is remarkably enhanced.
Owner:QINGDAO ZHANCHEN NEW MATERIAL
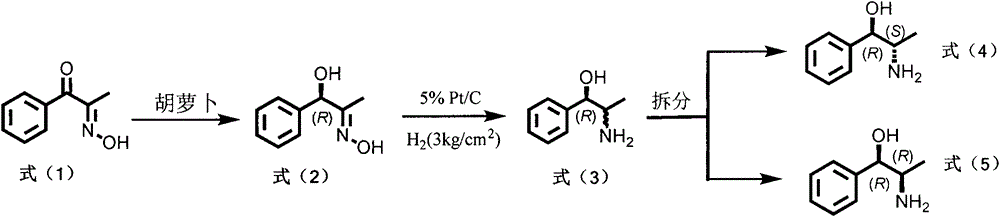
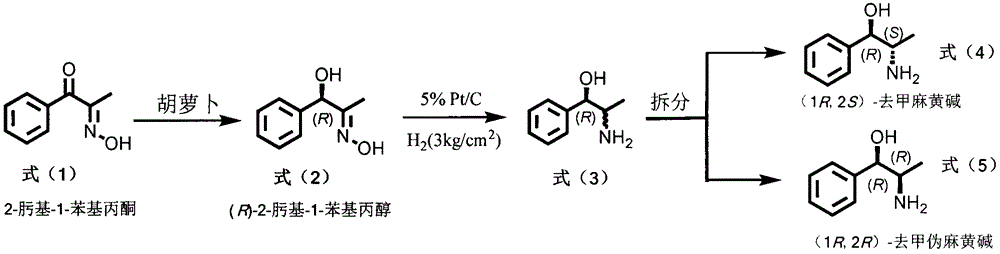
Method for preparing norephedrine and norpseudoephedrine
The invention relates to a method for preparing (1R, 2S)-norephedrine, (1R, 2R)-norpseudoephedrine and a midbody thereof. The method comprises the following steps: in the reaction process in the first step, by taking a carrot as a medium, carrying out bio-transforming on 2-oximido-1-phenylacetone and reducing into (R)-2-oximido-1-phenyl propyl alcohol; in the second step, reducing (R)-2-oximido-1-phenyl propyl alcohol into a mixture of (1R, 2S)-norephedrine and (1R, 2R)-norpseudoephedrine; adding a chiral reliquid reagent (R)-O-acetyl mandelic acid and decomposing, thereby acquiring an optical pure (1R, 2S)-norephedrine and (1R, 2R)-norpseudoephedrine monomeric compound.
Owner:CHINA PHARM UNIV
Synthesis of substituted methyl benzylketone
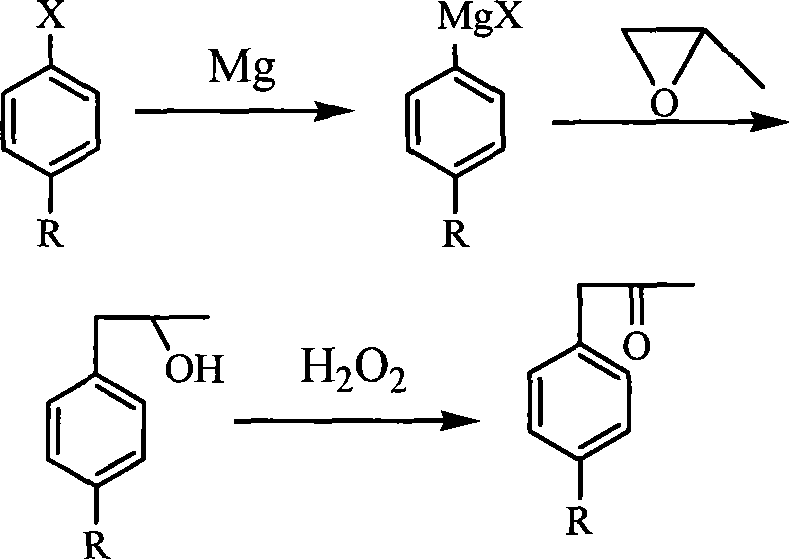
ActiveCN101367715ARaw materials are easy to getHigh yieldCarbonyl compound preparation by oxidationOrganic grignard reactionsArylPropanol
Owner:YIYUAN XINQUAN CHEM +1
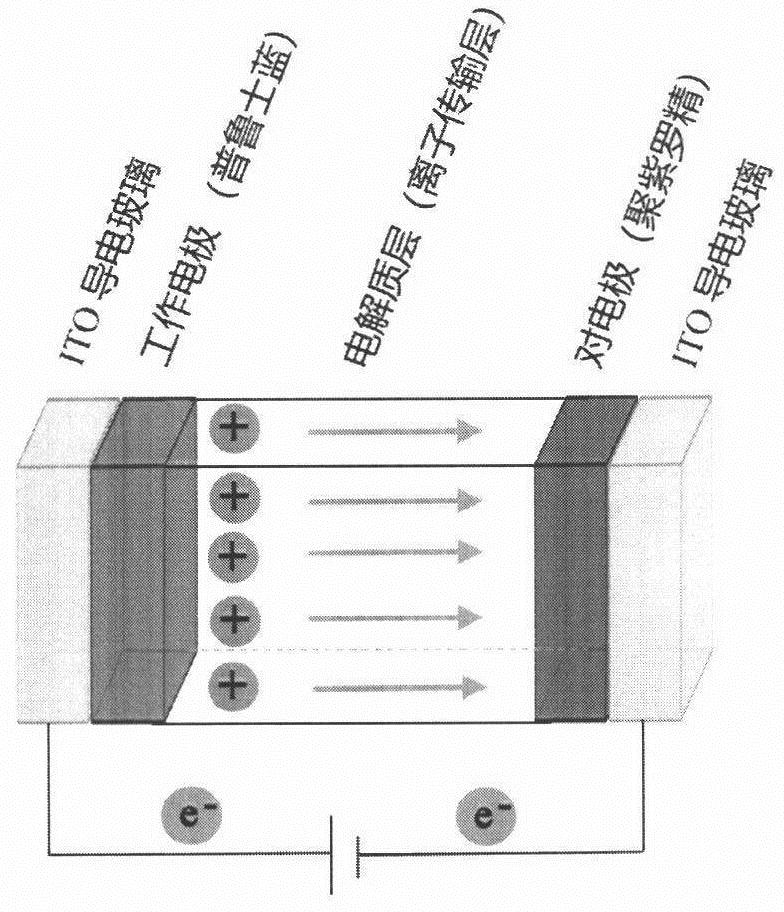
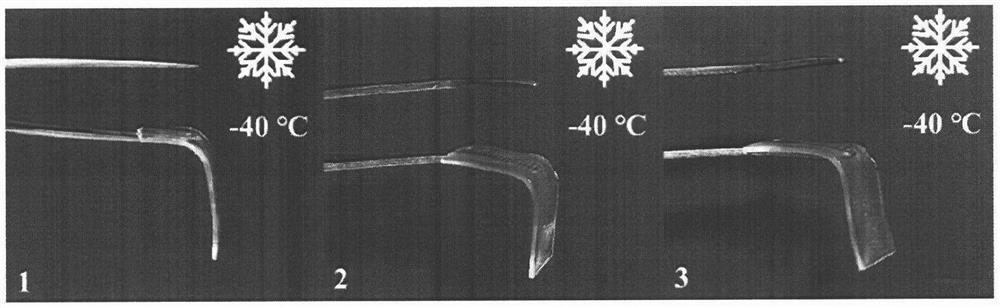
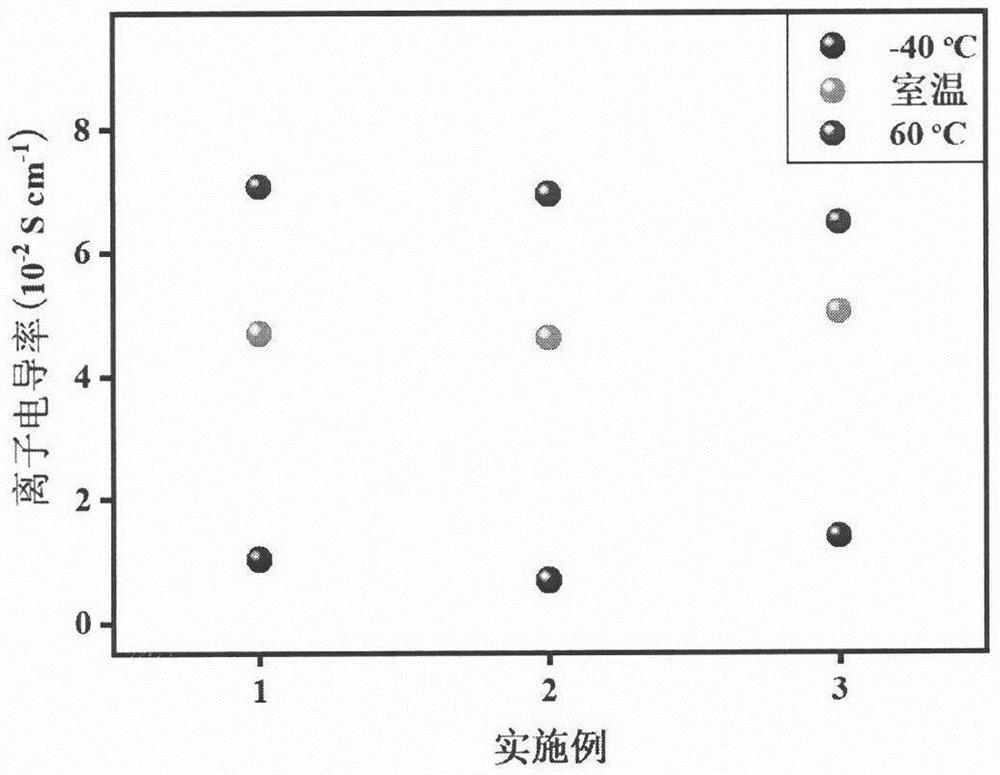
