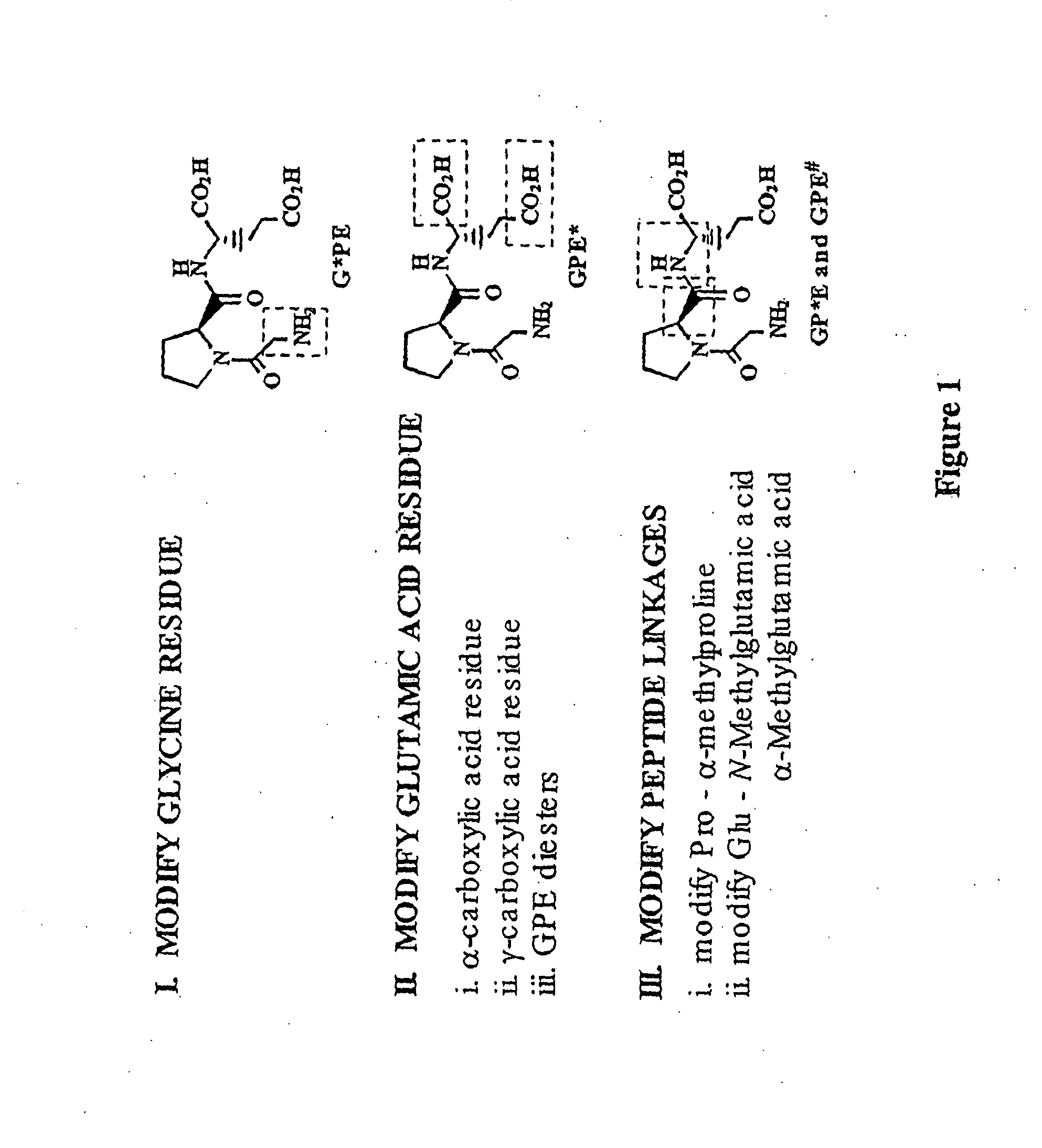
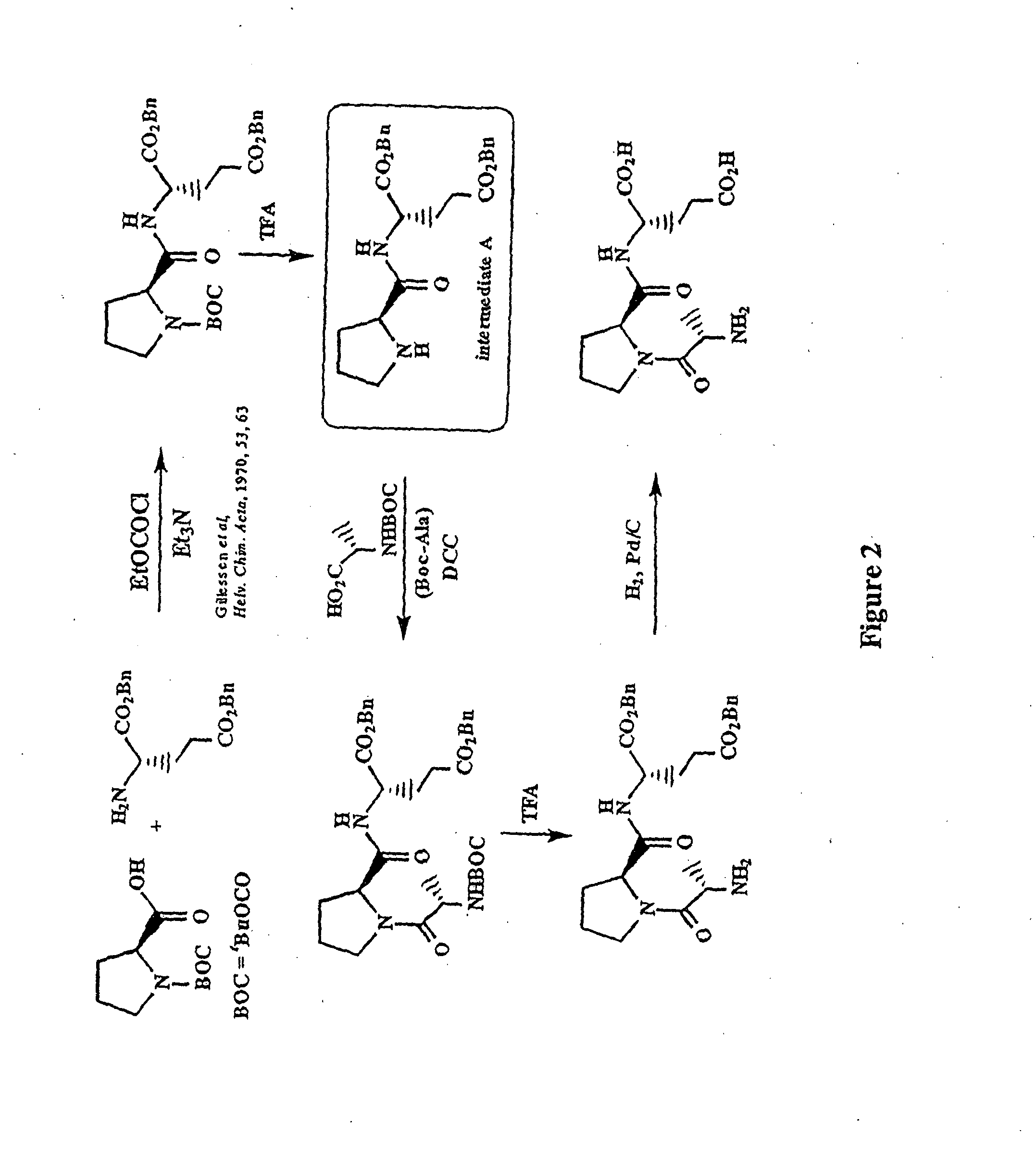
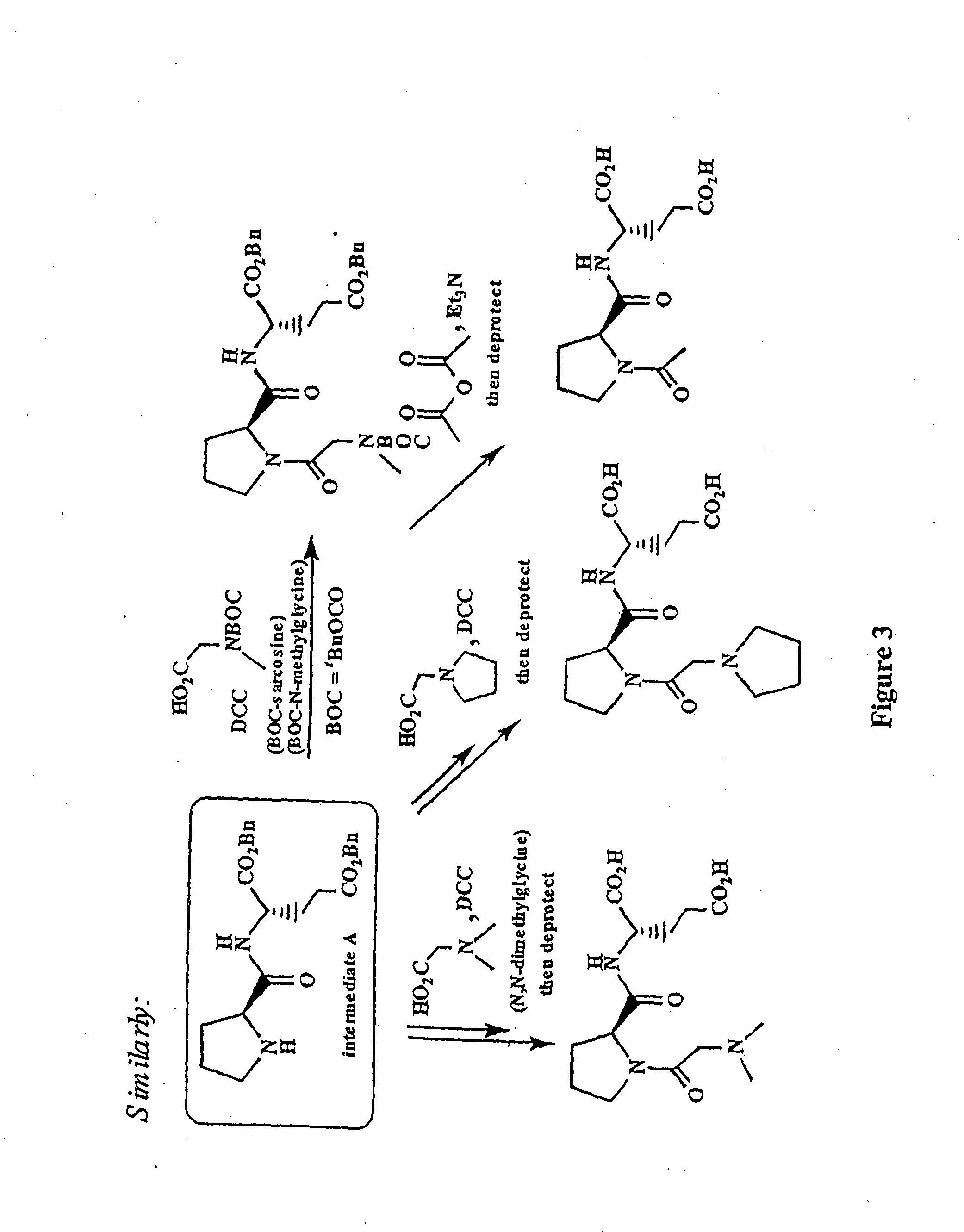
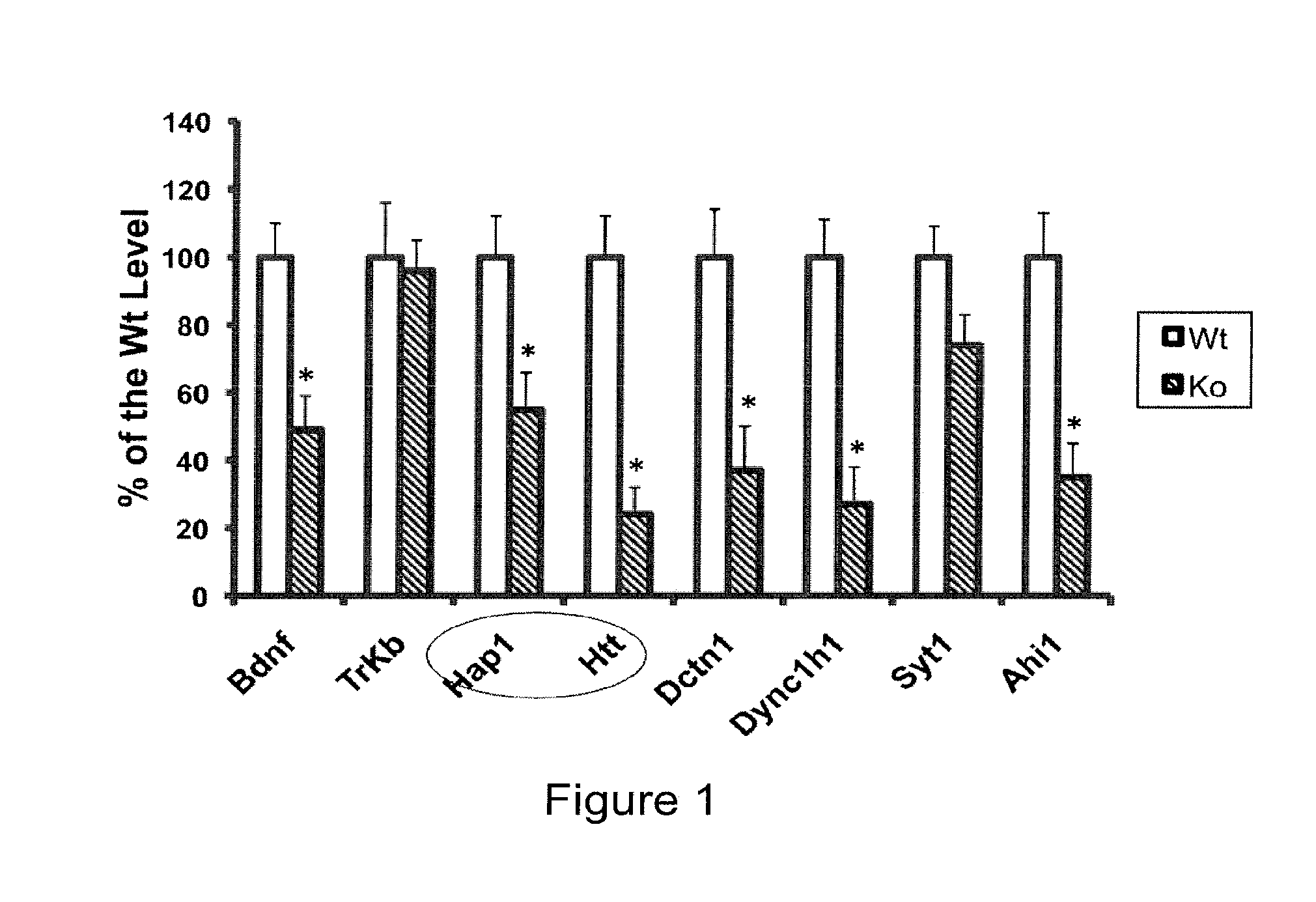
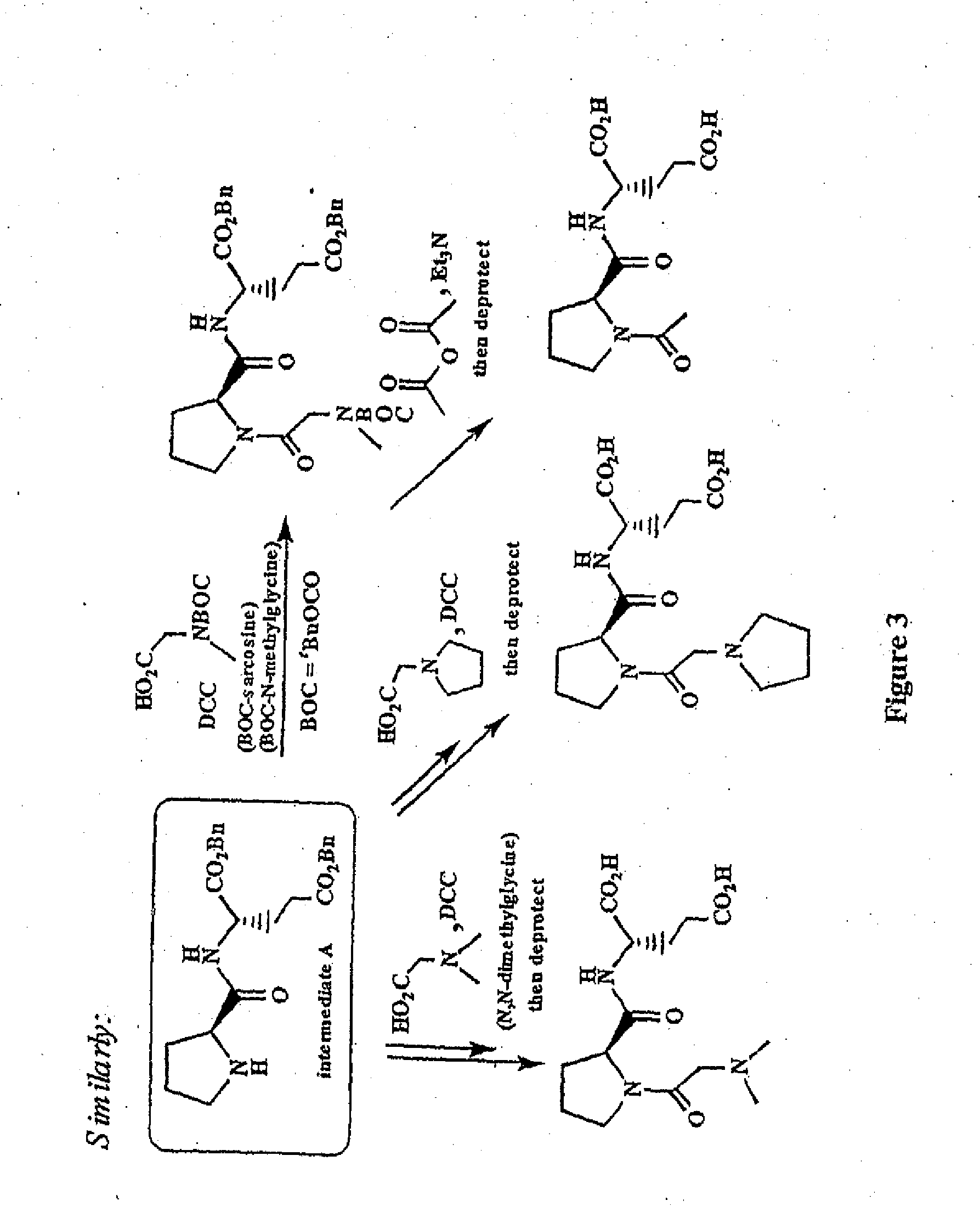
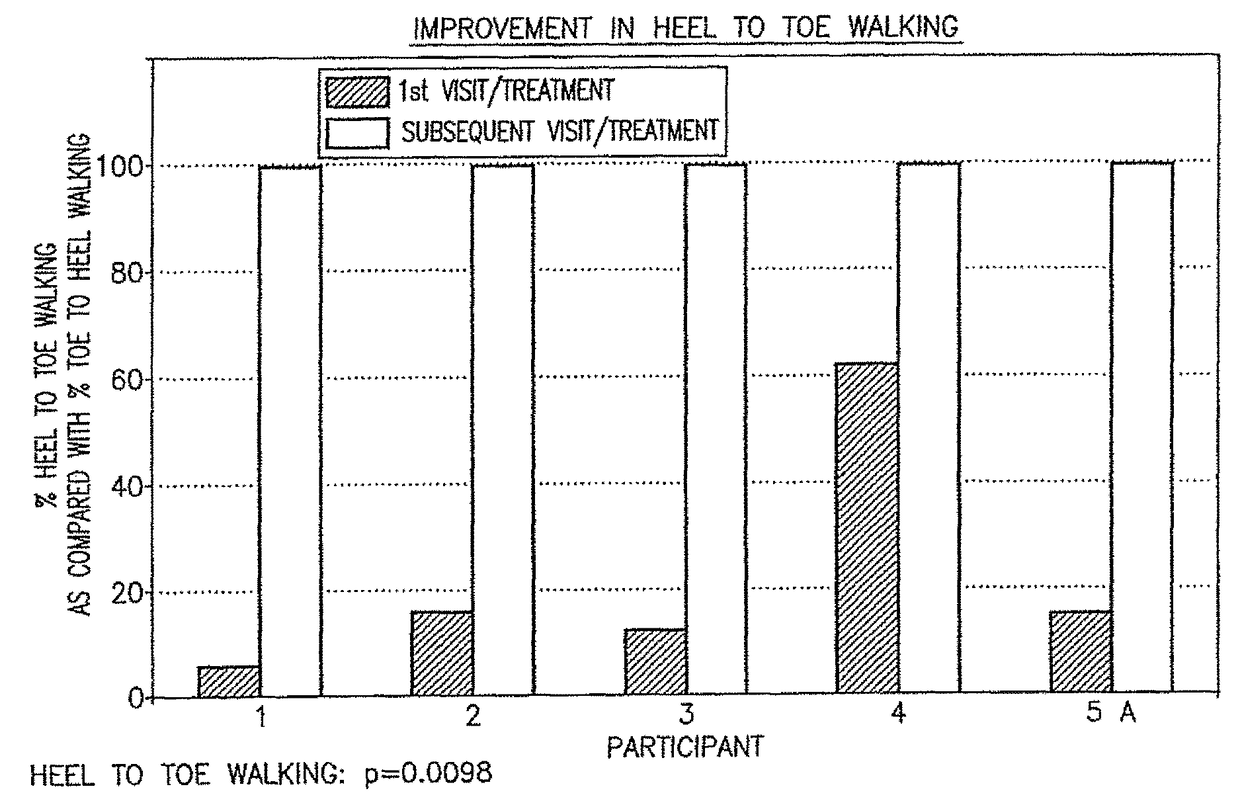
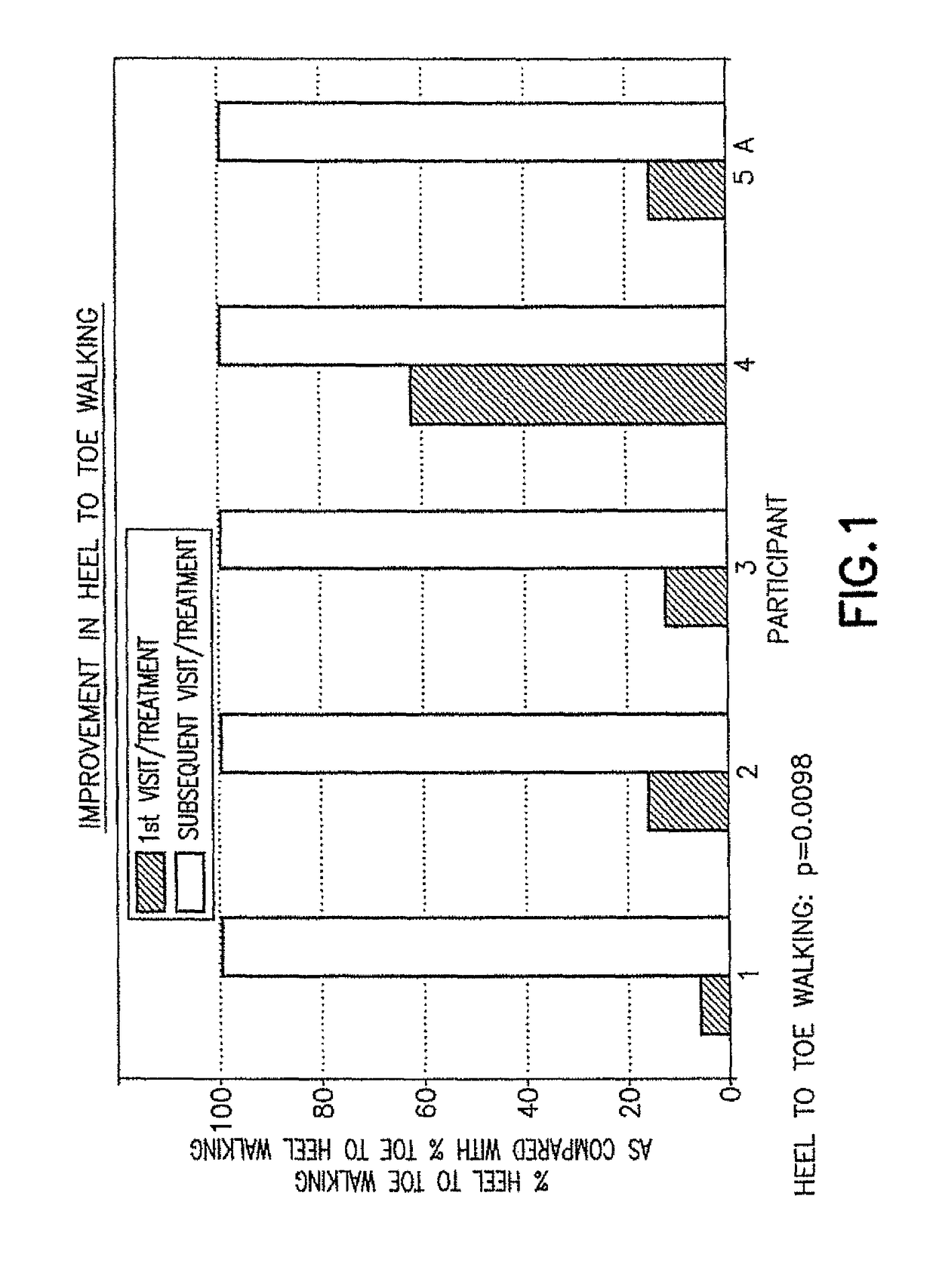
