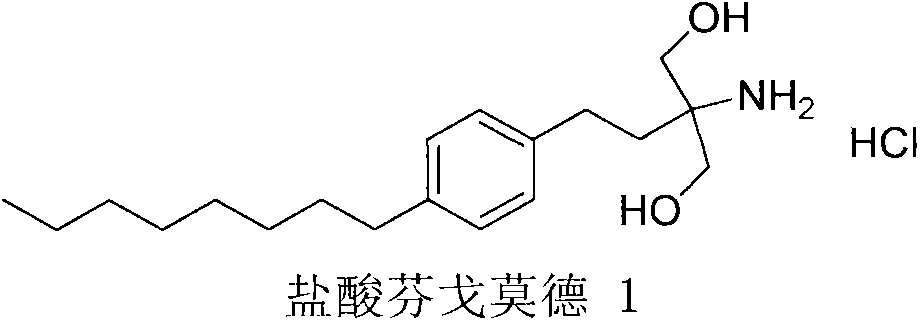
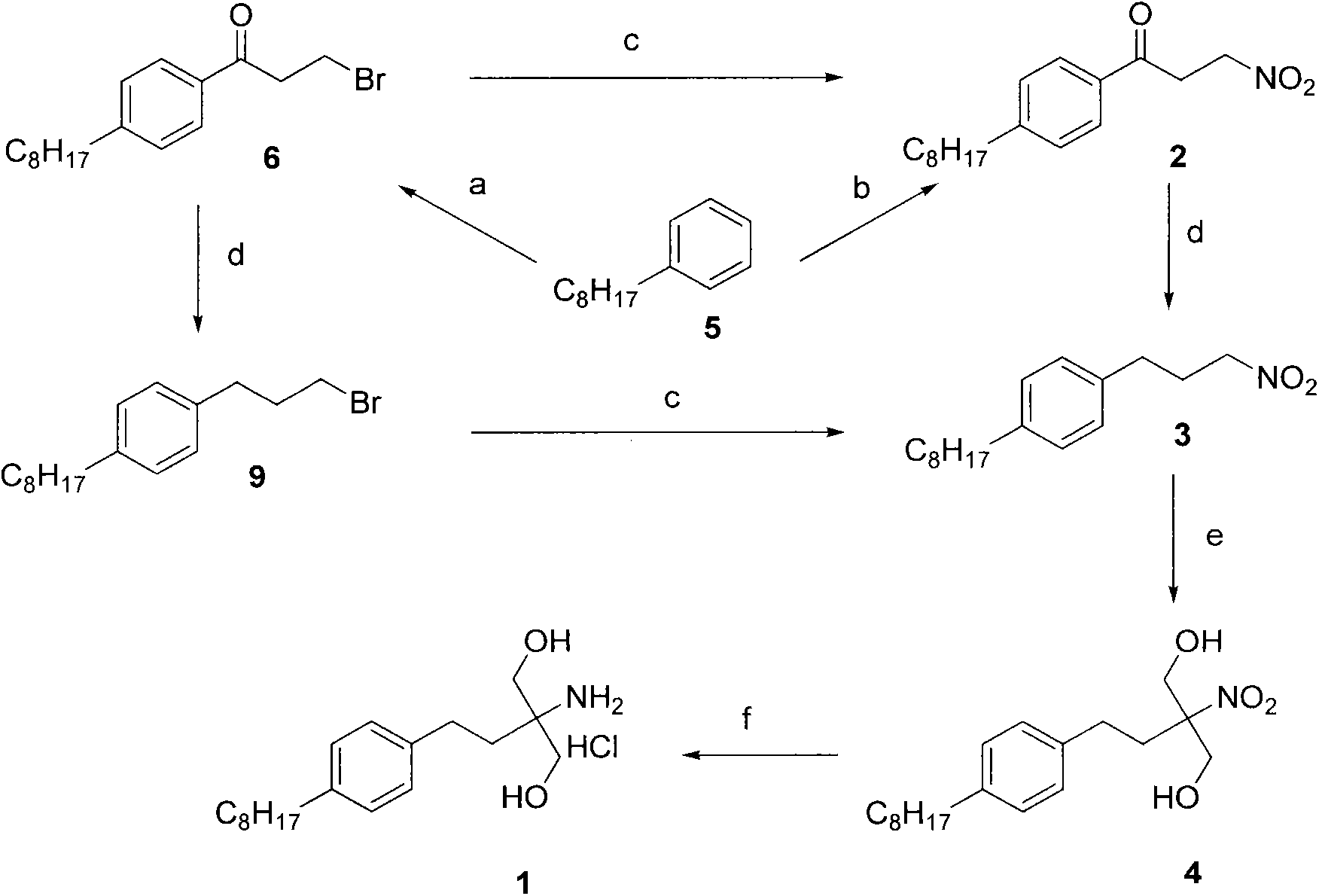
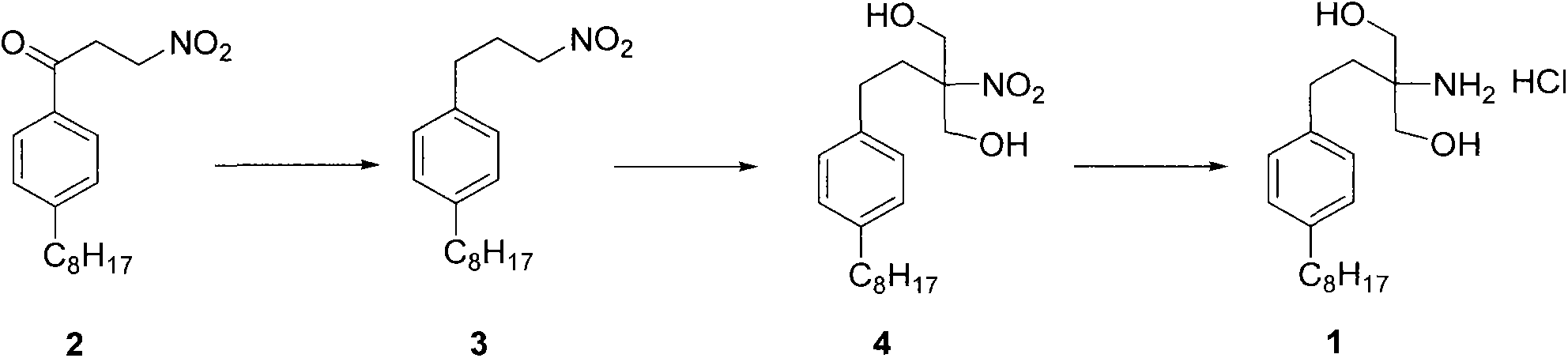
New synthetic method of fingolimod hydrochloride
A new synthesis technology of fingolimod hydrochloride, applied in the field of preparation of fingolimod hydrochloride, can solve the problems of harsh reaction conditions, excessively long reaction route, and difficulty in expanding production, and achieves mild synthesis conditions and easy synthesis of raw materials. The effect of obtaining and synthetic raw materials is cheap
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] 3-nitro-1-(4-octylphenyl)propan-1-one (2)
[0025] A mixture of 70 g (0.22 mol) of 3-bromo-1-(4-octylphenyl)propan-1-one (6) and 300 mL of dimethylformamide (DMF) was cooled on ice to below 20°C. 61 g (0.88 mol) of sodium nitrite was added to the above mixture, and the reaction was carried out at 20° C. for 2 h. After the reaction was completed, pour it into 1200mL of water under stirring, and a yellowish solid was precipitated, filtered, washed with water, and dried in vacuo. The crude product was decolorized with 450mL of n-hexane and 1g of activated carbon under reflux for 0.5h, filtered, and crystallized by cooling. Filtration and vacuum drying at room temperature gave 50 g of white solid, yield 80%, mp 54-56°C.
[0026] Compounding to 2 has the following NMR data:
[0027] 1 H MNR (CDCl 3 , δ): ppm7.89-7.91 (d, 2H, J=8.2Hz), 7.29-7.31 (d, 2H, J=8.2Hz), 4.81-4.84 (t, 2H, J=6.2Hz), 3.64- 3.67(t, 2H, J=6.2Hz), 2.66-2.69(t, 2H, J=7.6Hz), 1.61-1.65(m, 2H), 1.26-1.3...
Embodiment 2
[0029] 3-Bromo-1-(4-octylphenyl)propan-1-one (9)
