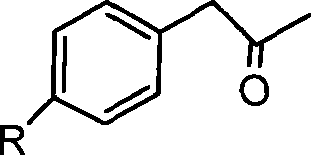
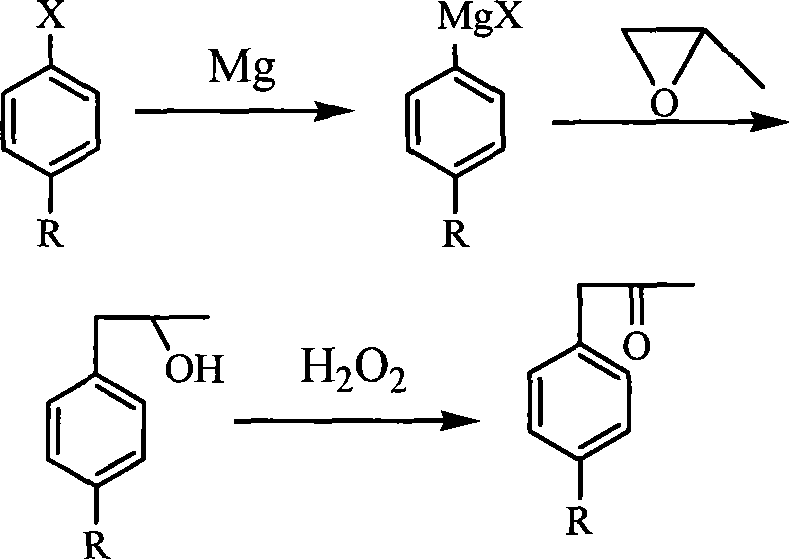
Synthesis of substituted methyl benzylketone
A synthetic method and technology of phenylacetone, applied in the direction of organic chemical methods, chemical instruments and methods, oxidation preparation of carbonyl compounds, etc., can solve the problems of high cost and low yield, and achieve wide application, high yield, and raw material Easy to get effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1: the synthesis of phenylacetone
[0019] In a three-necked reaction flask equipped with a reflux condenser, a nitrogen inlet and outlet, and a magnetic stirrer, add 300 mL of pure tetrahydrofuran, 17.0 g of magnesium powder, 67.5 g of chlorinated benzene, and several grains of iodine. Slowly heated to 65° C. under stirring in a nitrogen flow, and reacted at a constant temperature for 1 hour, then heated to 75° C., and reacted at a constant temperature for 4 hours. Cool the reaction solution to -5°C with ice water, slowly drop 45.0g of propylene oxide into the reaction solution from the dropping funnel, after dropping, keep stirring at 0°C for 1 hour, then naturally raise the temperature to 20°C, and stir for 0.5 Hour. After the reaction solution was post-treated by the usual method, 74.4 g of the product was obtained by distillation under reduced pressure, which was identified as 1-phenyl-2-propanol by GC-MS, with a purity of 99.1% and a yield of 91%.
[...
Embodiment 2
[0021] Embodiment 2: the synthesis of p-methoxyphenylacetone
[0022] In a three-neck reaction flask equipped with a reflux condenser, nitrogen inlet and outlet, and a magnetic stirrer, add 300mL of pure tetrahydrofuran, 17.0g of magnesium powder, 85.5g of p-methoxychlorobenzene, and several grains of iodine. Slowly heated to 65° C. under stirring in a nitrogen flow, and reacted at a constant temperature for 1 hour, then heated to 75° C., and reacted at a constant temperature for 4 hours. Cool the reaction solution to -5°C with ice water, slowly drop 45.0g of propylene oxide into the reaction solution from the dropping funnel, after dropping, keep stirring at 0°C for 1 hour, then naturally raise the temperature to 20°C, and stir for 0.5 Hour. After the reaction solution was post-treated by the usual method, 87.7 g of the product was obtained by distillation under reduced pressure, which was identified as 1-(p-methoxyphenyl)-2-propanol by GC-MS, with a purity of 98.0% and a yi...
Embodiment 3
[0024] Embodiment 3: the synthesis of p-fluorophenylacetone
[0025] In a three-necked reaction flask equipped with a reflux condenser, nitrogen inlet and outlet, and a magnetic stirrer, add 300 mL of pure tetrahydrofuran, 17.0 g of magnesium powder, 103.0 g of p-fluorobromobenzene, and several grains of iodine. Slowly heated to 65° C. under stirring in a nitrogen flow, and reacted at a constant temperature for 1 hour, then heated to 75° C., and reacted at a constant temperature for 4 hours. Cool the reaction solution to -5°C with ice water, slowly drop 45.0g of propylene oxide into the reaction solution from the dropping funnel, after dropping, keep stirring at 0°C for 1 hour, then naturally raise the temperature to 20°C, and stir for 0.5 Hour. After the reaction solution was post-treated by the usual method, 75.8 g of the product was obtained by distillation under reduced pressure, which was identified as 1-(p-fluorophenyl)-2-propanol by GC-MS, with a purity of 98.0% and a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com