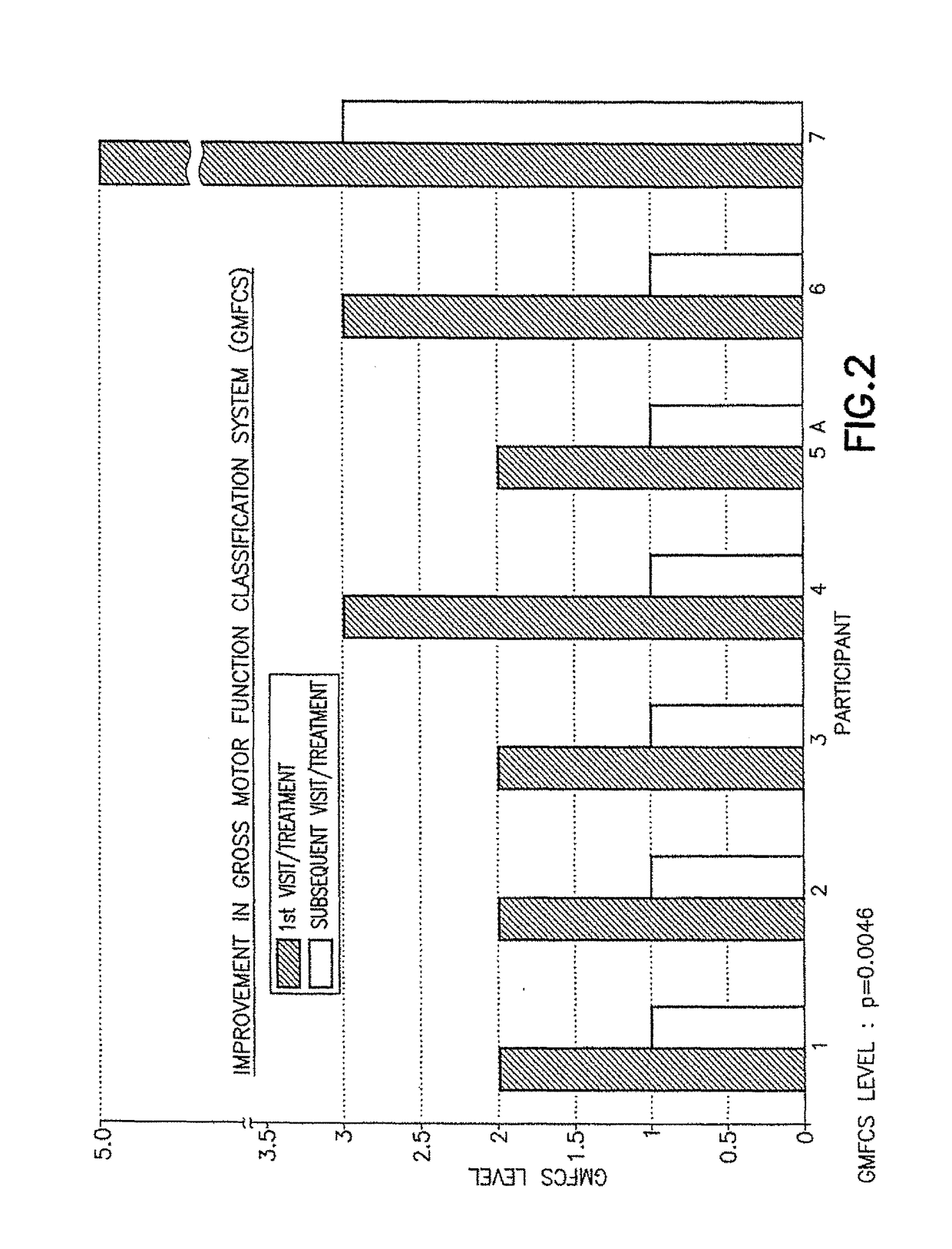
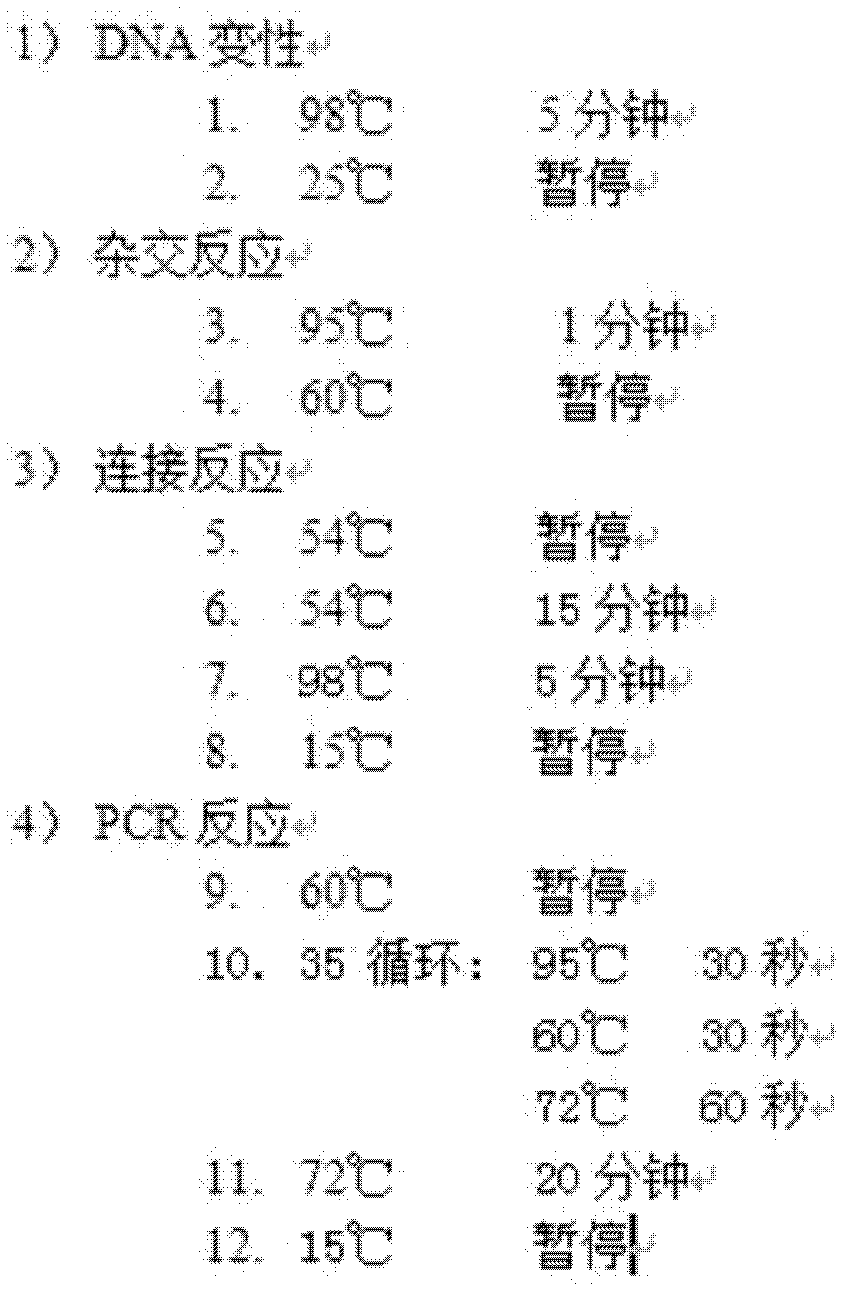Patents
Literature
81 results about "Rett syndrome" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A rare neurological genetic disorder that causes severe muscle movement disability.