[0030] Add 18.6ml of TFA and 7.92g (0.024mol) of 3-bromo-1-(4-octylphenyl)propan-1-one (6) into a 250ml single-necked bottle, stir magnetically, and add triethylsilane under ice-cooling 5.65g, stirred in the ice bath for 0.5h, removed the ice bath and continued to stir for 4h. After the reaction is detected by TLC, pour the reaction solution into an appropriate amount of ice water and stir, gradually add sodium bicarbonate solution, adjust to PH = 8, a slightly yellow oily substance appears on the liquid surface, extract with 100ml×3 petroleum ether, add anhydrous sodium sulfate Dry overnight, filter with suction, and rotary evaporate to obtain 14.29 g of a yellow liquid, which is passed through a column with petroleum ether as an eluent to obtain 7.46 g of a colorless liquid with a yield of 98.43%.
Embodiment 3
[0032] 1-(3-nitropropyl)-4-octylbenzene (3)
[0033] 9.59g (0.03mol) 3-bromo-1-(4-octylphenyl)propan-1-one (9) and 44ml DMF were added to a 250ml single-necked bottle, magnetically stirred, and 8.47g ( 0.12mol) sodium nitrite, the reaction solution changed from colorless to yellow, stirred in ice bath for 0.5h, then kept at 20°C for 6h, poured the reaction solution into an appropriate amount of ice water, stirred, and carried out with 100ml×3 petroleum ether Extraction, drying with anhydrous sodium sulfate overnight, suction filtration, and rotary evaporation to obtain 10.32 g of yellow liquid, which was passed through the column with PE:AE=20:1 as the eluent to obtain 7.20 g of yellow liquid, yield: 86.6%.
[0034] The combination of 3 has the following NMR data:
[0035] 1H MNR (CDCl3, δ): ppm7.08-7.16 (m, 4H), 4.34-4.37 (t, 2H, J = 6.9Hz), 2.66-2.70 (t, 2H, J = 7.5Hz), 2.55-2.57 (t, 2H, J=7.6Hz), 2.27-2.35(m, 2H), 1.55-1.61(m, 2H), 1.26-1.37(m, 10H), 0.86-0.89(t, 3H, J=7....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com