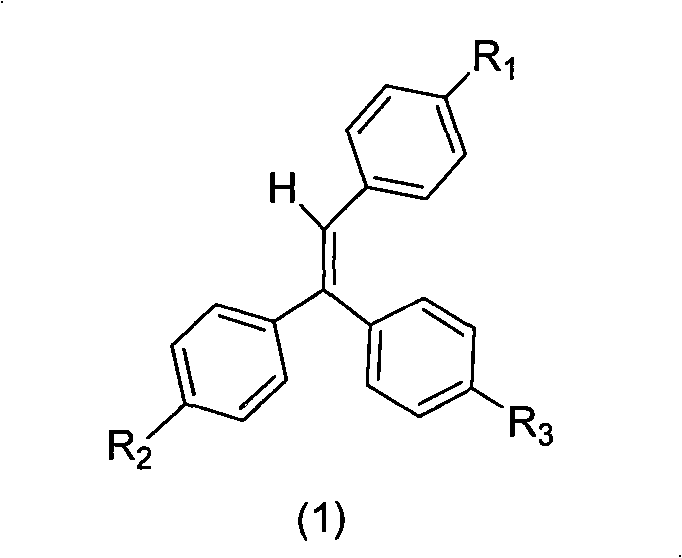
Gathering induced luminescence material containing triphenyl thylene structure, synthesis method and application thereof
A technology of aggregation-induced luminescence and triphenylethylene, which is applied in the direction of luminescent materials, styrene-based dyes, and material excitation analysis, can solve problems such as low glass transition temperature, limited compounds, and difficult molecular design, and achieve high luminous intensity and purification Easy, high glass transition temperature effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0017] The synthesis method of above-mentioned luminescent material, comprises the following steps:
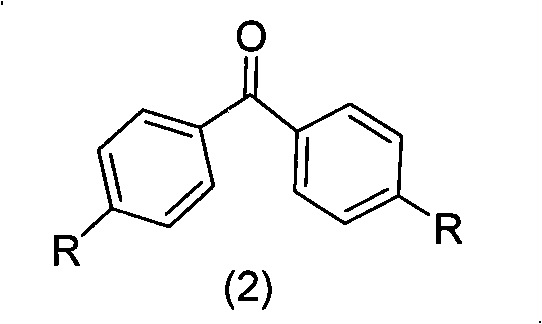
[0018] Step 1: Synthesis of Benzophenone Derivatives
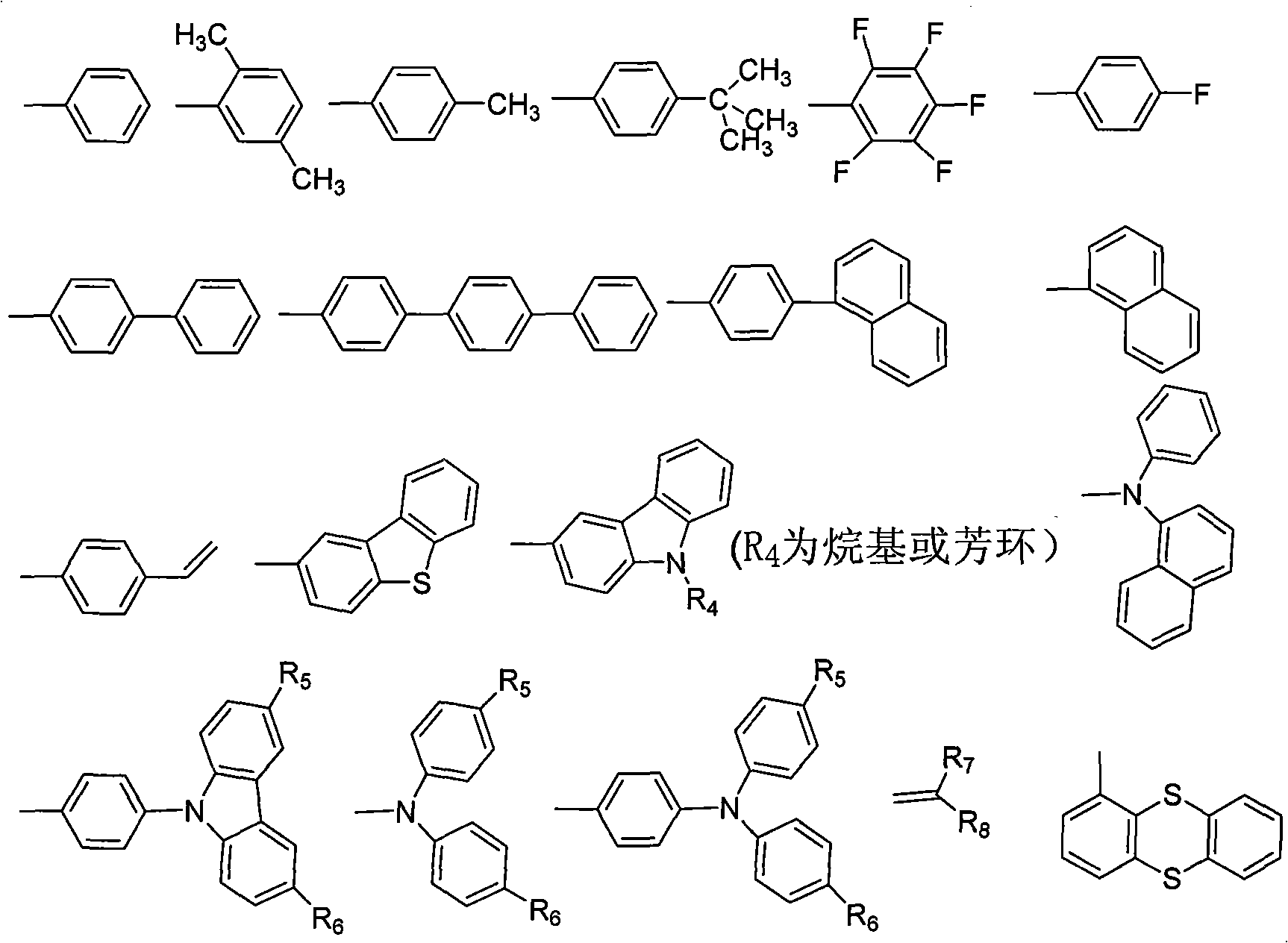
[0019] The synthesis of benzophenone derivatives is obtained by reacting 4,4'-dihalogenated benzophenone and 4,4'-methylbenzophenone with other aromatic compounds. The present invention preferably adopts 4,4'-difluorobenzophenone, 4,4'-dibromobenzophenone and 4,4'-methylbenzophenone as raw materials for preparing benzophenone derivatives, and the preparation process is simple, High yield is one of the main features of the present invention. For the synthesis of substituents, according to the mentioned R 4 , R 5 , R 6 , R 7 , R 8 The structure of the substituent is synthesized by conventional organic synthesis methods, including Friedel-Crafts alkylation, amine alkylation, halogenation, Suzuki reaction, Heck reaction, Wittig reaction, etc.