Treatment of rett syndrome and other disorders
ActiveUS20090099077A1Recovery functionRestore maturationNervous disorderDipeptide ingredientsDiseaseMedicine
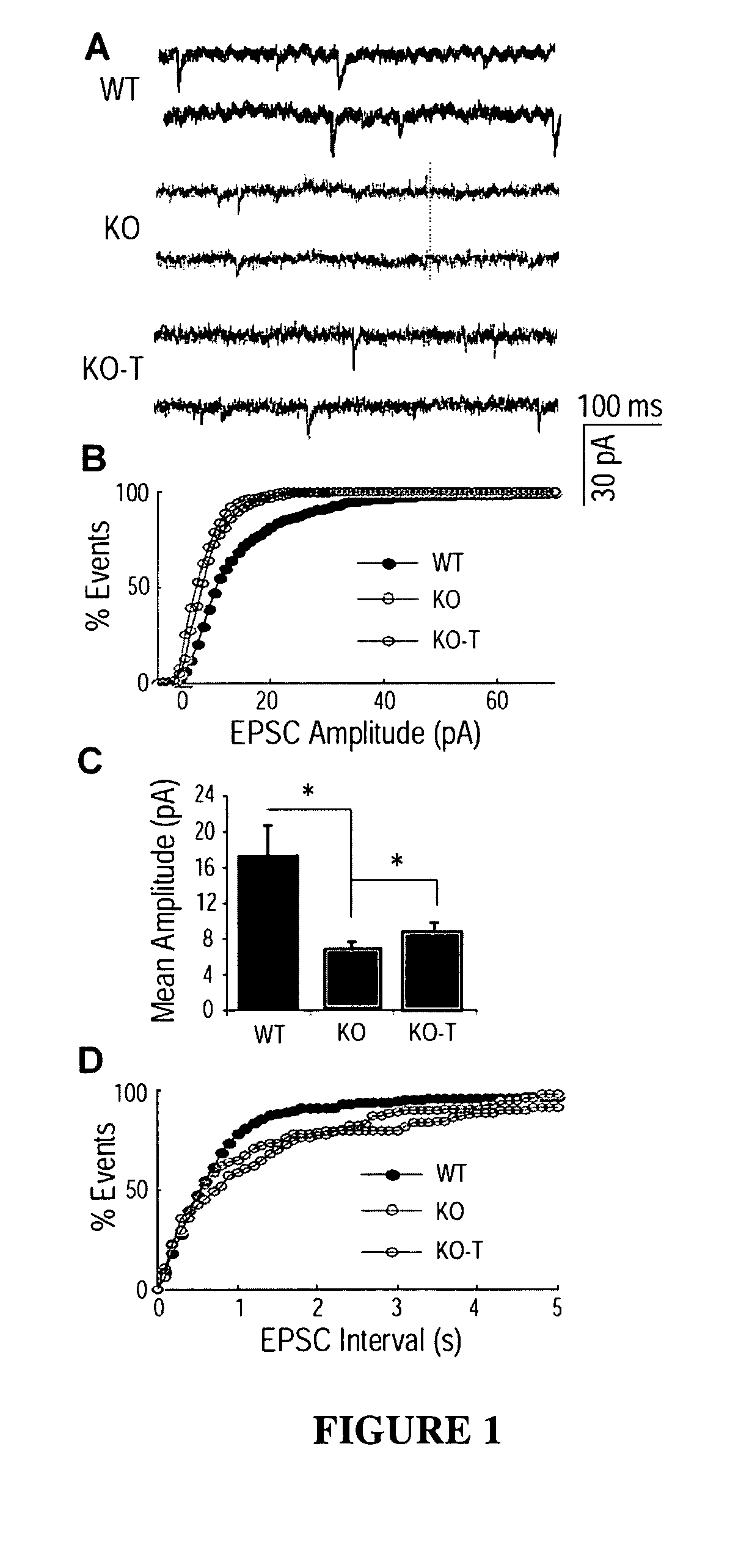
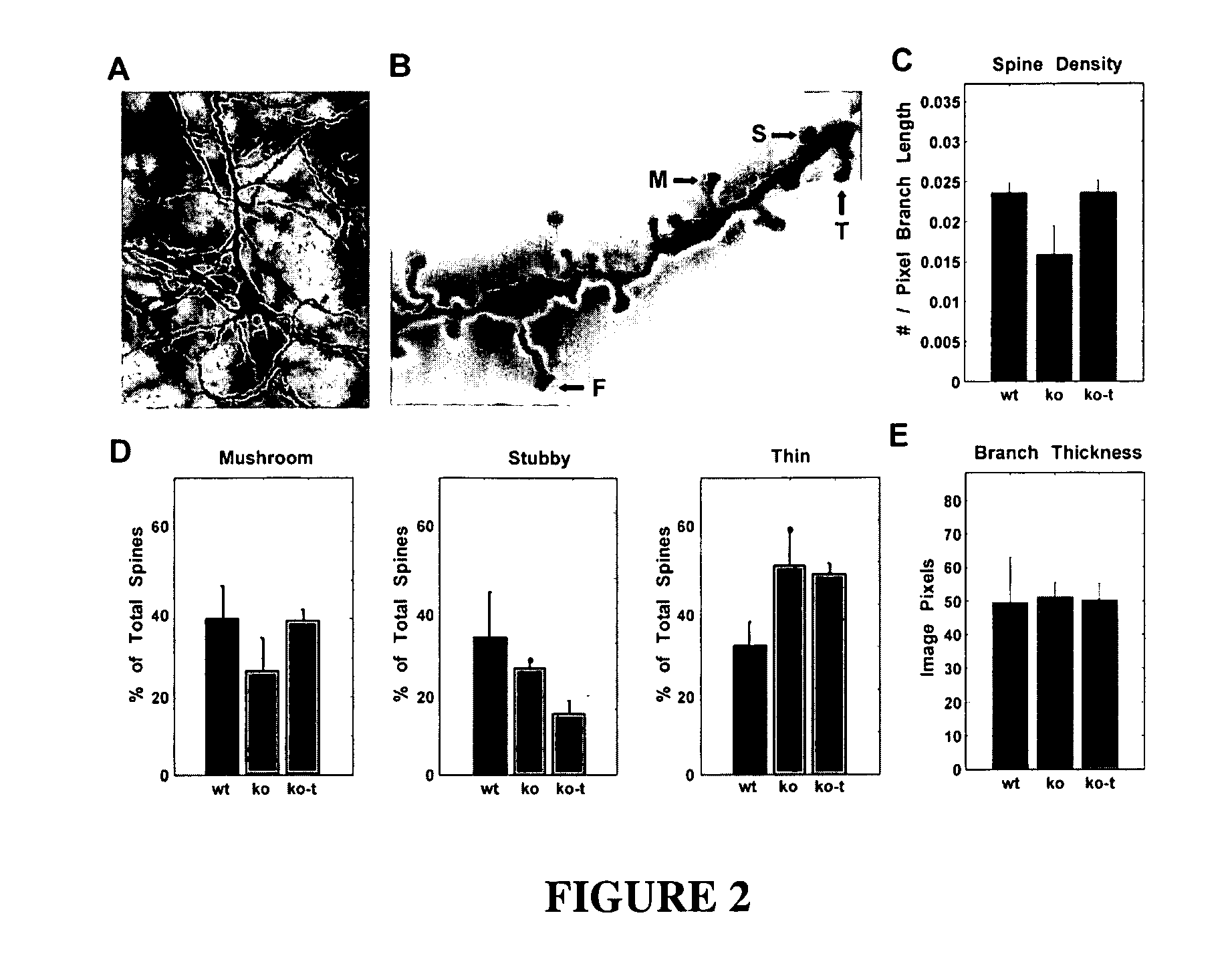
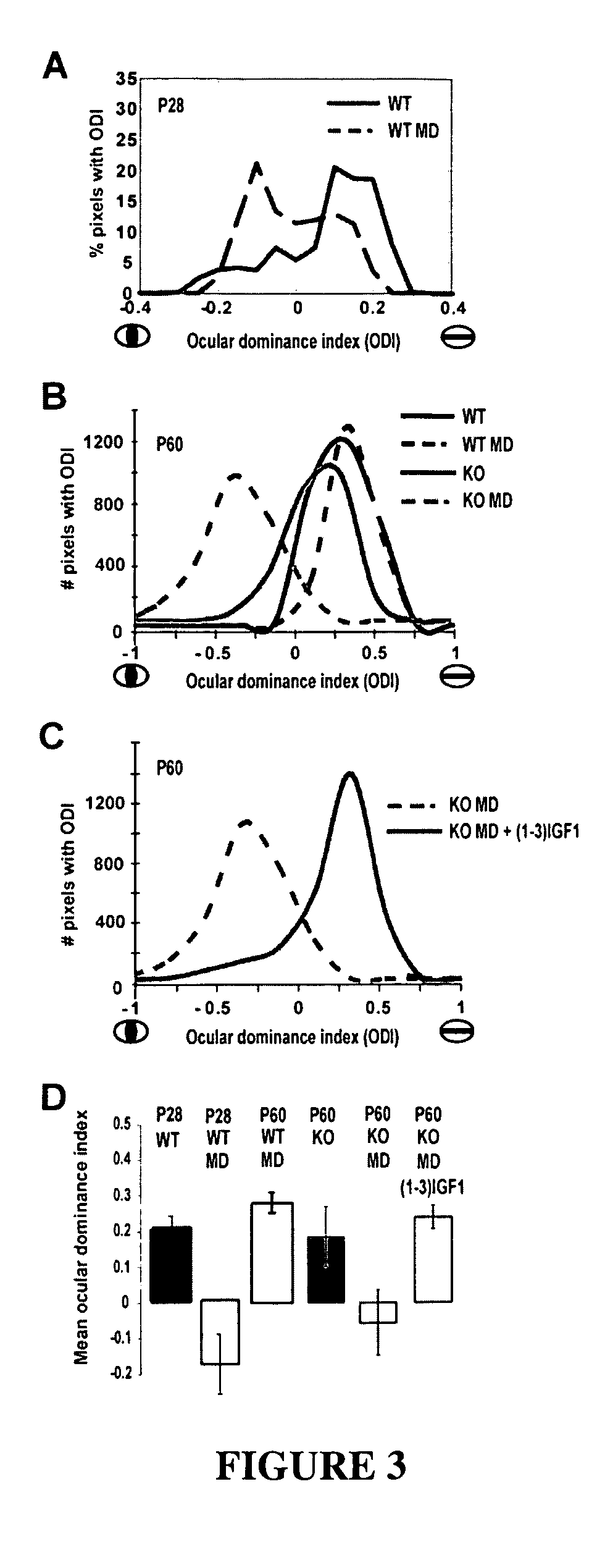
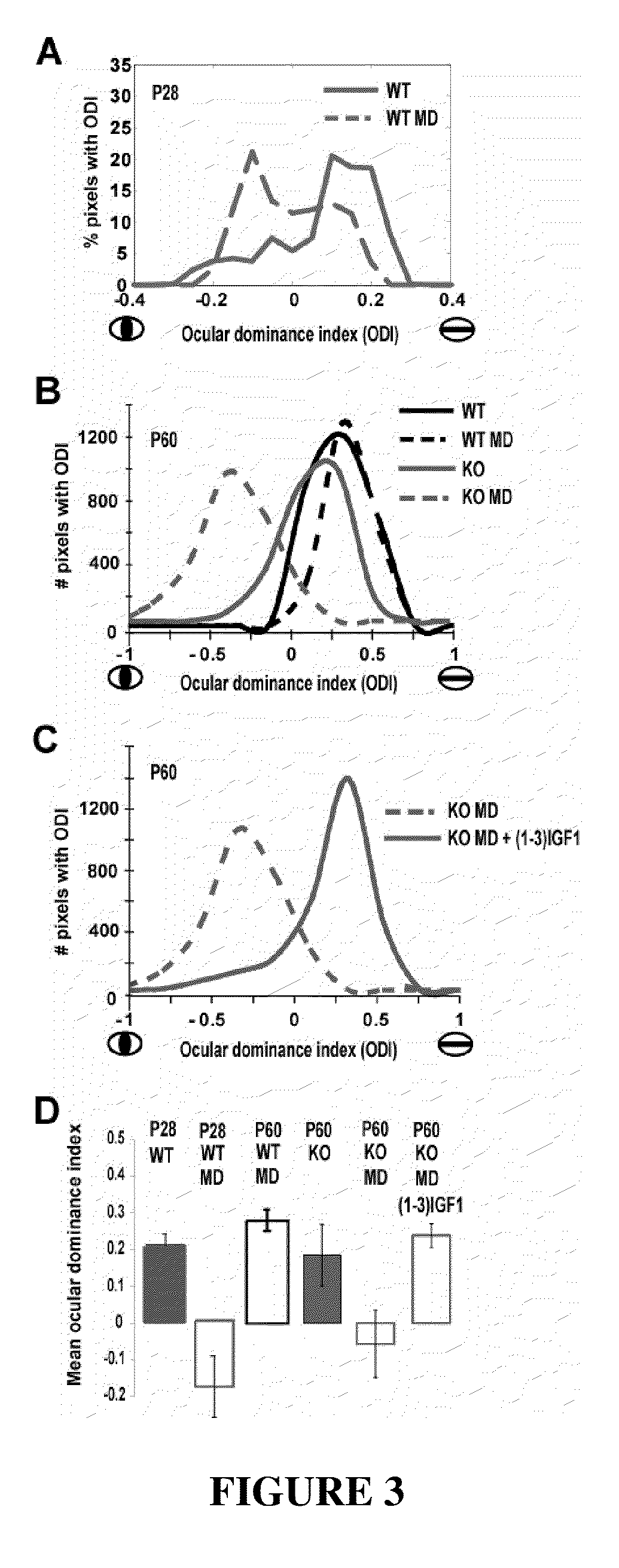
The invention relates to methods for treatment of Rett Syndrome and other disorders of synaptic function and maturation using IGF1, (1-3)IGF-1, (1-3)IGF-1 analog(s) and / or related therapeutic molecules.
Owner:WHITEHEAD INST FOR BIOMEDICAL RES +1
Di-substituted amides for enhancing glutamatergic synaptic responses
InactiveUS8013003B2Stable baselineIncrease amplitudeBiocideNervous disorderSIDS - Sudden infant death syndromeCentral sleep apnea
This invention relates to compounds, pharmaceutical compositions and methods for use in the prevention and treatment of cerebral insufficiency, including enhancement of receptor functioning in synapses in brain networks responsible for basic and higher order behaviors. These brain networks, which are involved in regulation of breathing, and cognitive abilities related to memory impairment, such as is observed in a variety of dementias, in imbalances in neuronal activity between different brain regions, as is suggested in disorders such as Parkinson's disease, schizophrenia, respiratory depression, sleep apneas, attention deficit hyperactivity disorder and affective or mood disorders, and in disorders wherein a deficiency in neurotrophic factors is implicated, as well as in disorders of respiration such as overdose of an alcohol, an opiate, an opioid, a barbiturate, an anesthetic, or a nerve toxin, or where the respiratory depression results form a medical condition such as central sleep apnea, stroke-induced central sleep apnea, obstructive sleep apnea, congenital hypoventilation syndrome, obesity hypoventilation syndrome, sudden infant death syndrome, Rett syndrome, spinal cord injury, traumatic brain injury, Cheney-Stokes respiration, Ondines curse, Prader-Willi's syndrome and drowning. In a particular aspect, the invention relates to compounds useful for treatment of such conditions, and methods of using these compounds for such treatment.
Owner:CORTEX PHARMA INC
Treatment of rett syndrome
ActiveUS7994127B2Recovery functionRestore maturationNervous disorderTetrapeptide ingredientsDiseaseMedicine
The invention relates to methods for treatment of Rett Syndrome and other disorders of synaptic function and maturation using IGF1, (1-3)IGF-1, (1-3)IGF-1 analog(s) and / or related therapeutic molecules.
Owner:WHITEHEAD INST FOR BIOMEDICAL RES +1
Molecular combination probe for diagnosing and screening chromosome microdeletion syndrome
InactiveCN103014142AEconomic screeningQuick screeningMicrobiological testing/measurementDNA/RNA fragmentationBiotechnologyPhosphorylation
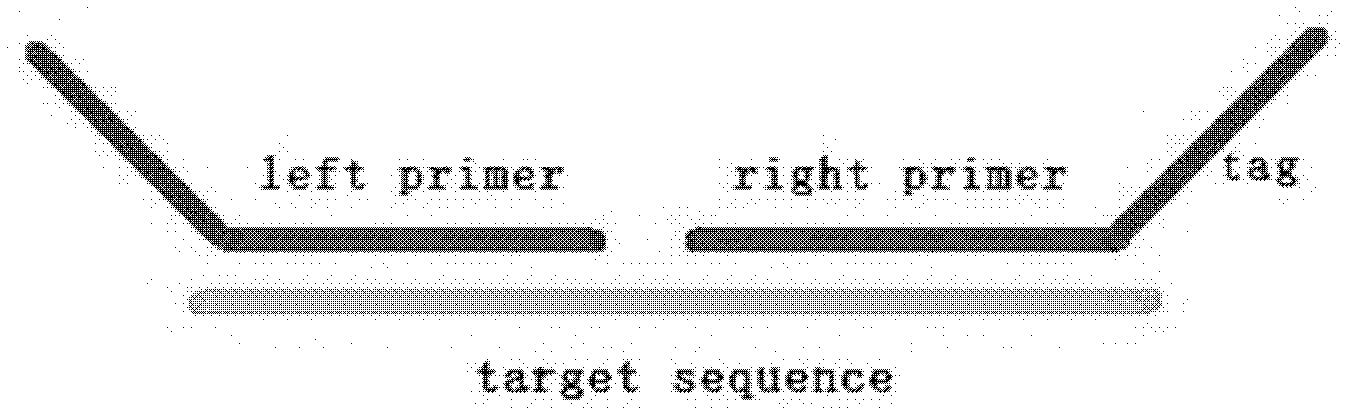
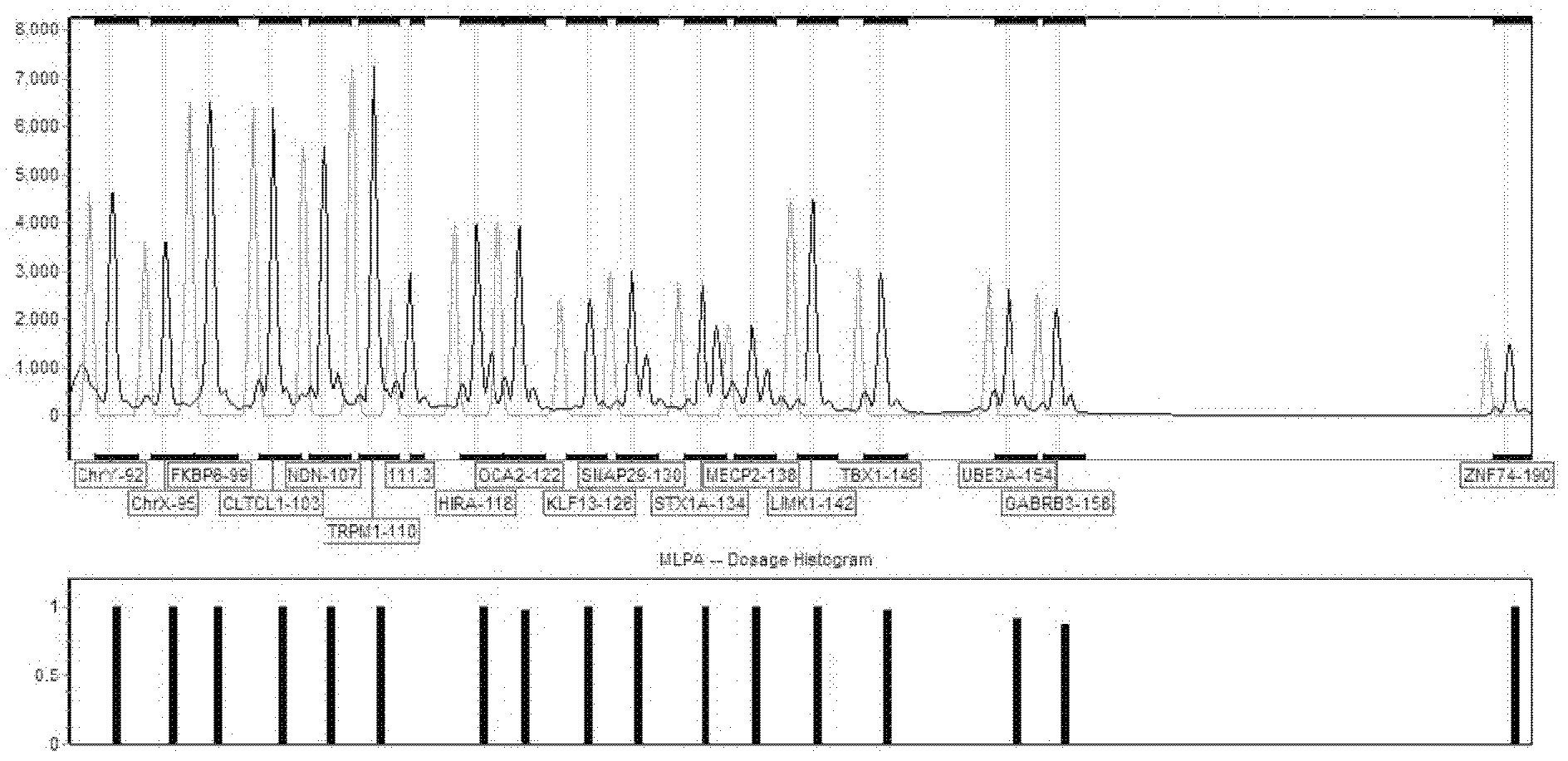
The invention belongs to the technical field of biology, and particularly relates to a molecular combination probe for diagnosing and screening chromosome microdeletion syndrome. The molecular combination probe is used for selecting the key gene of the Williams syndrome, the 22q11 microdeletion syndrome, the Prader-Willi syndrome, the Angelman syndrome, the 15q13.3 microdeletion syndrome and the Rett syndrome, or the gene within a critical area, or the gens arranged at two ends within a duplication / deletion fragment, selecting the sequence which meets a corresponding condition as a probe sequence according to the sequence of the gene, and adding a general primer sequence and adding a phosphorylation mark to the 5'end of a probe left-half sequence and the 3'end of a right-half probe to prepare the combination probe for the multiple continuous probe amplification technology. According to the combination probe provided by the invention, the defects of the fluorescent quantitative PCR (polymerase chain reaction) can be overcome, a plurality of sequences can be analyzed for once, and the molecular combination probe is higher in resolution ratio, sensitivity and repeatability. The probe can be used for the clinical molecular diagnosing and screening of the six-chromosome microdeletion syndrome.
Owner:FUDAN UNIV
Di-substituted amides for enhancing glutamatergic synaptic responses
InactiveUS20100120764A1Stable baselineIncrease amplitudeBiocideNervous disorderSIDS - Sudden infant death syndromeCentral sleep apnea
This invention relates to compounds, pharmaceutical compositions and methods for use in the prevention and treatment of cerebral insufficiency, including enhancement of receptor functioning in synapses in brain networks responsible for basic and higher order behaviors. These brain networks, which are involved in regulation of breathing, and cognitive abilities related to memory impairment, such as is observed in a variety of dementias, in imbalances in neuronal activity between different brain regions, as is suggested in disorders such as Parkinson's disease, schizophrenia, respiratory depression, sleep apneas, attention deficit hyperactivity disorder and affective or mood disorders, and in disorders wherein a deficiency in neurotrophic factors is implicated, as well as in disorders of respiration such as overdose of an alcohol, an opiate, an opioid, a barbiturate, an anesthetic, or a nerve toxin, or where the respiratory depression results form a medical condition such as central sleep apnea, stroke-induced central sleep apnea, obstructive sleep apnea, congenital hypoventilation syndrome, obesity hypoventilation syndrome, sudden infant death syndrome, Rett syndrome, spinal cord injury, traumatic brain injury, Cheney-Stokes respiration, Ondines curse, Prader-Willi's syndrome and drowning. In a particular aspect, the invention relates to compounds useful for treatment of such conditions, and methods of using these compounds for such treatment.