Anti-freezing heat-resistant hydrogel electrolyte for electrochromic device
ActiveCN112521555AFreeze and heat resistanceElectrolyte method is easyNon-linear opticsMeth-Polyethylene glycol
The invention relates to an anti-freezing heat-resistant hydrogel electrolyte for an electrochromic device. Deionized water is used as a solvent, carboxylated chitosan and acrylamide are used as mainmonomers, N, N-methylene bisacrylamide is used as a cross-linking agent, lithium salt is used as an electrolyte, polyethylene glycol and glycerol are used as additives, 2-hydroxy-2-methyl-1-phenyl- 1-acetone is used as a photoinitiator, free radical polymerization is carried out under irradiation of an ultraviolet lamp, and by adjusting the addition amount of polyethylene glycol and glycerol, thehydrogel electrolyte with good anti-freezing and heat-resisting properties is prepared. Furthermore, the hydrogel electrolyte is used for preparing a sandwich-structure electrochromic device, and thedevice has a wide working temperature range, shows good cycling stability in a temperature interval of -40-60 DEG C, and is suitable for preparing a flexible device. The novel hydrogel electrolyte provided by the invention is simple in preparation method, cheap and easily available in raw materials, high in ionic conductivity, high in transparency, high in stability and excellent in high and low temperature tolerance, and has a wide application prospect.
Owner:NANJING FORESTRY UNIV
Operative wound healing promoting gel dressing and preparation method thereof
InactiveCN108434508APromote healingPromote growthAbsorbent padsBandagesAntibacterial propertyPolycaprolactone
The invention provides operative wound healing promoting gel dressing and a preparation method thereof. The operative wound healing promoting gel dressing is prepared from a collagen solution and an electrospinning web, wherein the collagen solution is prepared from collagen powder, an acetic ethanol solution, vitamin E, 6-cyclohexyl-1-hydroxy-4-methyl-2(1H)-pyridone, N-hydroxyl succinimide and povidone-iodine; the electrospinning web is prepared from poly(lactic-co-glycolic acid), hydroxypropyl chitosan, polycaprolactone, medical chitosan, triethylene-glycol dimethacrylate and 2-hydroxyl-2-methyl-phenylacetone. The operative wound healing gel dressing disclosed by the invention has the advantages of biological degradability, very good physiological adaptation, strong antibacterial property, ability in promoting operative wound healing and epithelial cell growth and strong anti-adhesion property.