[0020] Step 2: Conversion of ketone carbonyl to double bond
[0021] Method...
Embodiment 1
[0028] Synthesis of p-naphthalene triphenylethylene:
[0029] (1) Synthetic intermediate p-bromotriphenylethylene
[0030] 4,4'-dibromobenzophenone (34.0g, 0.1mol), diethyl 4-bromobenzylidene phosphonate (30.7g, 0.1mol) were added in the there-necked flask, and 200mL of dry tetrahydrofuran was added, and Potassium tert-butoxide (11.2 g, 0.1 mol) was added under the protection of argon, stirred at room temperature for 12 h, and the reaction solution was poured into 500 mL of ethanol for precipitation. Suction filtration, washing with ethanol three times, and drying to obtain 46.8 g of white powder with a yield of 95%.
[0031]
[0032] (2) Synthesis of p-naphthalene triphenylethylene:
[0033] p-Bromotriphenylethylene (0.83g, 0.0017mol), 2-naphthaleneboronic acid (0.85g, 0.005mol) were added to the three-necked flask, and 20mL of toluene, 2M K 2 CO 3 Aqueous solution 5mL, TBAB 1g, after stirring and argon flow for 30min, add 0.01g Pd(PPh 3 ) 4 , The oil bath was heated...
Embodiment 2
[0036] The synthesis method of p-benzothiophene triphenylethylene refers to Example 1, and the boric acid used is 4-dibenzothiophene boronic acid. The pure product was an off-white powder with a yield of 67%.
[0037]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com