Owner:CORTEX PHARMA
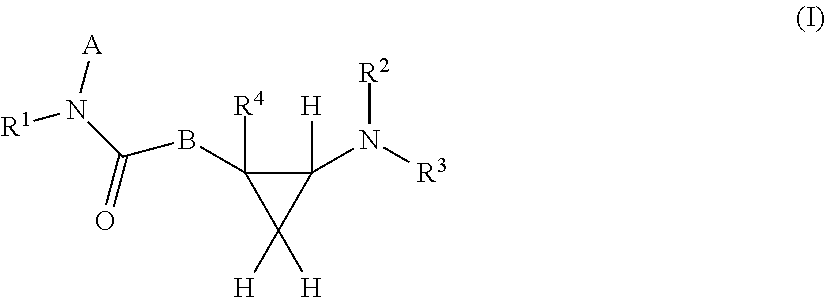
Derivatives of rufinamide and their use in inhibtion of the activation of human voltage-gated sodium channels
ActiveUS20170029382A1Improve actionGreatly reduces seizuresOrganic active ingredientsNervous disorderDiseaseEpileptic encephalopathy
The present invention provides compounds of formula I which are capable of inhibition of the activation of hNav1.1 or hNav1.6 sodium channels in neurons. Pharmaceutical compositions comprising these compounds are also provided. Methods for prevention and treatment of neurological disorders, including, for example, seizures and seizure disorders, including Lennox-Gastaut Syndrome, Dravet syndrome, epileptic encephalopathies, autism, Familial hemiplegic migraine (FHM), anxiety disorders, including Post-traumatic stress disorder (PTSD), panic disorder and obsessive-compulsive disorder, neuropathic pain, and Rett syndrome by administration of these compounds are also provided.
Owner:AES GLOBAL HLDG PTE LTD +1
Methods for the treatment of respiratory depression
InactiveUS20110003835A1Stable baselineIncrease amplitudeBiocideNervous disorderSIDS - Sudden infant death syndromeCentral sleep apnea
This invention relates to compounds, pharmaceutical compositions and methods for use in the prevention and treatment of cerebral insufficiency, including enhancement of receptor functioning in synapses in brain networks responsible for basic and higher order behaviors. These brain networks, which are involved in regulation of breathing, and cognitive abilities related to memory impairment, such as is observed in a variety of dementias, in imbalances in neuronal activity between different brain regions, as is suggested in disorders such as Parkinson's disease, schizophrenia, respiratory depression, sleep apneas, attention deficit hyperactivity disorder and affective or mood disorders, and in disorders wherein a deficiency in neurotrophic factors is implicated, as well as in disorders of respiration such as overdose of an alcohol, an opiate, an opioid, a barbiturate, an anesthetic, or a nerve toxin, or where the respiratory depression results form a medical condition such as central sleep apnea, stoke-induced central sleep apnea, obstructive sleep apnea, congenital hypoventilation syndrome, obesity hypoventilation syndrome, sudden infant death syndrome, Rett syndrome, spinal cord injury, traumatic brain injury, Cheney-Stokes respiration, Ondines curse, Prader-Willi's syndrome and drowning, hi a particular aspect, the invention relates to bicyclic amide compounds useful for treatment of such conditions, and methods of using these compounds for such treatment.
Owner:CORTEX PHARMA
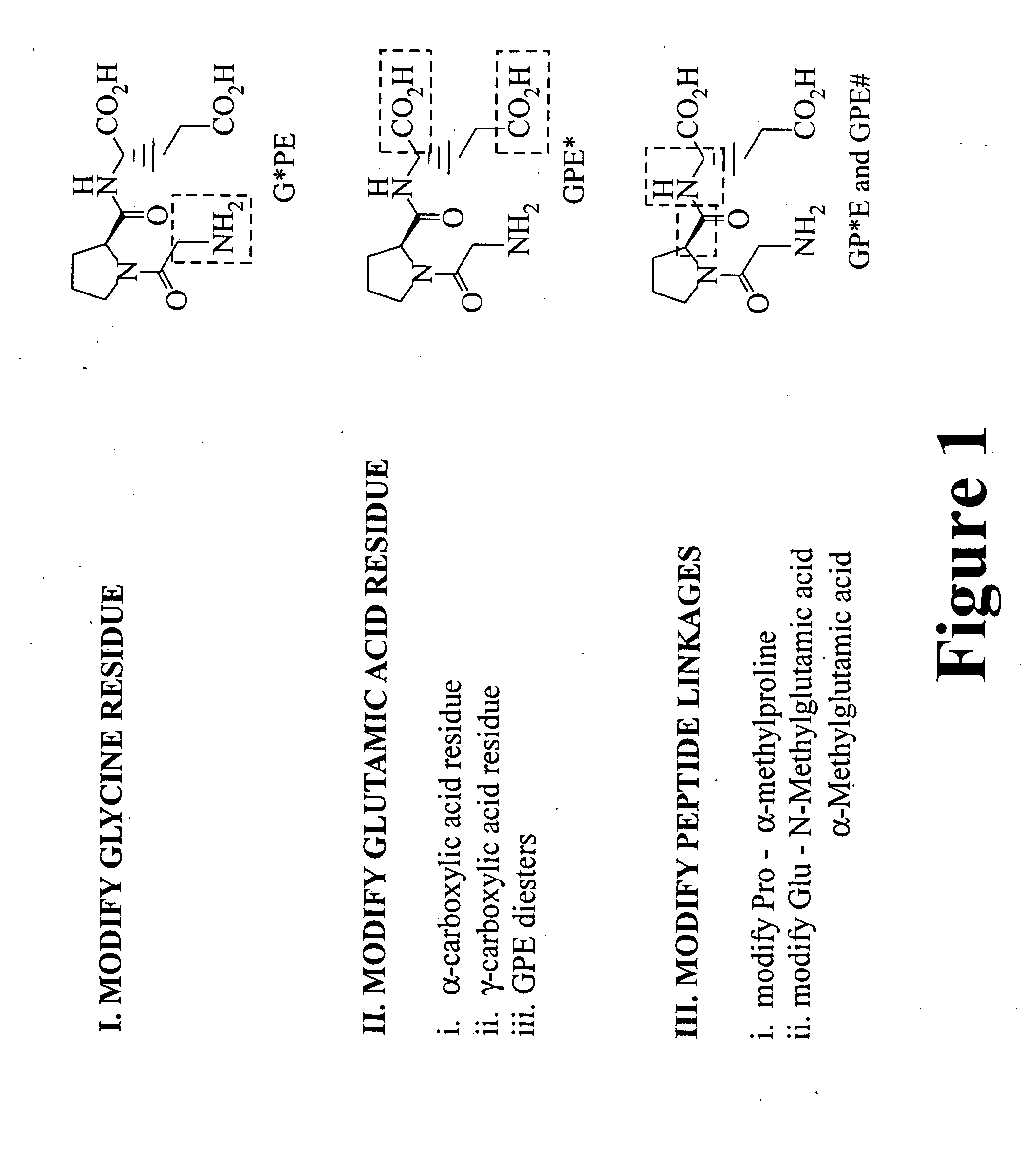
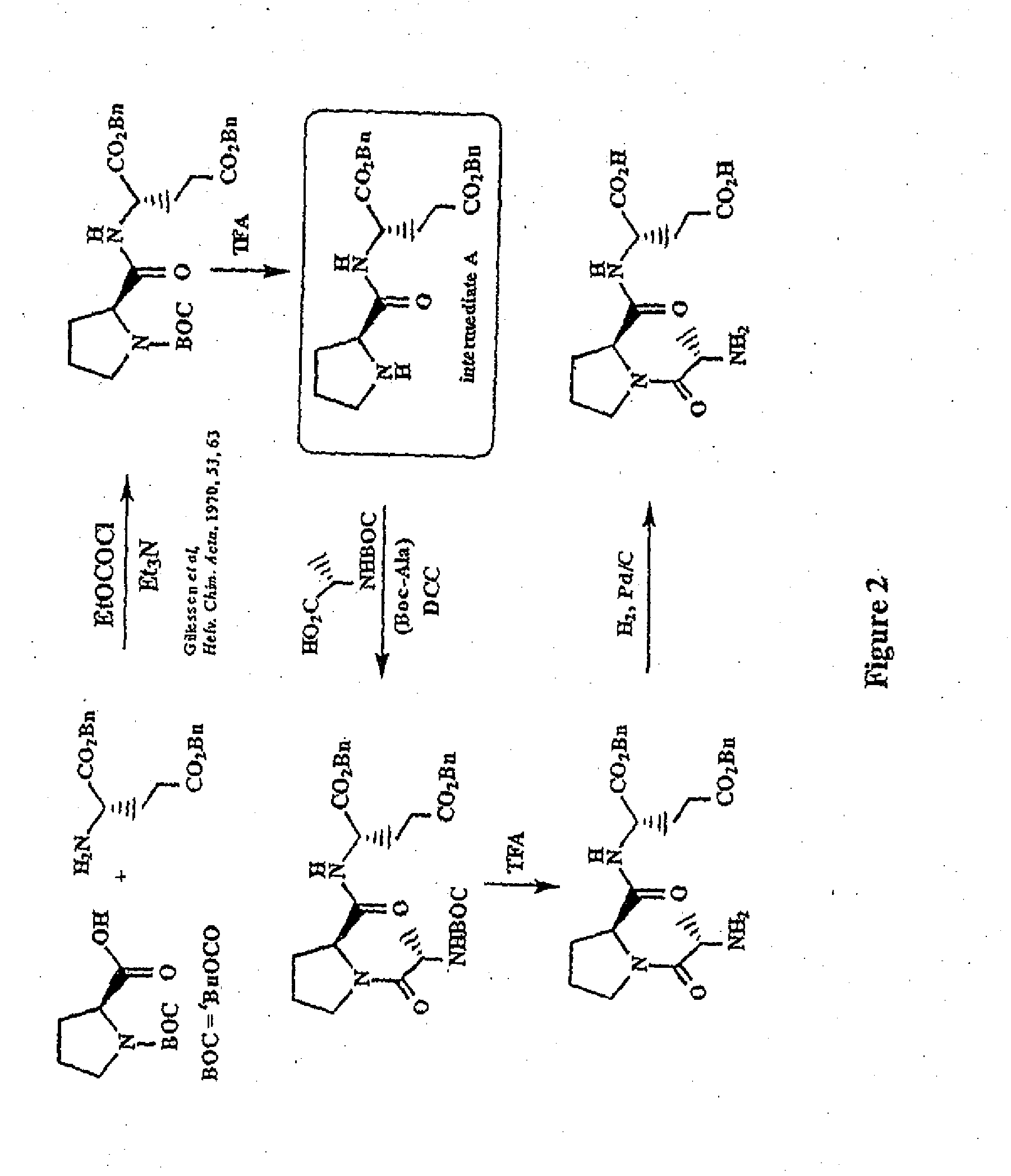
Treatment of autism spectrum disorders using glycyl-l-2-methylprolyl-l-glumatic acid
This invention provides compounds, compositions and methods for treating Autism Spectrum Disorders (ASD) using glycyl-2-methylprolyl-glutamic acid (G-2-MePE) and analogs thereof. Autism Spectrum Disorders include Autism, Autistic Disorder, Asperger Syndrome, Childhood Disintegrative Disorder, Pervasive Developmental Disorder-Not Otherwise Specified (PDD-NOS), Fragile X Syndrome, and Rett Syndrome. Compositions containing compounds include water-soluble formulations, water-in-oil micro-emulsions, water-in-oil coarse emulsions, water-in-oil liquid crystals, nanocapsules, tablets, and orally administered gels. The compounds and compositions of this invention can be administered intravenously, intraventricularly, parenterally, or orally, and can be effective in treating neurodegeneration, promoting neurological function, treating seizure activity and other symptoms of ASD, and can prolong life in animals including human beings having Autism Spectrum Disorders.
Owner:NEUREN PHARMA LTD
Treatment of autism spectrum disorders using glycyl-l-2-methylprolyl-l-glutamic acid
This invention provides compounds, compositions and methods for treating Autism Spectrum Disorders (ASD) using glycyl-2-methylprolyl-glutamic acid (G-2-MePE) and analogs thereof. Autism Spectrum Disorders include Autism, Autistic Disorder Asperger Syndrome, Childhood Disintegrative Disorder, Pervasive Developmental Disorder—Not Otherwise Specified (PDD-NOS), Fragile X Syndrome, and Rett Syndrome. Compositions containing compounds include water-soluble formulations, water-in-oil micro-emulsions, water-in-oil coarse emulsions, water-in-oil liquid crystals, nanocapsules, tablets, and orally administered gels. The compounds and compositions of this invention can be administered intravenously, intraventricularly, parenterally, or orally, and can be effective in treating neurodegeneration, promoting neurological function, treating seizure activity and other symptoms of ASD, and can prolong life in animals including human beings having Autism Spectrum Disorders.
Owner:NEUREN PHARMA LTD
Treatment of MeCP-2 Associated Disorders
The invention relates to the use of cystamine, cysteamine, or a salt thereof, or of calcineurin inhibitors for treating a MeCP2-associated disorder such as Rett syndrome.
Owner:UNIV DAIX MARSEILLE +3
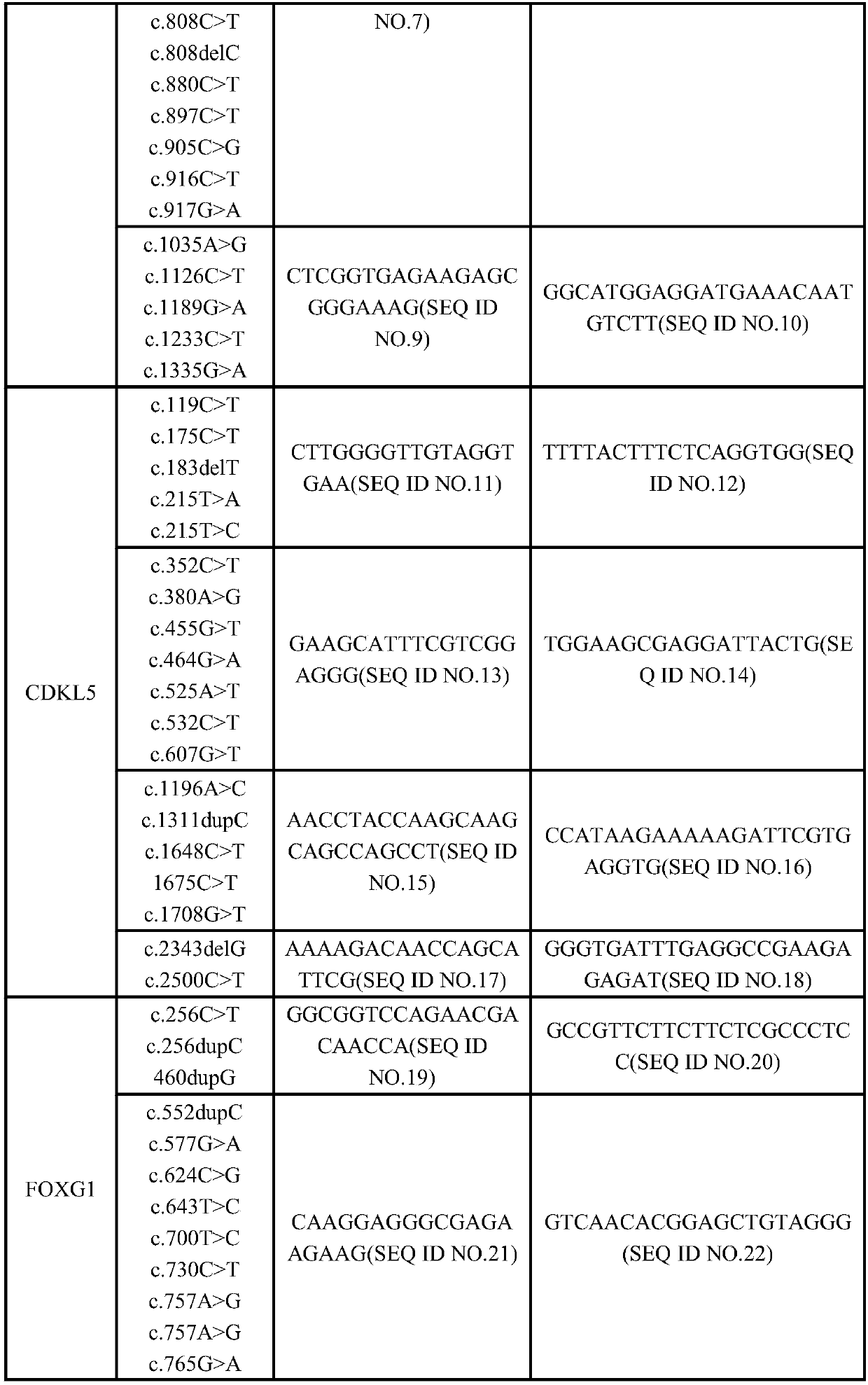
Kit for detecting Rett syndrome pathogenic gene SNP loci based on next generation sequencing and detection method thereof
InactiveCN105950774AAccurate diagnosisHigh detection throughputMicrobiological testing/measurementRett syndromeTrue positive rate
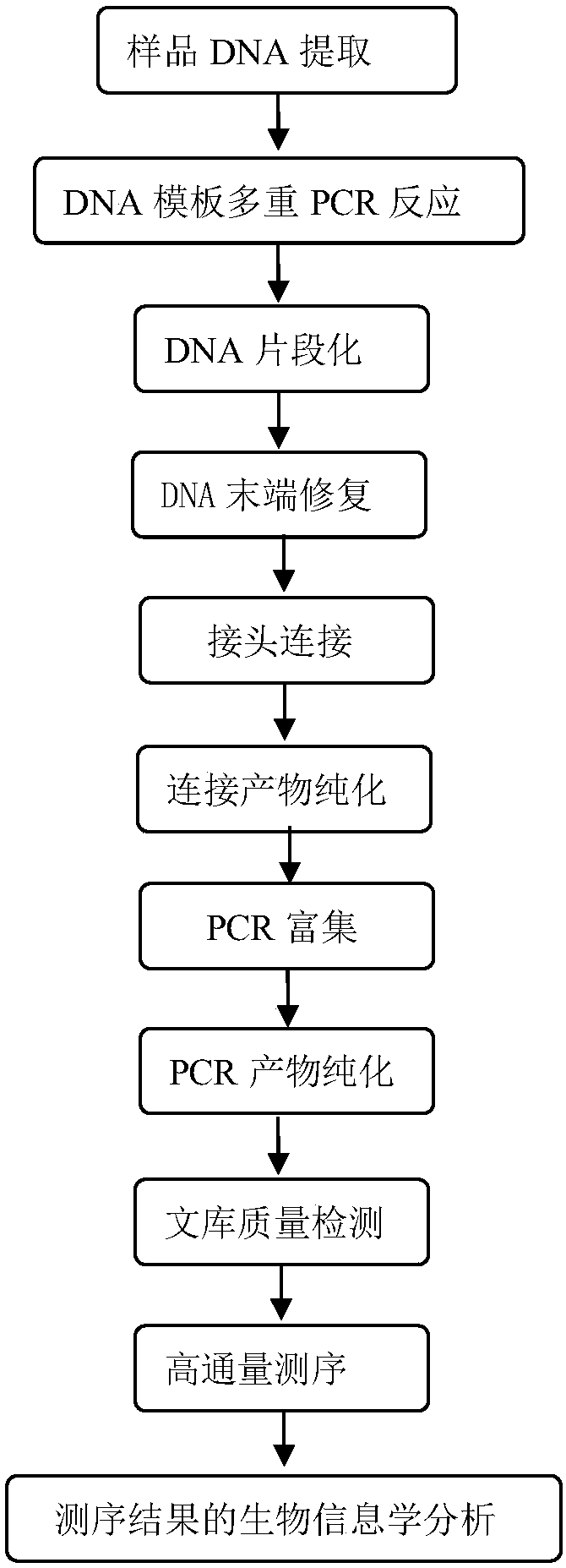
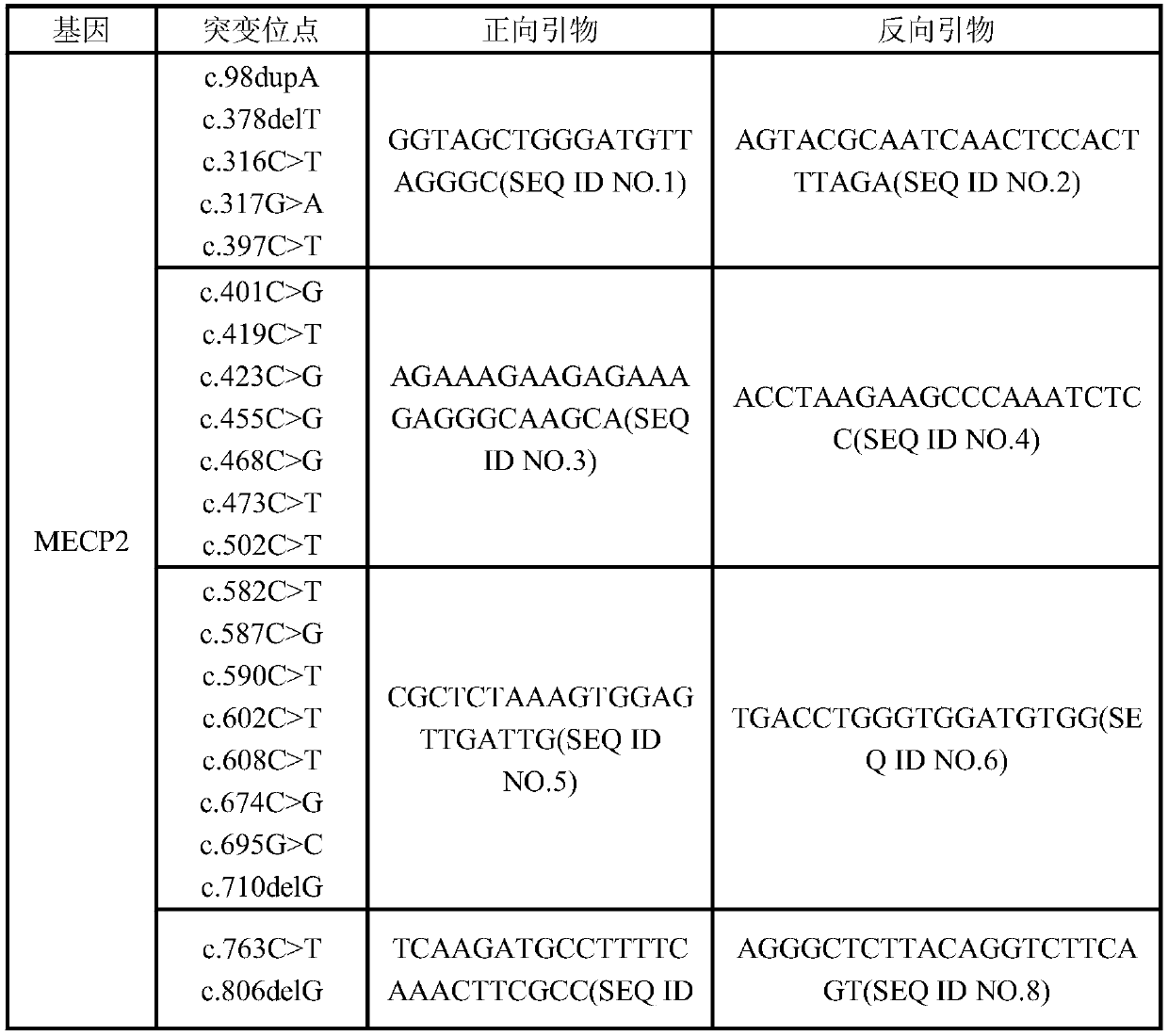
The invention discloses a kit for detecting Rett syndrome pathogenic gene SNP loci based on next generation sequencing and a detection method thereof and belongs to the field of gene detection. The detection method comprises the following steps: (1) extracting sample DNA; (2) carrying out multiplex PCR reaction on a DNA template; (3) carrying out DNA fragmentation; (4) repairing DNA terminal; (5) carrying out joint connection; (6) purifying a connection product; (7) carrying out PCR enrichment; (8) purifying a PCR product; (9) detecting quality of library; (10) carrying out high-throughput sequencing; and (11) analyzing bioinformatics of sequencing results. The kit is high in detection throughput, strong in sensitivity and specificity, simple and safe to operate; the operation is more fast, accurate and complete; the kit is free of toxic and harmful substances and is free of harm to test workers and the environment.