Owner:胡张艳
Weatherproof bronzing UV gloss oil
The invention relates to all-weather UV gloss oil capable of bronzing. Raw material consists of the following compositions in percentage by weight: 40 to 60 percent of amino-acrylics, 1 to 2 percent of 1-hydroxy cyclohexyl phenylacetone, 30 to 40 percent of 2-hydroxy acrylics, 1 to 2 percent of A-hydroxy isobutyrophenone, 8 to 18 percent of epoxy acrylic resin. The UV gloss oil has the advantage of long-term effect, is not limited by the light-curing condition in use and can be gilded after being solidified and placed for a long time.
Owner:HEBEI ANTAI PLASTIC PACKAGING PROD LTD
Polymerizable hydroxyalkyl benzophenone derivative photoinitiator and preparation method thereof
InactiveCN105646224AInitiation efficiency is highSimple processPreparation from carboxylic acid halidesCoatingsPolymer scienceMeth-
The invention discloses a polymerizable hydroxyalkyl benzophenone derivative photoinitiator and a preparation method thereof. 2-hydroxyl-2-methyl-1-phenylacetone (Darocur1173) is modified with (methyl) acryloyl chloride under the ice bath condition, and carbon-carbon double bonds which can participate in a photocuring reaction is introduced after hydroxyl esterification of Darocur1173, so that the photoinitiator which can participate in curing is prepared, the part, which is not photolyzed, of the initiator can be effectively prevented from being migrated to the surface of a coating, the product quality is guaranteed, the requirements of the food packaging field and the drug packaging field can be met, and the initiation efficiency of the modified initiator is high.
Owner:GUANGDONG UNIV OF TECH
Energy curable bonding resin
InactiveUS20140220367A1Reduce manufacturing costImprove reliabilityLamination ancillary operationsLaminationPolymer scienceMeth-
An energy curable bonding resin composition that prevents corrosion in metallic optical structures including DVDs includes at least one monomer, acrylated epoxidized soya bean oil, and a photoinitiator of among 2,4,6-trimethylbenzoyl-diphenylphosphine oxide (TPO); Phosphine oxide, phenylbis 2,4,6-trimethyl benzoyl; oligo[2-hydroxy-2-methyl-1-[4-(1-methylvinyl)phenyl]propanone]; alpha-hydroxy ketone, difunctional; or combinations thereof.
Owner:SUN CHEM CORP
Method of cell culture
Owner:PFIZER INC
In-tank type electrochemical synthesizing method of p-methoxy phenylacetone
InactiveCN1710150ALow priceAvoid pollutionElectrolysis componentsElectrolytic organic productionSupporting electrolyteAcetic acid
The invention discloses a tank-interior electrochemistry composite technique of methoxylbenzyl-ketone relates to electrochemistry technique field. Adopt Pt filament as positive pole and stainless fin as negative pole in bi-room electrolysis tank, acetic acid-acetic acid anhydride as disslovant, kalium acetate as electrolysis support, at certain temperature, in the electrolysis tank in positive pole room the anethole and lead acetate both as electrolysis basal material. The anethole directly electrolysis reaction on the positive pole takes place at the same time the indirect oxidation with lead tertraacetic as intermediate reactor develops. Hydrolyric decomposite the obtained product with sulfuric acid and further process to acquire the final product methoxylbenzyl-ketone. The invention has got advantages such as simple and easy reaction material, environmental reaction process, safe operation, high productivity and low cost, is a technique of high added value.
