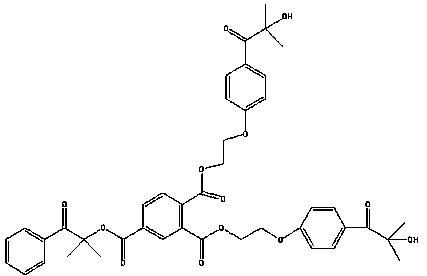
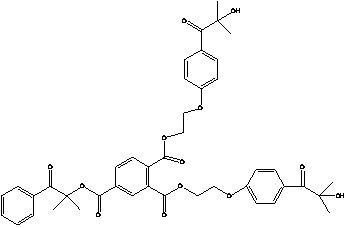
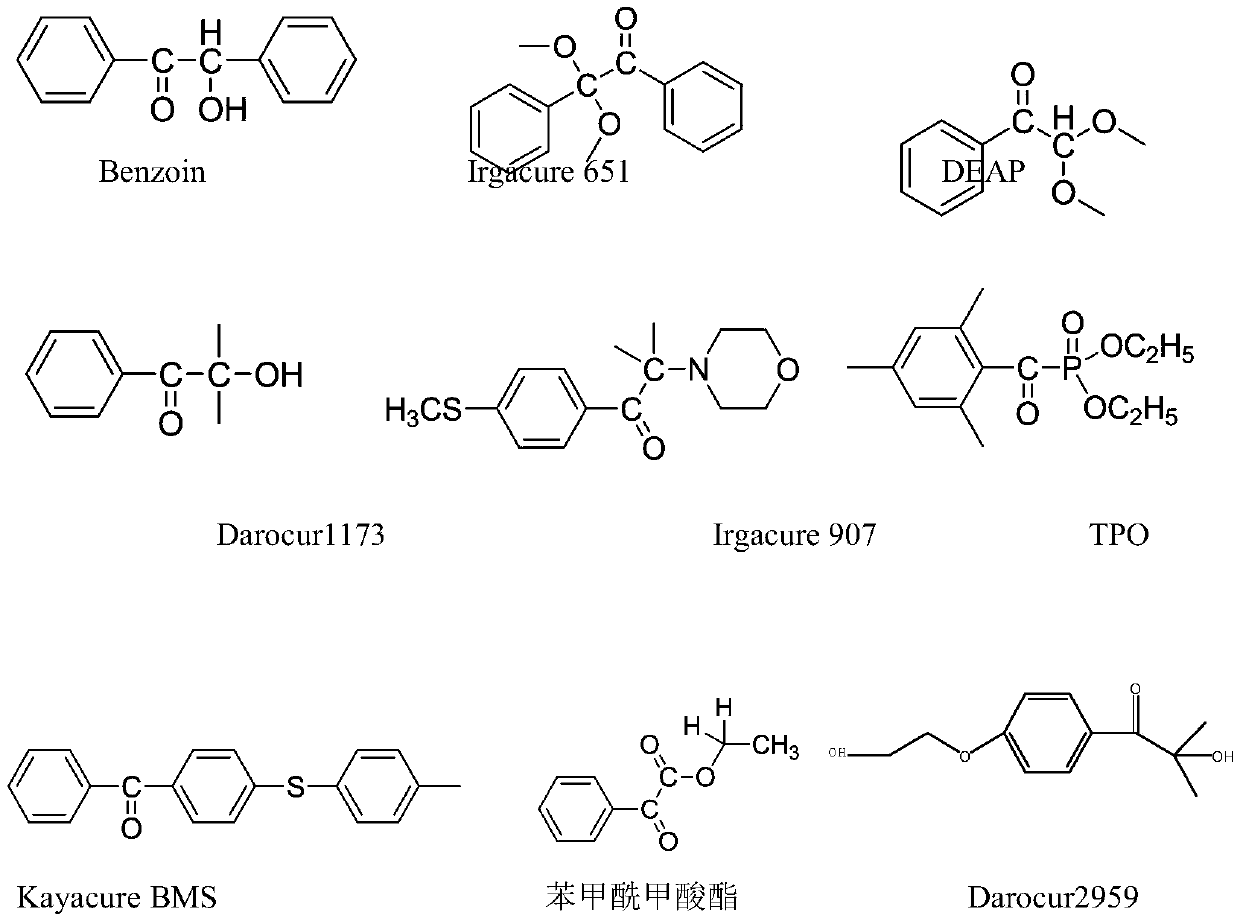
Trifunctional photoinitiator and preparation method thereof
A technology of photoinitiator and trifunctionality, which is applied in the field of trifunctionality photoinitiator and preparation, can solve the problems of reducing photoinitiator mobility and volatility, low photoinitiation activity, low mobility, etc. The effect of initiating activity, simple preparation of raw materials, and easy synthesis conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) Add 3.28g (0.02mol) 1173 photoinitiator and 2.25g (0.022mol) triethylamine in a three-necked flask, and magnetically stir the dichloromethane dissolved in 50ml at room temperature. To react at room temperature, dissolve 4.63g (0.022mol) of chlorinated hemiphthalic anhydride in 20ml of dichloromethane, add it to a constant pressure dropping funnel, and slowly drop it into a three-necked flask. After the dropwise addition, continue to stir and react overnight, remove triethylamine hydrochloride by filtration, and purify the dried organic phase again by column chromatography. The developer of the corresponding silica gel column is a solvent mixed with petroleum ether and ethyl acetate. The specific ratio is v (petroleum ether): v (ethyl acetate) = 2:1, and 5.10 g of the product yellow liquid is obtained.
[0034] (2) Weigh 3.38G of the above intermediate, 4.48g of 2959 photoinitiator, dimethylformamide (DMF) and methanesulfonic acid, heat up to 105°C under stirring and...
Embodiment 2
[0036] (1) Add 6.56g (0.04mol) of 1173 photoinitiator and 4.5g (0.044mol) of triethylamine in a three-necked flask, and magnetically stir the dichloromethane dissolved in 100ml at room temperature. To react at room temperature, dissolve 9.26g (0.044mol) of chlorinated hemiphthalic anhydride in 40ml of dichloromethane, add a constant pressure dropping funnel, and slowly drop it into a three-necked flask. After the dropwise addition, continue to stir and react overnight, filter to remove triethylamine hydrochloride, wash the filtrate 3 times with 50ml deionized water, and purify the dried organic phase again by column chromatography. The developing agent for the corresponding silica gel column is mixed petroleum Ether and ethyl acetate solvent. The specific ratio is v (petroleum ether): v (ethyl acetate) = 2:1, and the obtained product is 11.0 g of yellow liquid.
[0037] (2) Weigh 6.76g of the above-mentioned intermediate, 9.96g of 2959 photoinitiator, 100ml of dimethylformami...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com