Owner:JIANGSU YINUOWAN CELL CLINIC CO LTD
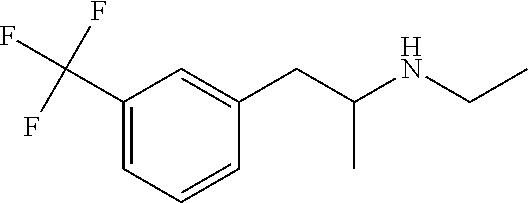
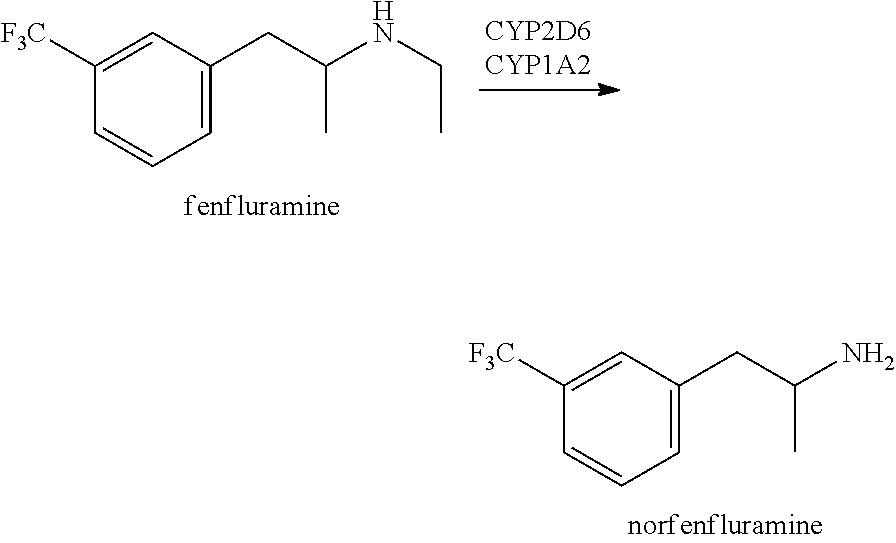
Methods of treating rett syndrome using fenfluramine
PendingUS20220008389A1Improving cognitive deficitExtended durationNervous disorderHydroxy compound active ingredientsFenfluramineRett syndrome
A method of treating and / or preventing symptoms of Rett syndrome (RTT) in a patient such as a patient previously diagnosed with Rett syndrome, by administering an effective dose of a 5-HT1D, 5-HT2A, 5-HT2C or sigma-1 receptor agonist (e.g., fenfluramine or its pharmaceutically acceptable salt) to that patient. RTT patients are treated at a preferred dose of less than about 1.0 mg / kg / day and may be administered as fenfluramine in an amount of between 0.2 to 0.8 mg / kg / day, to a maximum of 30 mg / day in a liquid oral dose.
Owner:ZOGENIX INT
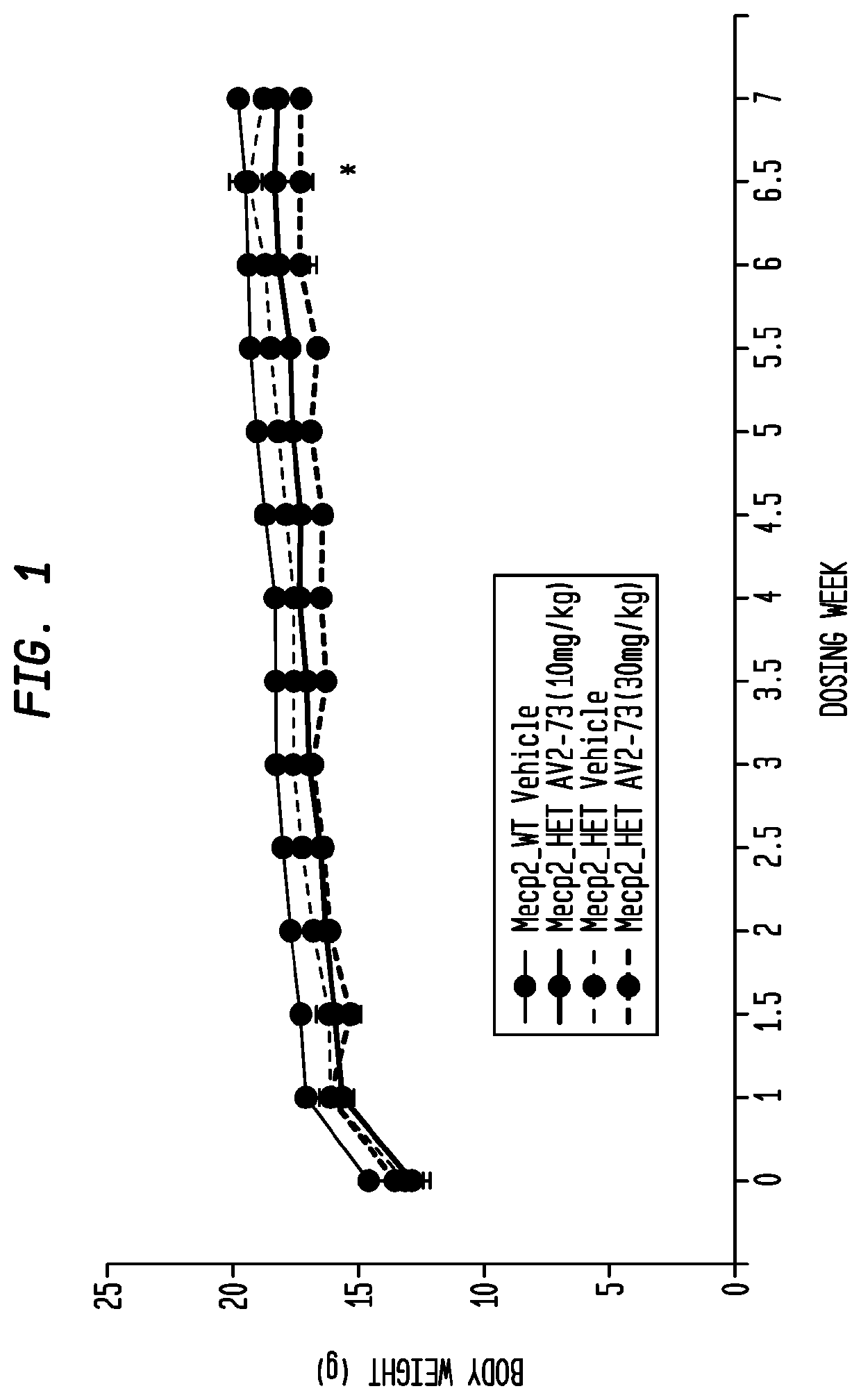
Neurodevelopmental disorder therapy
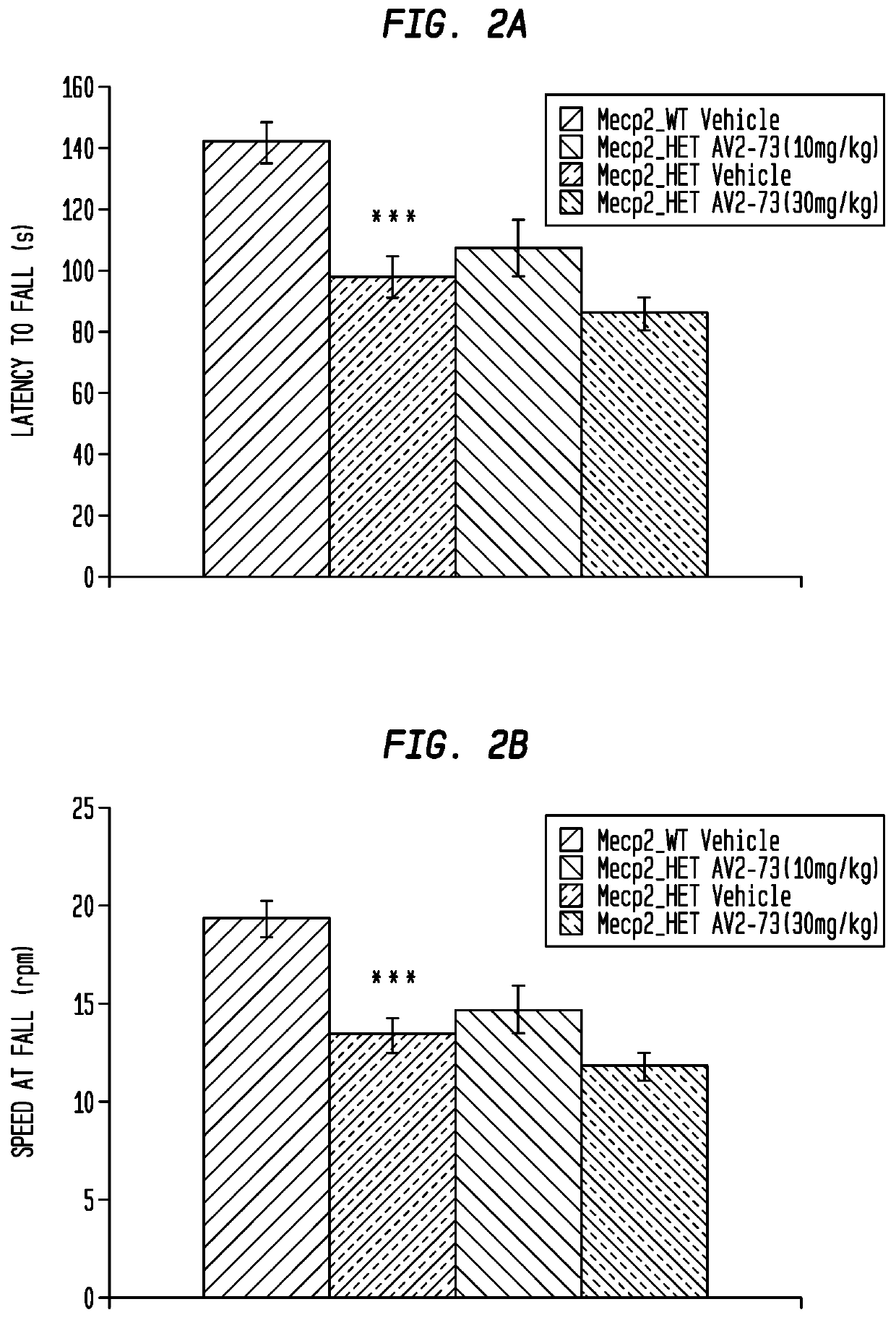
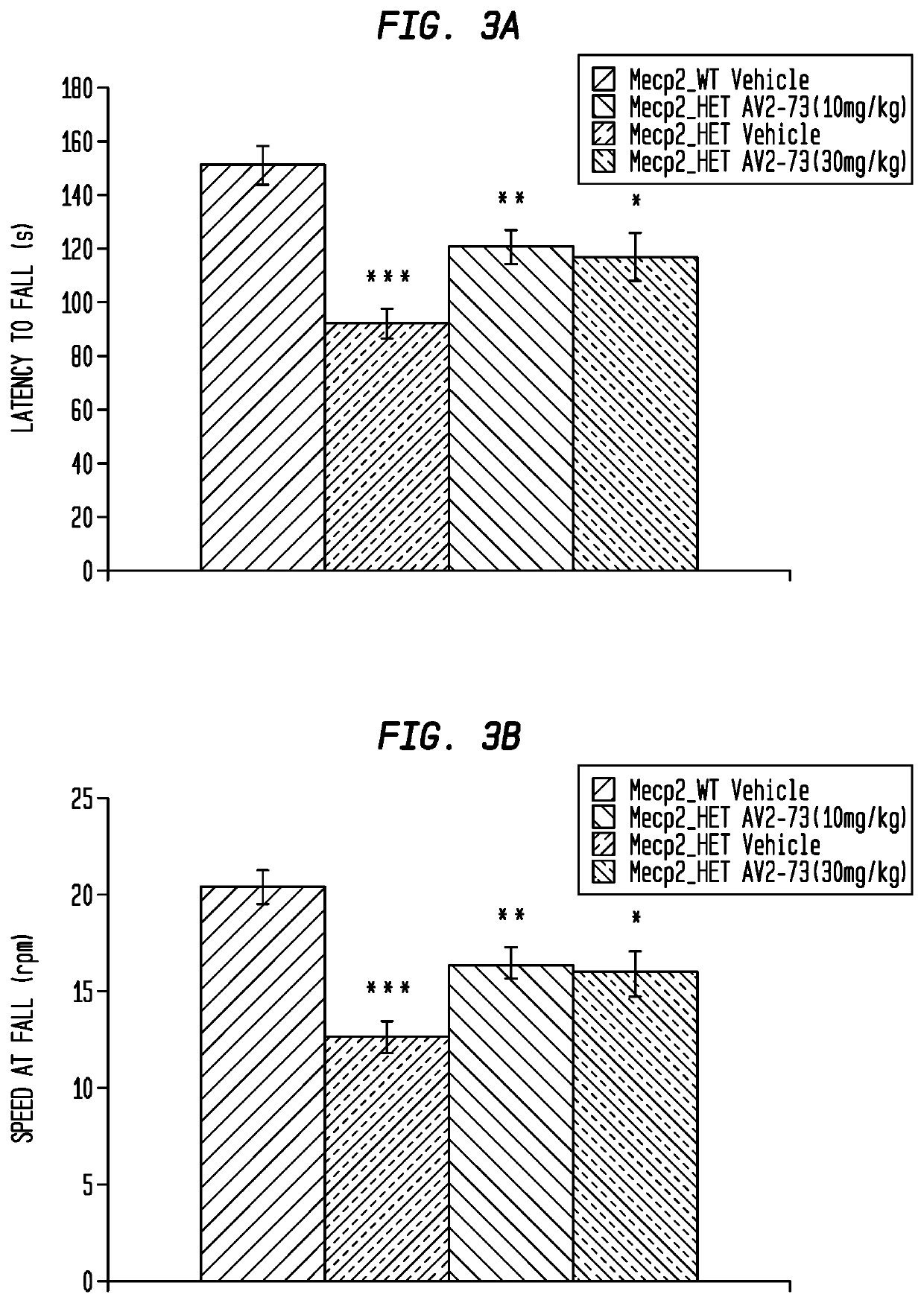
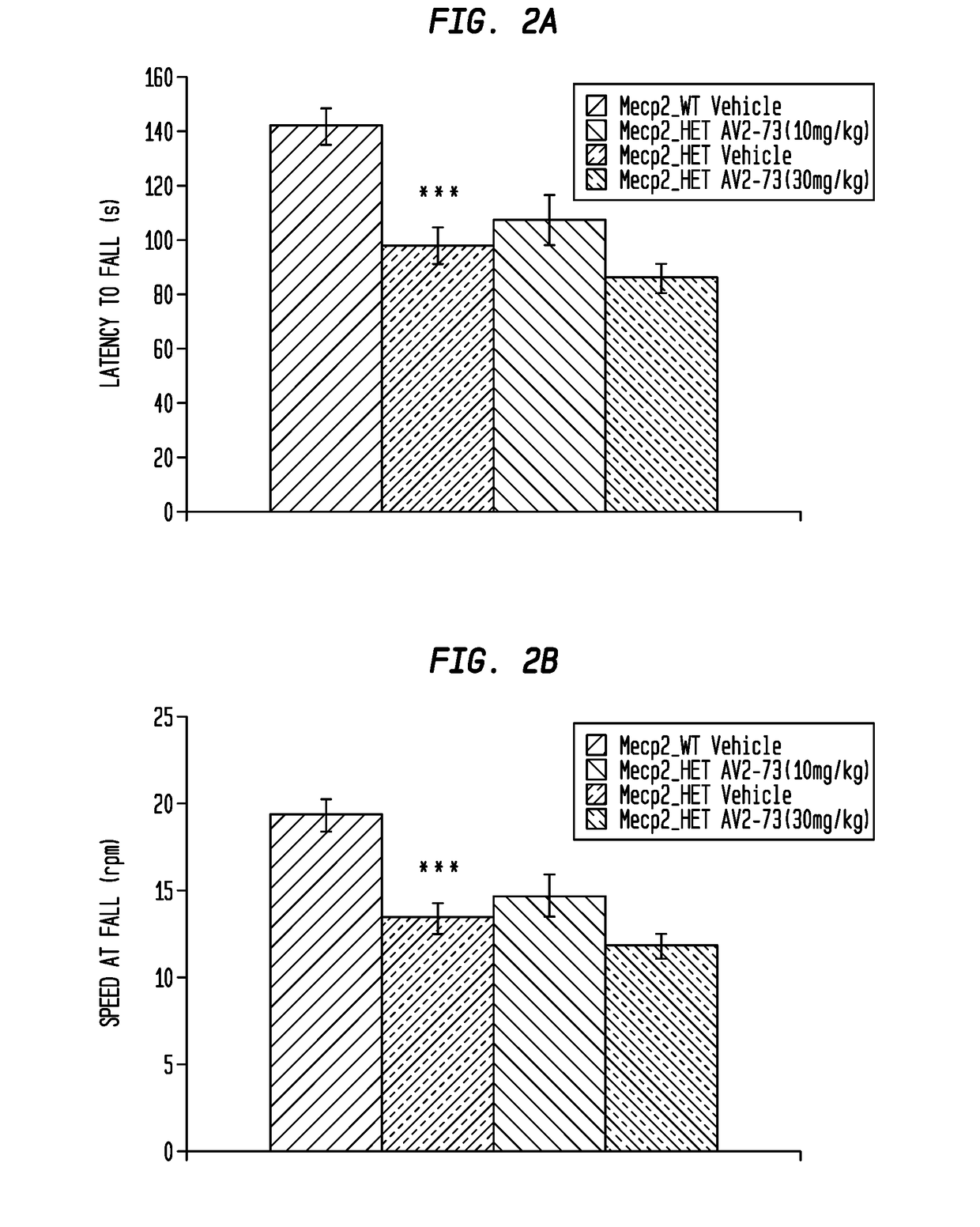
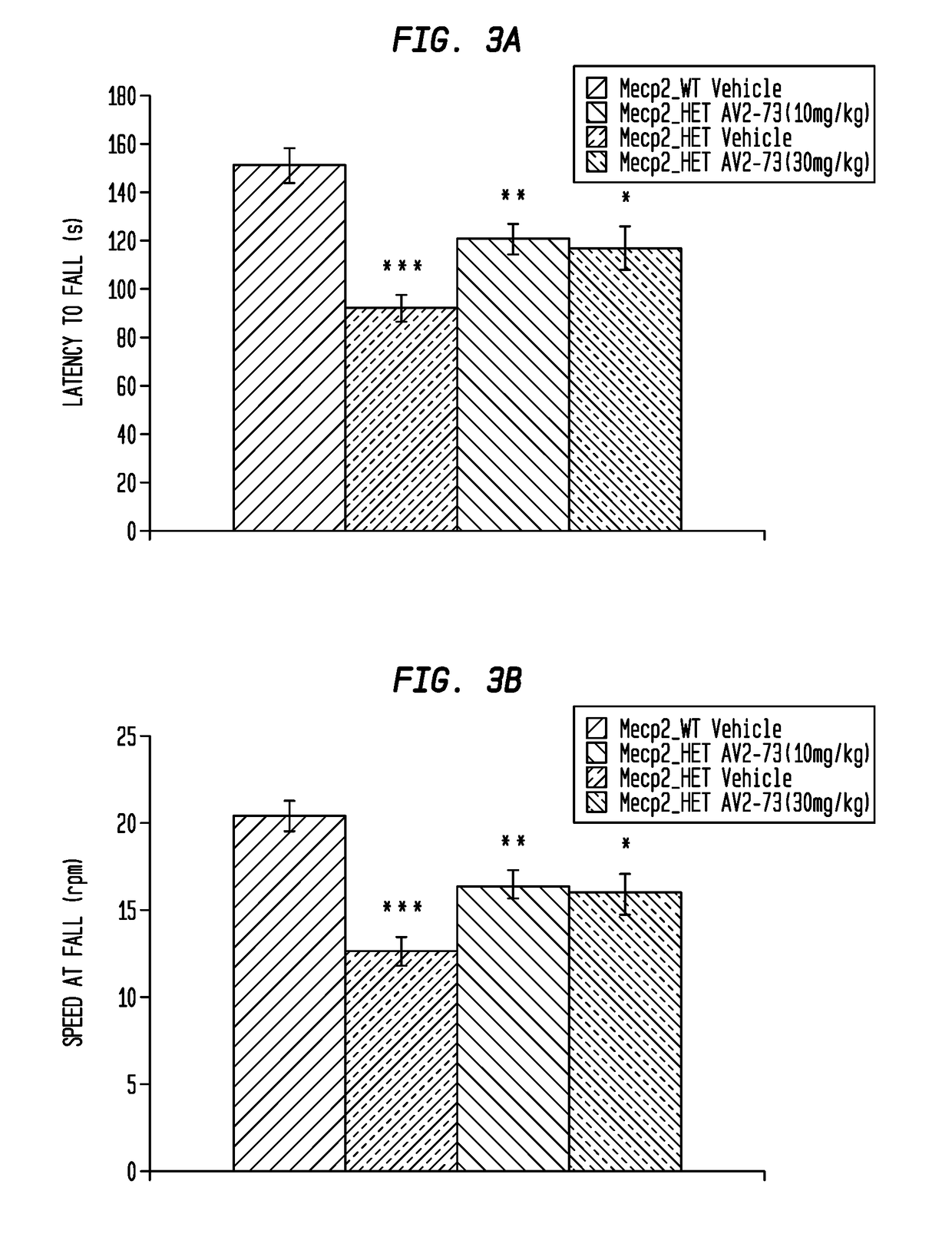
This invention addresses tetrahydro-N,N-dimethyl-2,2-diphenyl-3-furanmethanamine hydrochloride (ANAVEX2-73, AV2-73, or A2-73) in a method of treatment for neurodevelopmental disorders. Particular reference is made to the treatment of autism spectrum disorder, cerebral palsy, Rett syndrome, Angelman syndrome, Williams syndrome, pervasive developmental disorder not otherwise specified (PDD-NOS), childhood disintegrative disorder, and Smith-Magenis syndrome. Additional reference is made to multiple sclerosis.
Owner:ANAVEX LIFE SCI CORP
Treatment of Autism Spectrum Disorders Using Glycyl-L-2-Methylprolyl-L-Glutamic Acid
Owner:NEUREN PHARMA LTD
Pre-frontal cortex processing disorder gait and limb impairments treatment