Owner:EAST CHINA NORMAL UNIV
Method for synthesizing Janus nano particles by using Janus nano emulsion as template
ActiveCN113289560ASimple preparation processIncrease productivityMicroballoon preparationMicrocapsule preparationActive agentUltraviolet lights
The invention discloses a method for synthesizing Janus nano particles by using a Janus nano emulsion as a template, and relates to the technical field of chemistry. The method comprises the steps: mixing an acrylate monomer, 1-hydroxycyclohexyl phenylacetone, oleic acid, silicone oil and a surfactant, and dropwise adding ultrapure water at the same time to form a molecular ordered combination body with an amphiphilic double-layer structure; continuously dropwise adding water to obtain the Janus nano emulsion with the double-sided structure, wherein the water content of the Janus nano-emulsion is 90-95 wt%; and putting the obtained product under ultraviolet light to initiate polymerization, and carrying out centrifugal washing and drying so as to obtain the Janus nano particles. The method is mild in preparation condition and simple and convenient to operate, and can be prepared by dropwise adding ultrapure water under stirring at room temperature and low speed and then performing ultraviolet irradiation, so that the large-batch production requirement is met.
Owner:YANGZHOU UNIV
A self-initiating UV-curable oligomer and its preparation method
ActiveCN107915829BHigh glass transition temperatureHigh hardnessPolyurea/polyurethane coatingsMeth-Ptru catalyst
The invention provides a self-triggered ultraviolet light-cured oligomer and a preparation method thereof. The invention provides a synthetic method of the ultraviolet light-cured self-triggered oligomer. The oligomer is synthesized from 2-hydroxy-2-methyl-1-phenyl-1-propanone, an isocyanate compound, acrylic acid (methylacrylic acid)hydroxyl ester, a catalyst, an aid and the like, is capable