Method for preparing norephedrine and norpseudoephedrine
A kind of technology of norephedrine and norephedrine, applied in the field of preparing norephedrine and norephedrine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
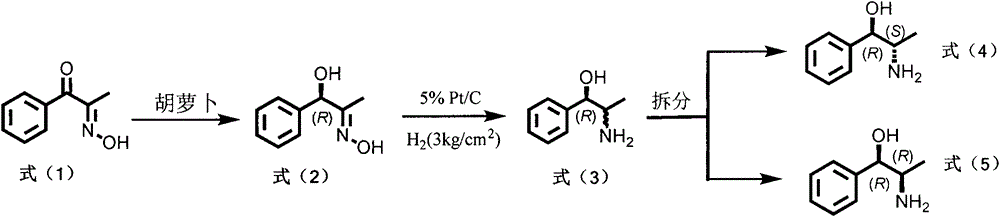
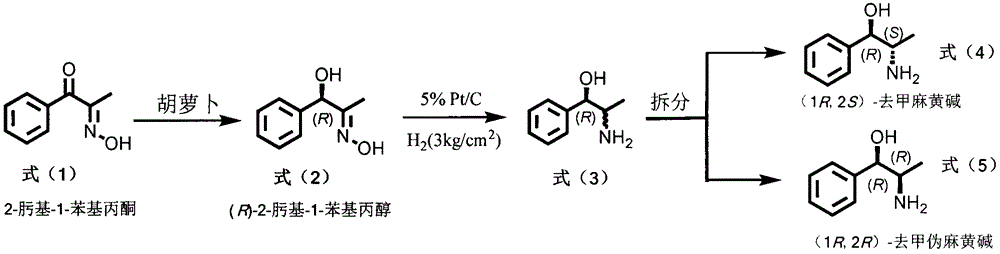
[0007] Preparation of formula (2) compound
[0008] Add 250mL of phosphate buffered saline solution (0.1mol / L, pH 6.5) and about 60g of chopped fresh carrots into a 500ml Erlenmeyer flask, stir and react at 30°C for about 10min in a water bath constant temperature reactor, and make the plant cells After the oxidoreductase reaches the reaction temperature, 100 mg of the compound of formula (1) is added, and the reaction is continued at 30° C. for 72 hours. The reaction process is monitored by thin-layer chromatography and high-performance liquid chromatography. After the reaction is complete, filter, and the filtrate is extracted three times with 250 mL of ethyl acetate, the organic layers are combined, dehydrated with anhydrous sodium sulfate, concentrated under reduced pressure, and the solvent is removed to obtain the crude product of formula (2), which is separated through a silica gel column to obtain the formula (2) Pure product, yield 85%, 92% ee.
[0009] Compound of f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com