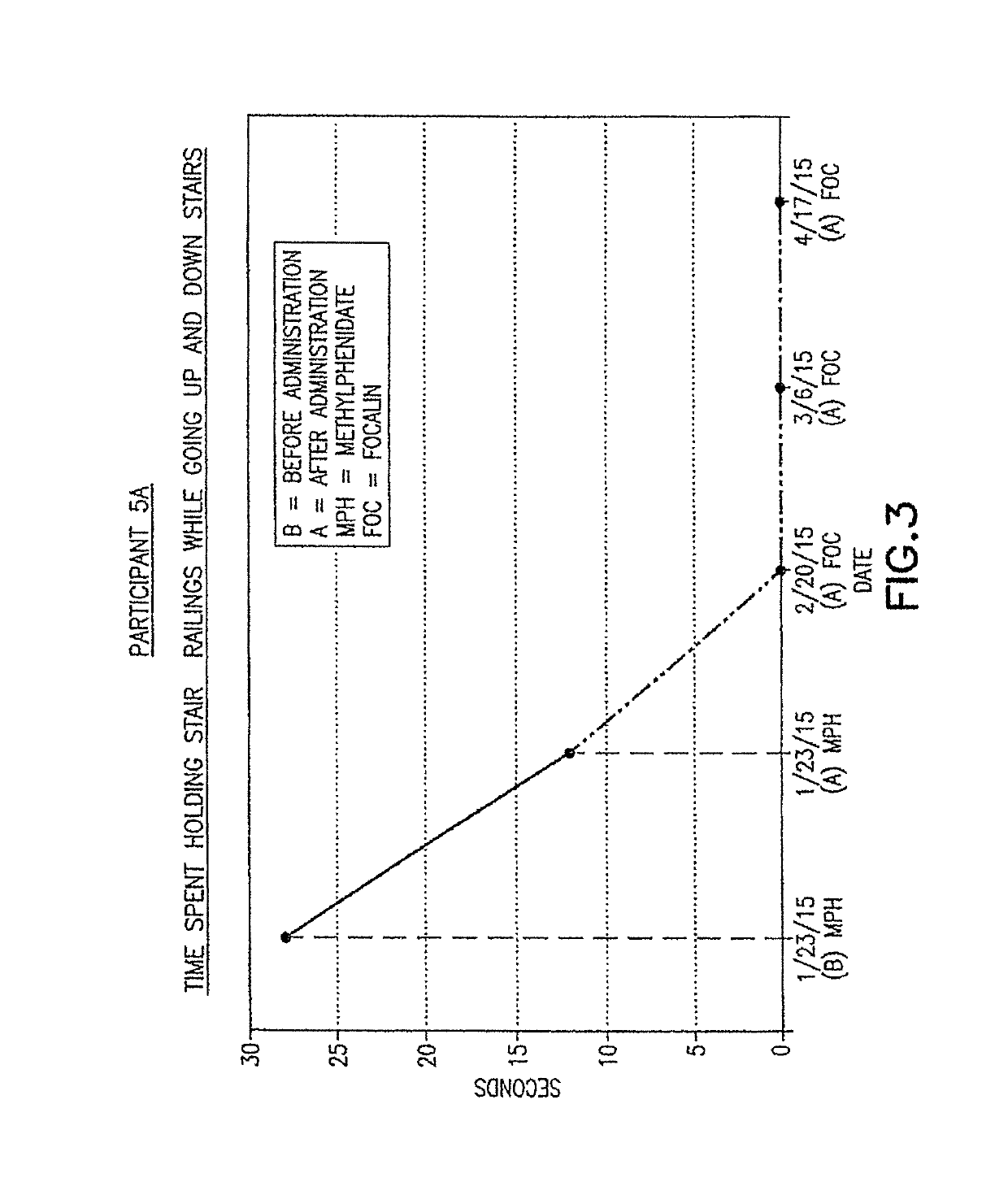
ActiveUS9682073B2Easy to controlConsequential diminishment in the speech, gait or limb impairmentOrganic active ingredientsDiagnostic recording/measuringDiseaseFragile X chromosome
A methylphenidate, particularly including dextro-threo-methylphenidate, is administered to a subject to treat a speech, gait or limb impairment secondary to a genetically acquired pre-frontal cortex processing disease or disorder, particularly including multiple sclerosis, cerebral palsy, Angelman syndrome, Rett syndrome and Fragile-X syndrome.
Owner:GILROSE PHARMA
Pre-frontal cortex processing disorder gait and limb impairments treatment
ActiveUS20160199366A1Easy to controlConsequential diminishment in the speech, gait or limb impairmentBiocideOrganic chemistryDiseaseFragile X chromosome
A methylphenidate, particularly including dextro-threo-methylphenidate, is administered to a subject to treat a speech, gait or limb impairment secondary to a genetically acquired pre-frontal cortex processing disease or disorder, particularly including multiple sclerosis, cerebral palsy, Angelman syndrome, Rett syndrome and Fragile-X syndrome.
Owner:GILROSE PHARMA
Neurodevelopmental disorder therapy
ActiveUS20190022052A1Organic active ingredientsNervous disorderSmith–Magenis syndromeCerebral palsied
This invention addresses tetrahydro-N,N-dimethyl-2,2-diphenyl-3-furanmethanamine hydrochloride (ANAVEX2-73, AV2-73, or A2-73) in a method of treatment for neurodevelopmental disorders. Particular reference is made to the treatment of autism spectrum disorder, cerebral palsy, Rett syndrome, Angelman syndrome, Williams syndrome, pervasive developmental disorder not otherwise specified (PDD-NOS), childhood disintegrative disorder, and Smith-Magenis syndrome. Additional reference is made to multiple sclerosis.