of generating free radicals under ultraviolet irradiation and can be automatically cross-linked and cured. An additional ultraviolet light initiator does not need to be added during the ultraviolet lightcuring of the oligomer, so that the oligomer has low odor, and micromolecule fragment is not produced; and after the oligomer is cured into a film, the hardness is of the film is high, and the curingdegree can reach 90%.
Owner:瑞通高分子科技(浙江)有限公司
Wear-resistant ultraviolet curing magnetic roller coating and preparation method thereof
InactiveCN111286265AHigh hardnessImprove wear resistancePolyurea/polyurethane coatingsEpoxy resin coatingsPhosphoric Acid EstersEpoxy
The invention discloses coating, and particularly relates to wear-resistant ultraviolet curing magnetic roller coating and a preparation method thereof. The coating is composed of the following raw materials by weight: 8 to 18 parts of graphene; 0.2 to 1.2 parts of methyl silicone oil; 2 to 12 parts of organosilicon resin; 0.2 to 1 part of organic modified polysiloxane acrylic acid type leveling agent; 0.2 to 2.5 parts of fumed silica, 10 to 32 parts of urethane acrylate, 0.01 to 15 parts of hexanediol diacrylate, 1 to 8 parts of 2-hydroxy-2-methyl-1-phenyl-1-acetone, 2 to 16 parts of inositolhexaphosphate, 0.25 to 2.5 parts of a titanate coupling agent, 5 to 15 parts of alicyclic epoxy resin and 10 to 18 parts of zinc powder. The coating is prepared by multi-step mixing and dispersing. The coating can conduct electricity after being subjected to ultraviolet curing, and has enough hardness and excellent wear resistance and tensile strength.
Owner:中山市锝力打印机设备有限公司
New synthetic method of fingolimod hydrochloride
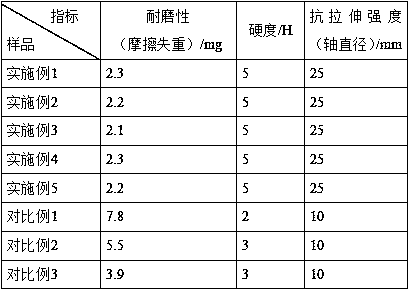
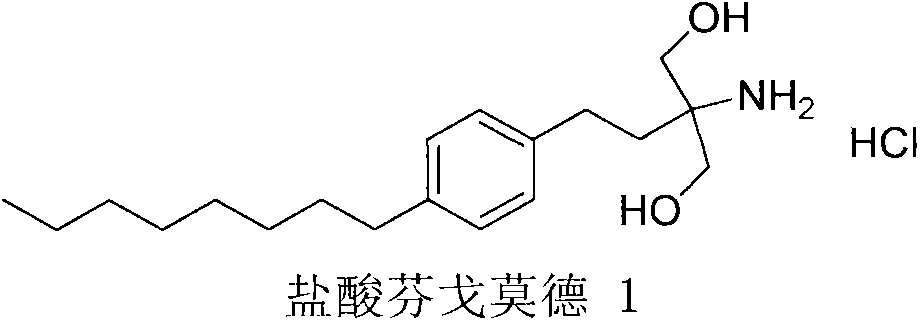
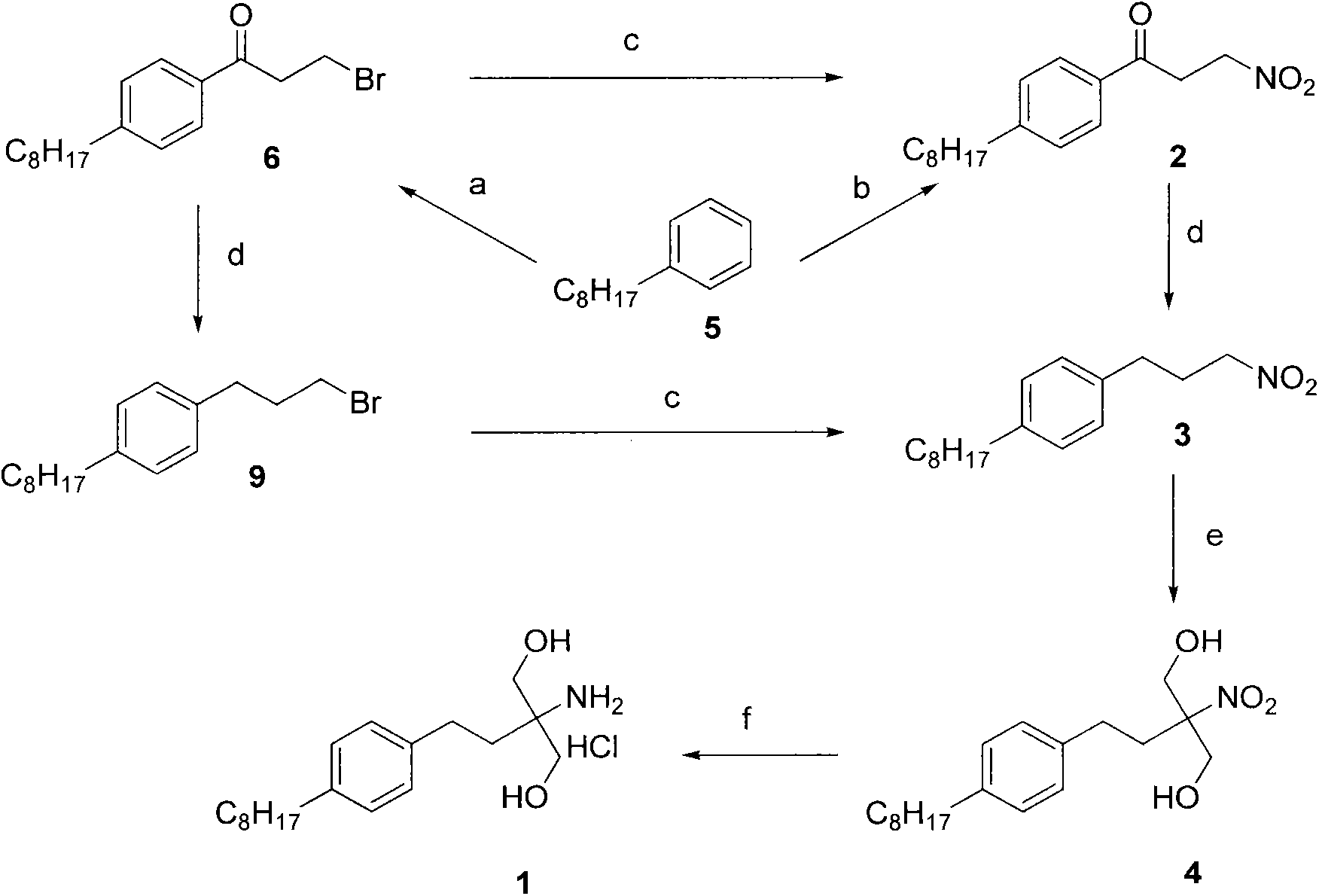
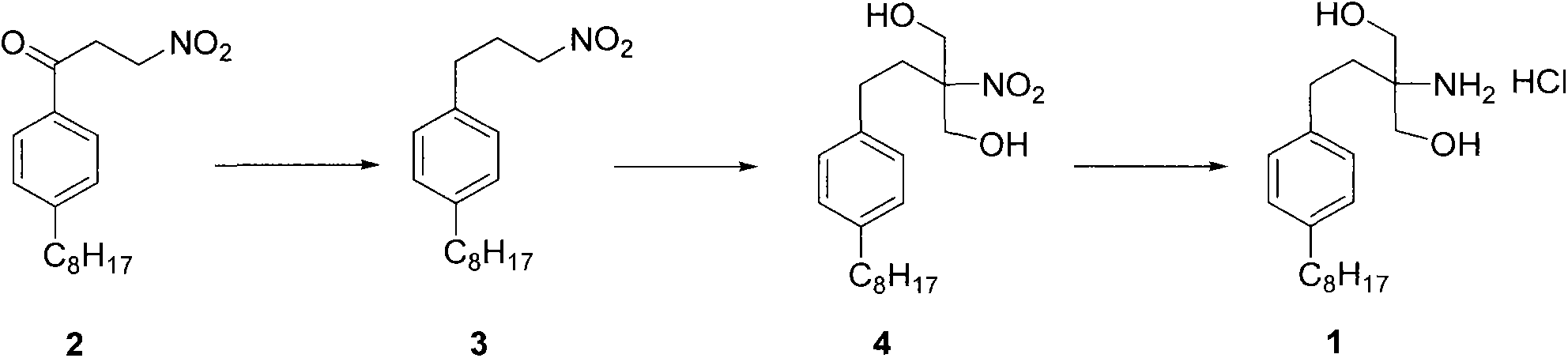
