A self-initiating UV-curable oligomer and its preparation method
An ultraviolet light and self-initiation technology, applied in polyurea/polyurethane coatings, coatings, etc., can solve the problems of irritating odor, low initiation efficiency, easy migration, etc., and achieve high glass transition temperature, high initiation efficiency, and solidification high degree of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 Synthesis of self-initiated UV-curable oligomer R1
[0032] Table 1, synthetic formula table
[0033]
[0034] Add components 1, 2, 3, and 4 into the flask, add A11 dropwise at less than 45°C, and control the temperature at 60-65°C after the dropwise addition
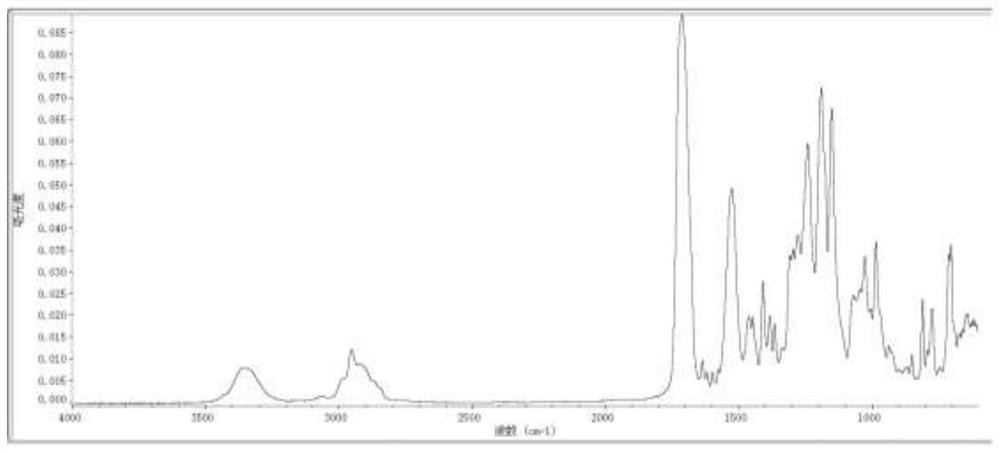
[0035] After reacting for 3 hours, HEA was added dropwise, and the isocyanate (-NCO) value was detected to be less than 0.1% after reacting at 70-75° C. for 4 hours to obtain the UV-curable self-initiated synthesis product R1. Infrared spectra of the obtained composites are shown as figure 1 shown.
Embodiment 2
[0036] Example 2 Synthesis of self-initiated UV-curable oligomer R2
[0037] Table 2, synthetic formula table
[0038]
[0039]
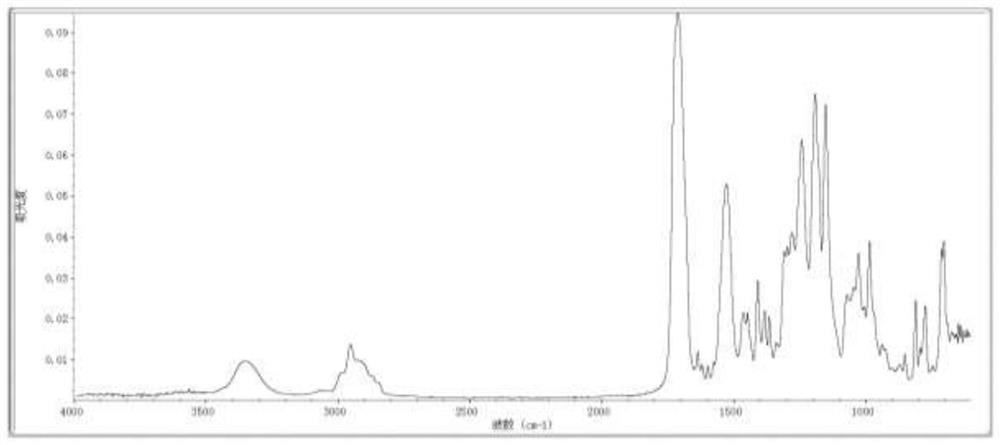
[0040] Add components 1, 2, 3, and 4 into the flask, add A11 dropwise at less than 45°C, after the dropwise addition, control the temperature at 60-65°C for 3 hours, then add HPA dropwise, at 70-75°C After reacting for 4 hours, it is detected that the isocyanate (-NCO) value is less than 0.1%, and the synthetic product R2 which is self-initiated by ultraviolet curing is obtained. The infrared spectra of the synthesized compounds are shown in figure 2 .
Embodiment 3
[0041] Example 3 Synthesis of self-initiated UV-curable oligomer R3
[0042] Table 3, synthetic formula table
[0043]
[0044]Add components 1, 2, 3, and 4 into the flask, add A11 dropwise at less than 45°C, after the dropwise addition, control the temperature at 60-65°C for 3 hours, then add HEA dropwise, at 70-75°C After reacting for 4 hours, the isocyanate (-NCO) value was detected to be less than 0.1%, and the UV-curable self-initiated synthesis product R3 was obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com