Owner:ANAVEX LIFE SCI CORP
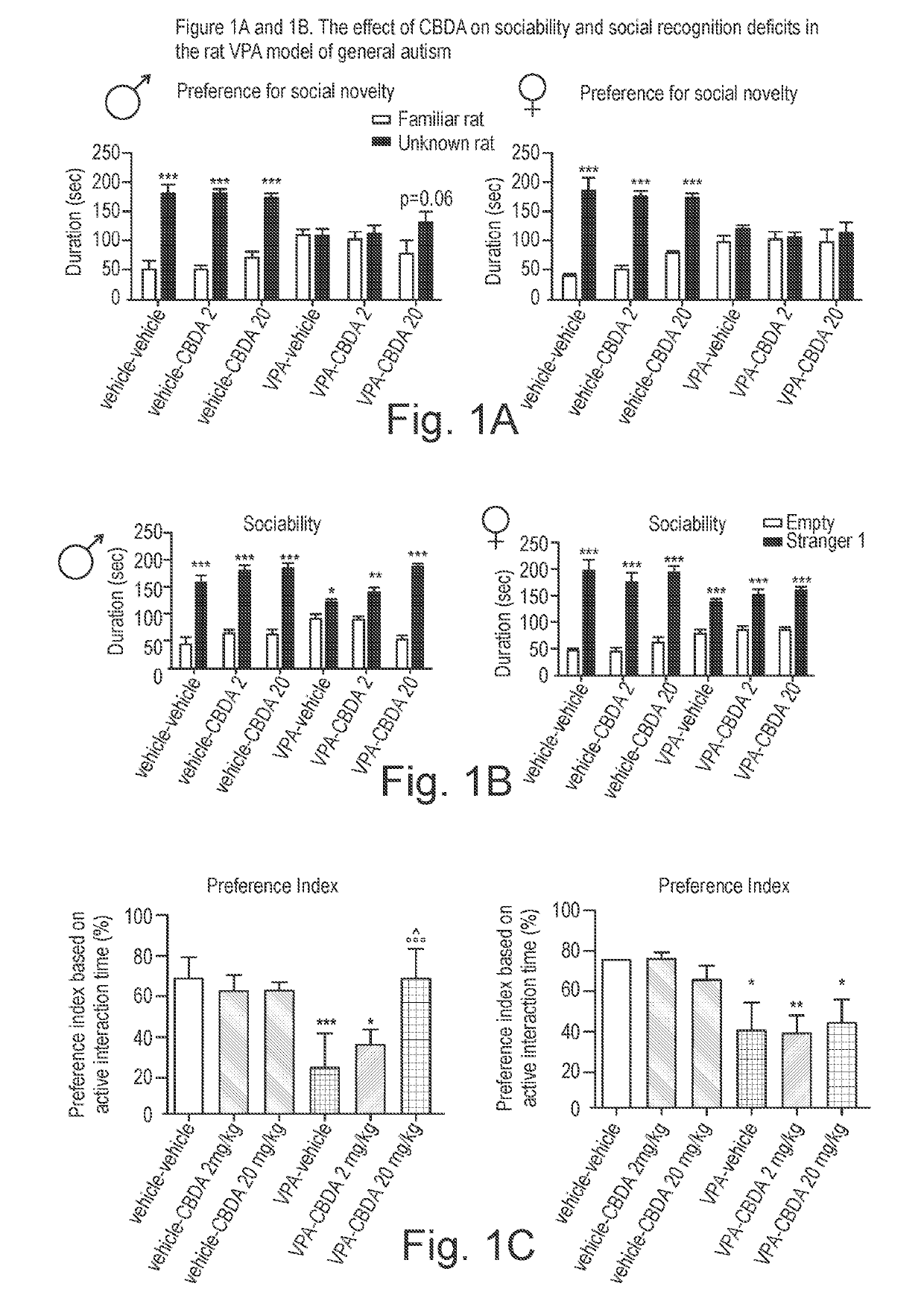
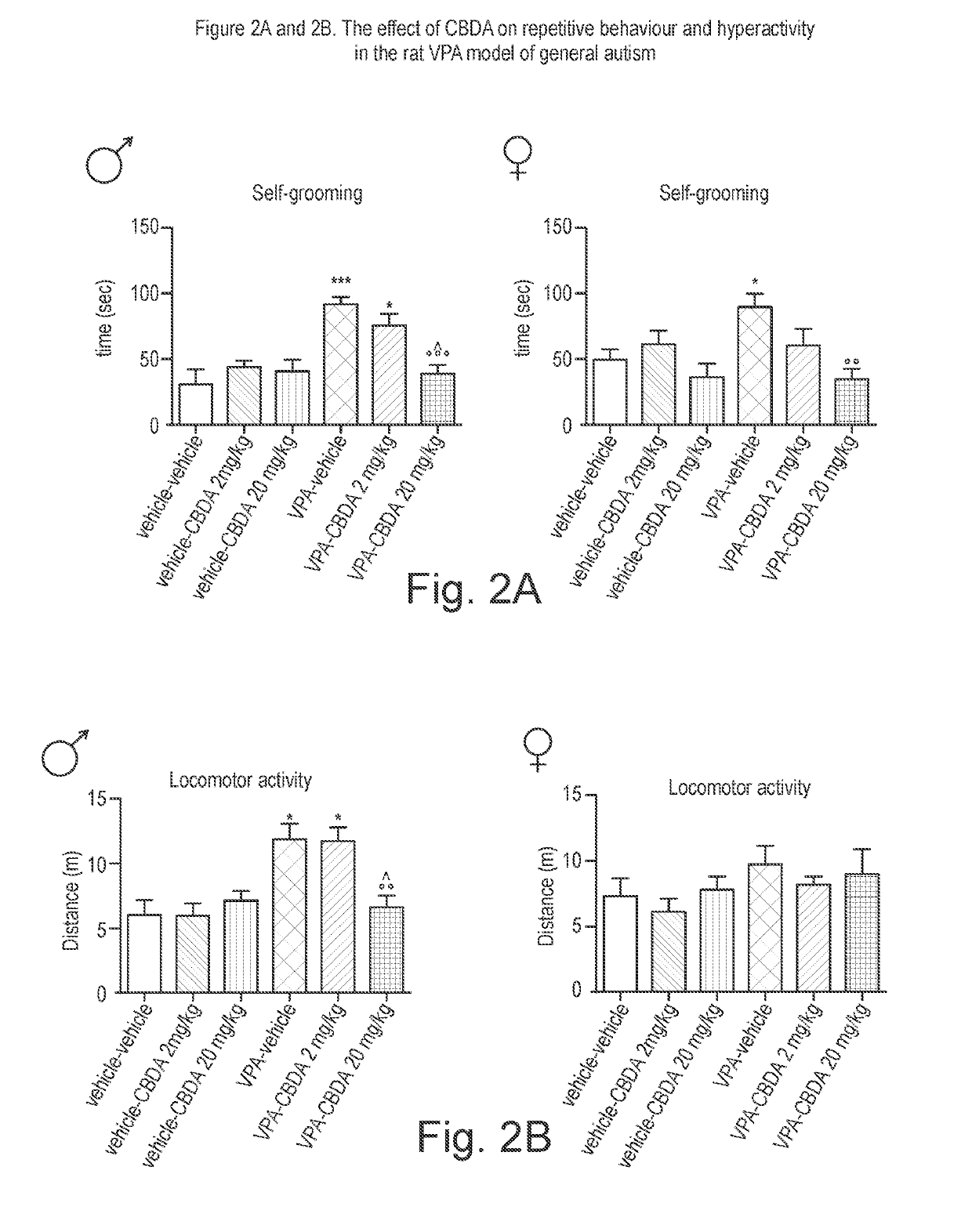
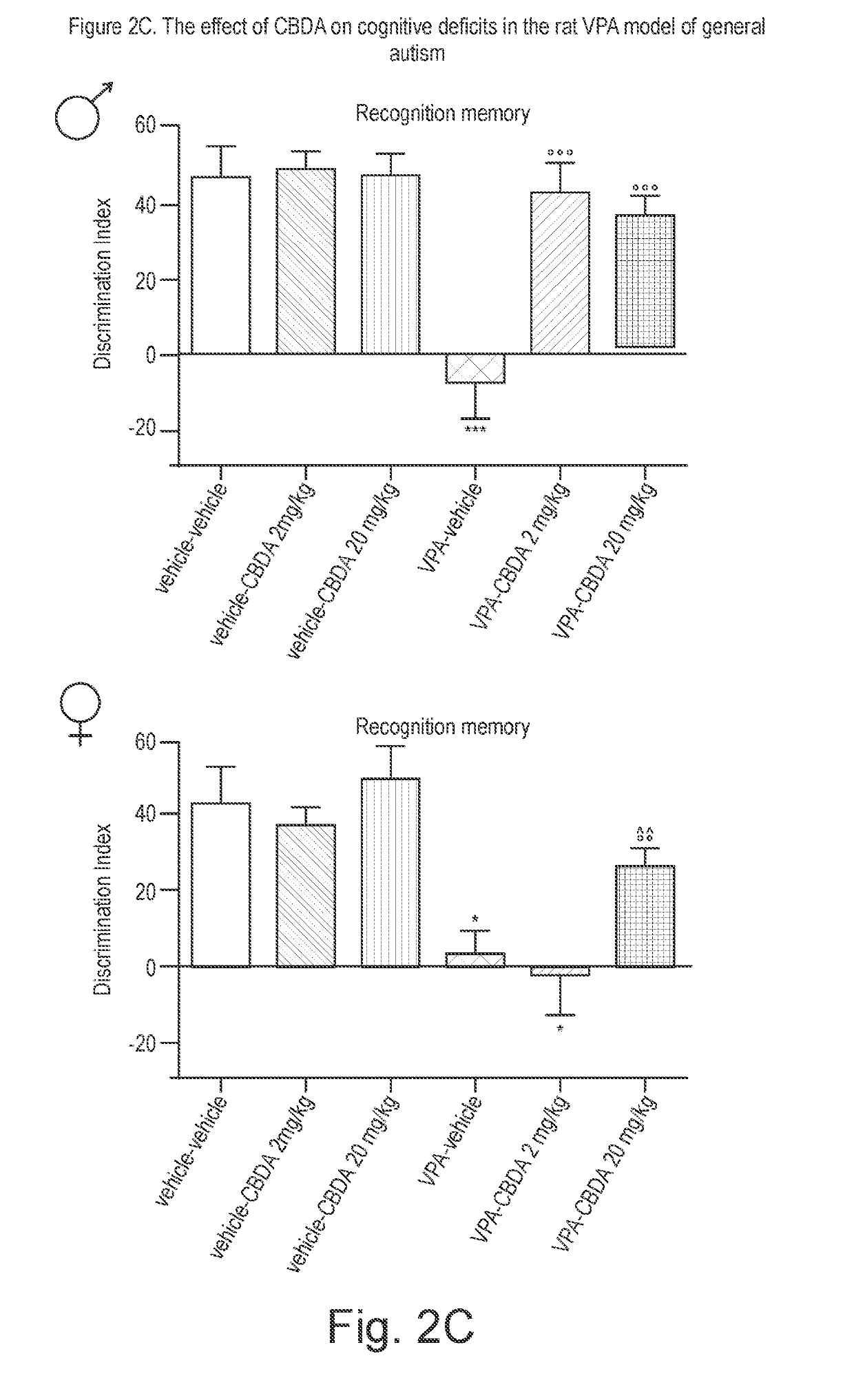
Use of cannabidiolic acid in the treatment of autism spectrum disorder and associated disorders
ActiveUS20190117619A1Symptoms improvedNervous disorderHydroxy compound active ingredientsDiseaseRodent model
The present invention relates to the use of cannabidiolic acid (CBDA) in the treatment of autism spectrum disorder (ASD) and ASD-associated disorders, such as Fragile X syndrome (FXS); Rett syndrome (RS); or Angelman syndrome (AS). CBDA has been shown to be particularly effective in improving cognitive dysfunction in rodent models of ASD, FXS, RS and AS. The CBDA is preferably substantially pure. It may take the form of a highly purified extract of cannabis such that the CBDA is present at greater than 95% of the total extract (w / w) and the other components of the extract are characterised. Alternatively, the CBDA is synthetically produced.
Owner:GW RES LTD
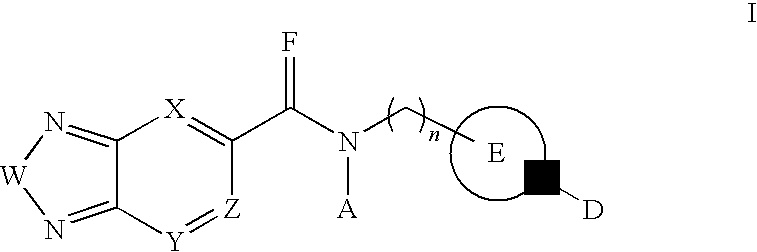
Use of aminoglycoside analogs in the treatment of rett syndrome
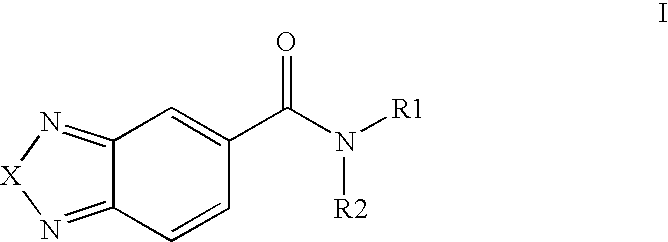
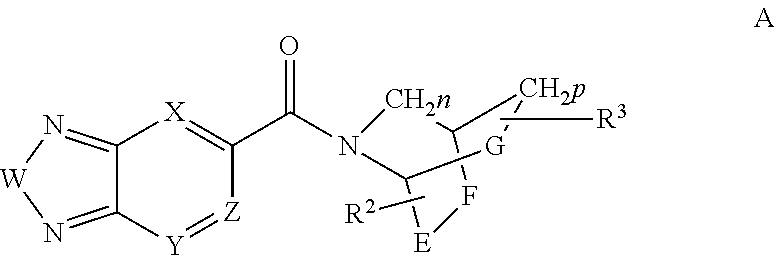
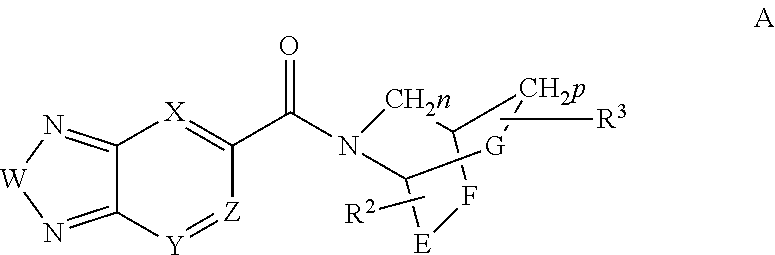
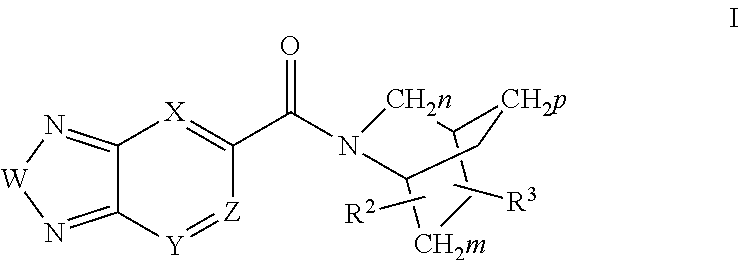
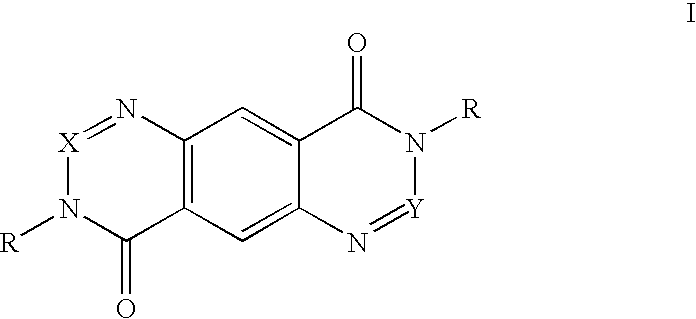
Compounds, methods and uses of pseudo-trisaccharide aminoglycosides represented by the general formula I:or a pharmaceutically acceptable salt thereof, wherein R1 is selected from the group consisting of alkyl, cycloalkyl and aryl; and all other variables and features are as described in the specification, in the treatment of Rett syndrome are disclosed.
Owner:ELOXX PHARM LTD
Cyclopropanamine compound and use thereof
ActiveUS20150291577A1Superior LSD inhibitory actionHigh LSD selectivityBiocideNervous disorderNoonan syndromeNodular sclerosis
The present invention provides a compound having a lysine-specific demethylase-1 inhibitory action, and useful as a medicament such as a prophylactic or therapeutic agent for schizophrenia, developmental disorders, particularly diseases having intellectual disability (e.g., autistic spectrum disorders, Rett syndrome, Down's syndrome, Kabuki syndrome, fragile X syndrome, Kleefstra syndrome, neurofibromatosis type 1, Noonan syndrome, tuberous sclerosis), neurodegenerative diseases (e.g., Alzheimer's disease, Parkinson's disease, spinocerebellar degeneration (e.g., dentatorubural pallidoluysian atrophy) and Huntington's disease), epilepsy (e.g., Dravet syndrome) or drug dependence, and the like. A compound represented by the formulawherein each symbol is as defined in the present specification, or a salt thereof.
Owner:TAKEDA PHARMA CO LTD
Bicyclic amide derivatives for enhancing glutamatergic synaptic responses
ActiveUS8119632B2Stable baselineIncrease amplitudeOrganic active ingredientsNervous disorderSIDS - Sudden infant death syndromeCentral sleep apnea
Owner:CORTEX PHARMA INC
Carbamoyl cyclohexane derivatives for treating autism spectrum disorder
The present invention relates to trans-N-[4-[2-[4-(2,3-dichlorophenyl)piperazin-1-yl]ethyl]cyclohexyl]-N′,N′-dimethylurea (cariprazine), its salts, close analogs, derivatives, pharmaceutical compositions, metabolites and combinations for use in the treatment of symptoms of autism spectrum disorder in general, and preferably the object of the present invention is to treat one or more symptoms of autism.Furthermore, it was also found that cariprazine, its salts, close analogs, derivatives, pharmaceutical compositions, metabolites and combinations are suitable for treatment of conditions such as Asperger's syndrome, atypical autism (otherwise known as pervasive developmental disorder not otherwise specified; PDD-NOS), Rett syndrome, childhood disintegrative disorder, attention deficit hyperactivity disorder (ADHD) and sensory integration dysfunction.
Owner:RICHTER GEDEON NYRT
Pre-frontal cortex processing disorder speech, gait and limb impairments treatment
ActiveUS10085414B2Easy to controlConsequential diminishment in the speech, gait or limb impairmentAnimal housingHeterocyclic compound active ingredientsExtremity injuryFragile X chromosome
A methylphenidate, particularly including dextro-threo-methylphenidate, is administered to a subject to treat a speech, gait or limb impairment secondary to a genetically acquired pre-frontal cortex processing disease or disorder, particularly including multiple sclerosis, cerebral palsy, Angelman syndrome, Rett syndrome and Fragile-X syndrome.