InactiveCN104292115AShort synthetic routeSynthetic raw materials are cheapOrganic compound preparationAmino-hyroxy compound preparationKetoneDiol
The invention provides a preparation method of fingolimod hydrochloride (1). The method comprises the following steps: carrying out a reducing reaction on 3-nitro-1(4-octylphenyl)propyl-1-one (2) to obtain a compound 1-(3-nitropropyl)-4-octylbenzene (3); reacting 1-(3-nitropropyl)-4-octylbenzene (3) with formaldehyde to generate 2-(4-octylphenylethyl)-2-nitro-propane-1,3-diol (4); and reducing the compound 4, and carrying out salt formation on the reduced compound 4 and hydrochloric acid to generate the 2-(4-octylphenylethyl)-2-amino-propane-1,3-diol hydrochloride (1) which is the fingolimod hydrochloride. The method has the advantages of short synthetic route, cheap and easily available synthetic raw materials, no obvious pollution in the synthetic process, and total yield reaching 40-45%, is a simple and economic method for preparing the fingolimod hydrochloride. The invention also provides a synthetic method of intermediates.
Owner:YANTAI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Method for synthesizing 2-methyl-1-[4-(methylthio)phenyl]-2-(4-morpholinyl)-1-propanon Method for synthesizing 2-methyl-1-[4-(methylthio)phenyl]-2-(4-morpholinyl)-1-propanon](https://images-eureka.patsnap.com/patent_img/404513ee-a36e-4edc-bc71-3f9c73e78624/A20081011887200031.PNG)
![Method for synthesizing 2-methyl-1-[4-(methylthio)phenyl]-2-(4-morpholinyl)-1-propanon Method for synthesizing 2-methyl-1-[4-(methylthio)phenyl]-2-(4-morpholinyl)-1-propanon](https://images-eureka.patsnap.com/patent_img/404513ee-a36e-4edc-bc71-3f9c73e78624/A20081011887200041.PNG)
![Method for synthesizing 2-methyl-1-[4-(methylthio)phenyl]-2-(4-morpholinyl)-1-propanon Method for synthesizing 2-methyl-1-[4-(methylthio)phenyl]-2-(4-morpholinyl)-1-propanon](https://images-eureka.patsnap.com/patent_img/404513ee-a36e-4edc-bc71-3f9c73e78624/A20081011887200042.PNG)