Owner:GILROSE PHARMA
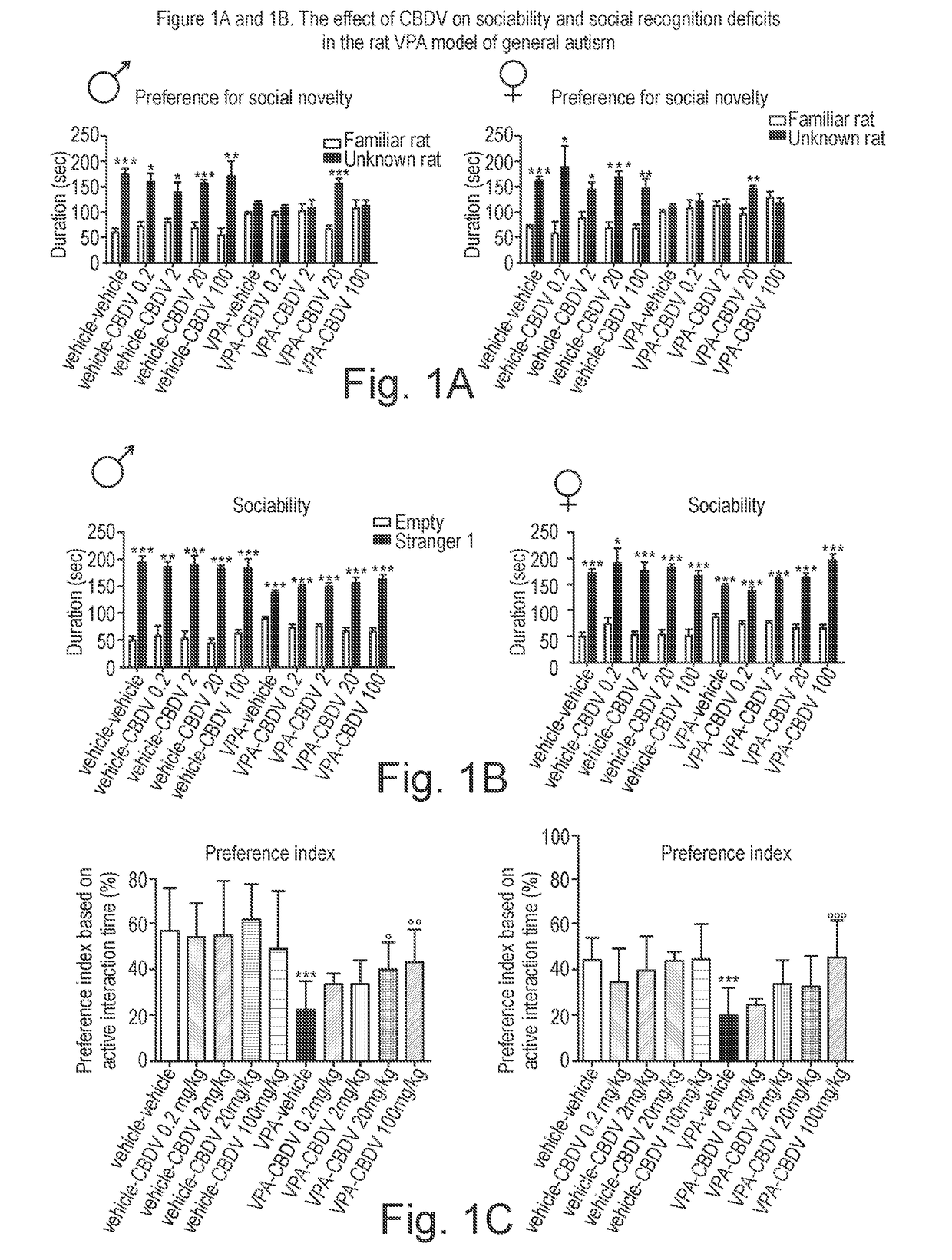
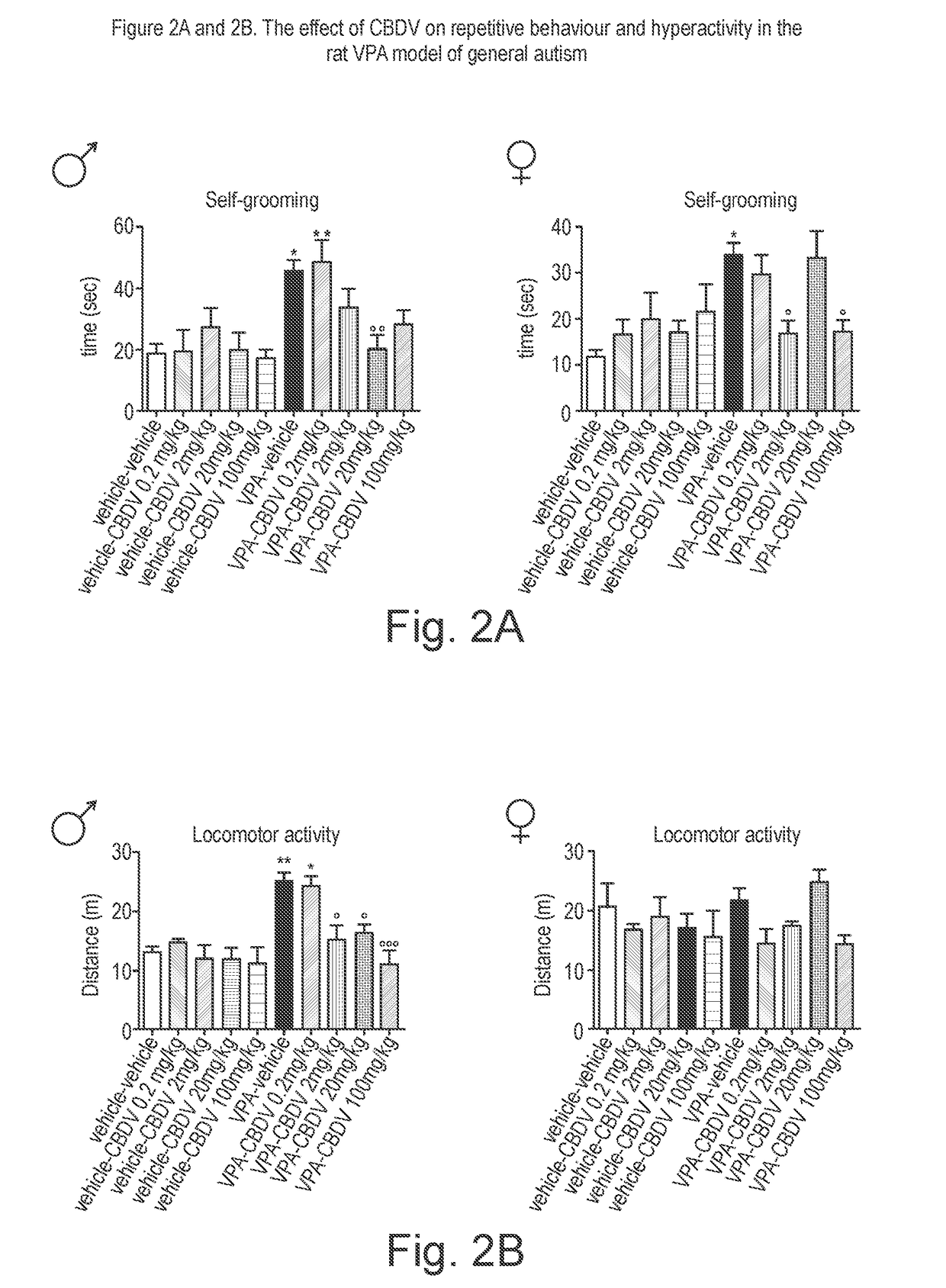
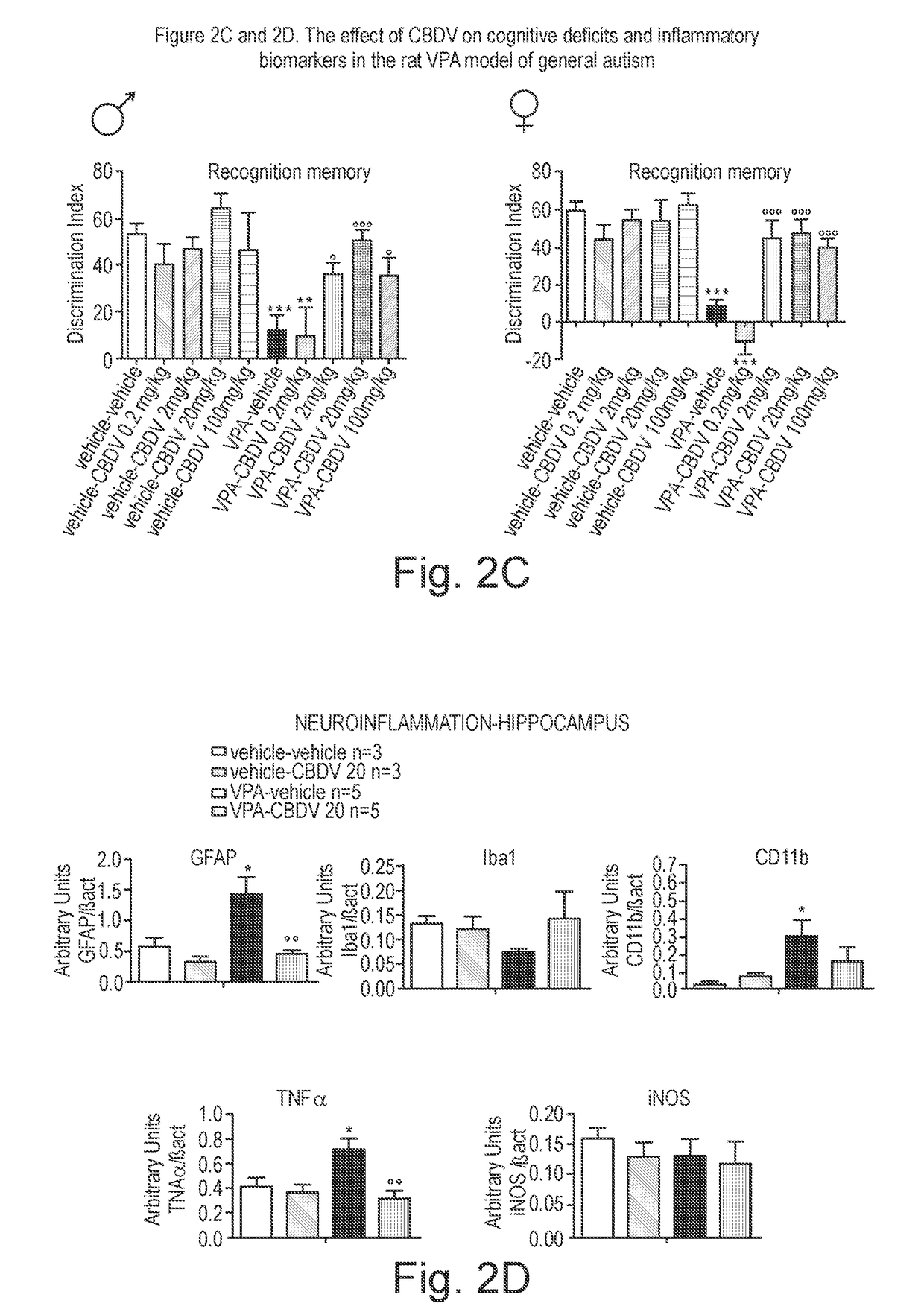
Use of cannabidivarin in the treatment of autism spectrum disorder, associated disorders and schizophrenia
InactiveUS20190070128A1Symptoms improvedNervous disorderHydroxy compound active ingredientsCannabisDisease
The present invention relates to the use of cannabidivarin (CBDV) in the treatment of autism spectrum disorder (ASD) and ASD-associated disorders such as Fragile X syndrome (FXS); Rett syndrome (RS); or Angelman syndrome (AS). In a further embodiment the invention relates to the use of CBDV in the treatment of schizophrenia. CBDV has been shown to be particularly effective in improving cognitive dysfunction in rodent models of ASD, FXS, RS, AS and schizophrenia. The CBDV is preferably substantially pure. It may take the form of a highly purified extract of cannabis such that the CBDV is present at greater than 95% of the total extract (w / w) and the other components of the extract are characterised. Alternatively, the CBDV is synthetically produced.
Owner:GW RES LTD
Rufinamide and derivatives and their use in modulating the gating process of human voltage-gated sodium channels
ActiveUS20150336904A1Slow recoveryImprove actionBiocideNervous disorderEpileptic encephalopathyNeurological disorder
The present invention provides compounds of formula I which are capable of inhibition of the activation of hNav1.1 or hNav1.6 sodium channels in neurons. Pharmaceutical compositions comprising these compounds are also provided. Methods for prevention and treatment of neurological disorders, including, for example, seizures and seizure disorders, including Lennox-Gastaut Syndrome, Dravet syndrome, epileptic encephalopathies, autism, Familial hemiplegic migraine (FHM), anxiety disorders, including Post-traumatic stress disorder (PTSD), panic disorder and obsessive-compulsive disorder, neuropathic pain, and Rett syndrome by administration of these compounds are also provided.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Pre-frontal cortex processing disorder speech, gait and limb impairments treatment
ActiveUS10420318B2Easy to controlConsequential diminishment in the speech, gait or limb impairmentAnimal housingHeterocyclic compound active ingredientsCerebral palsiedRett syndrome
Owner:GILROSE PHARMA
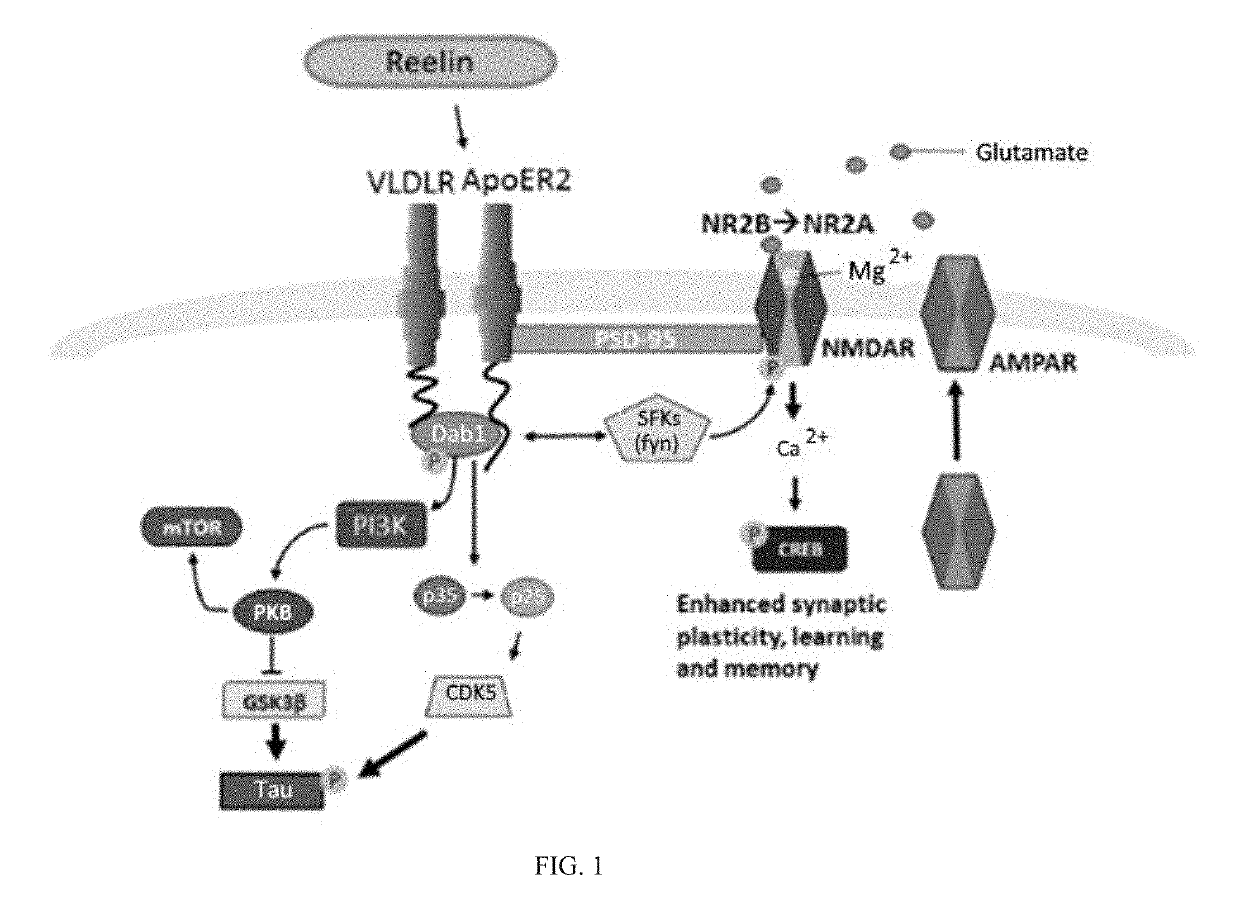
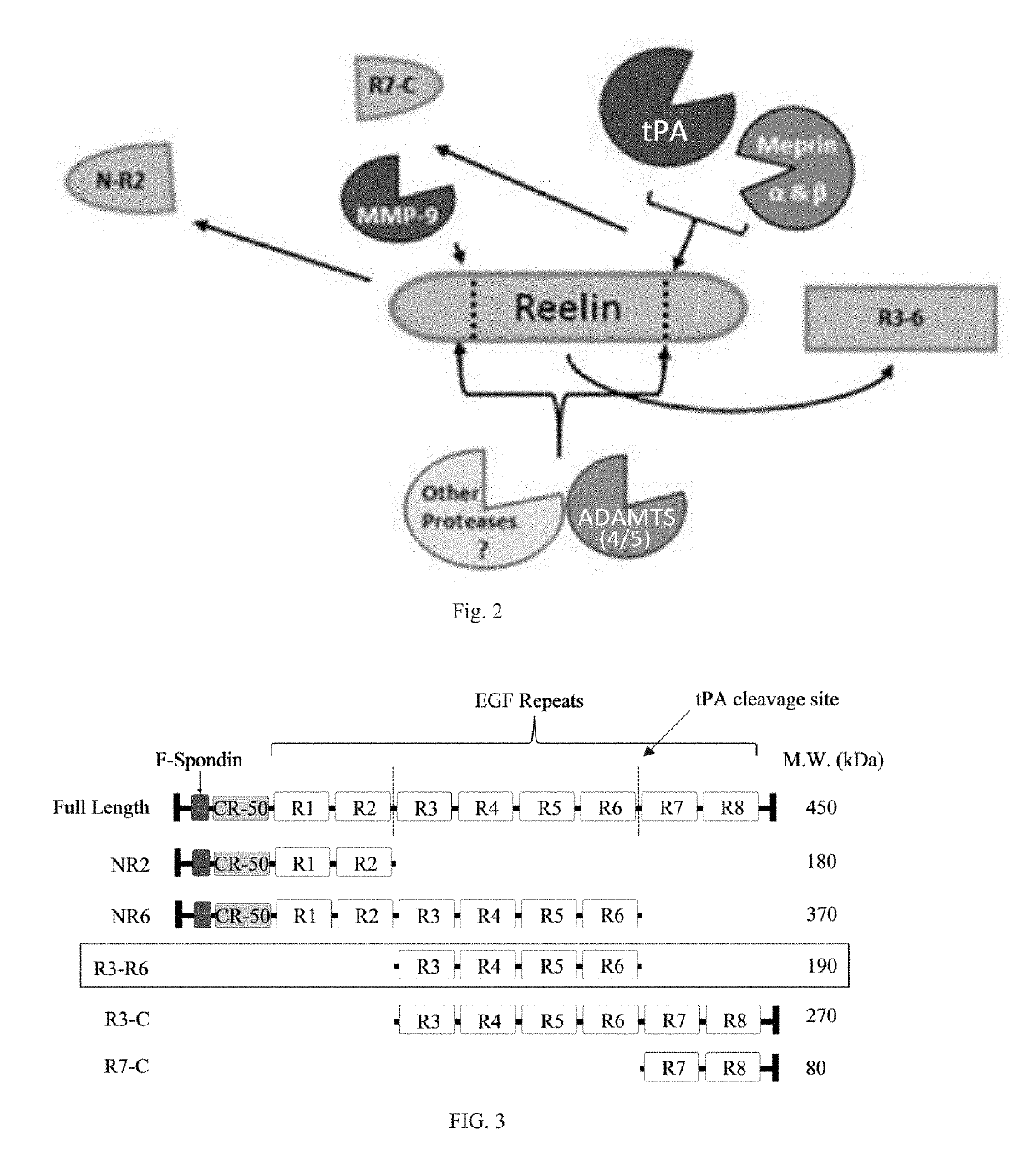
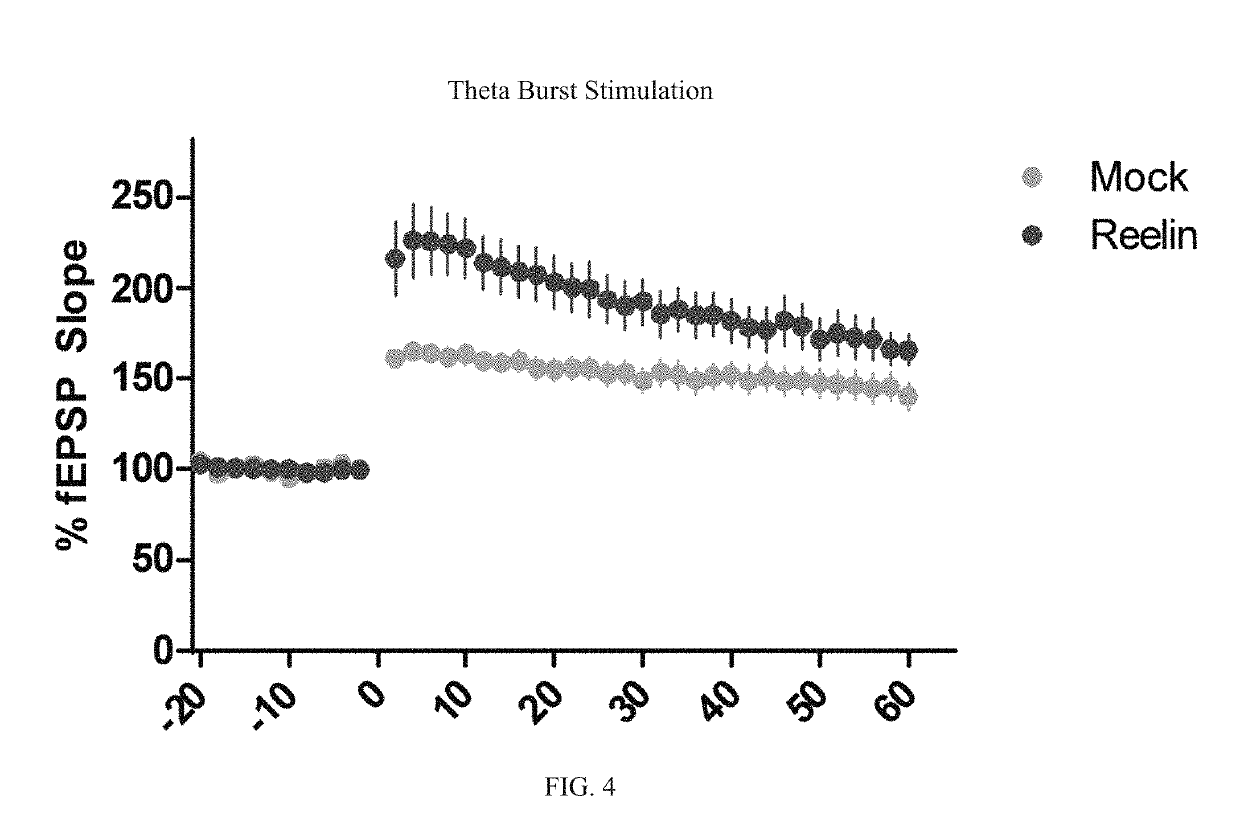
Reelin compositions for treatment of neurological disorders
PendingUS20190169246A1Improve plasticityImprove cognitive functionNervous disorderPeptide/protein ingredientsFragile X chromosomeReelin
Changes in Reelin levels as well as Reelin signaling alter cognitive function. This can be accomplished by administering a therapeutically effective amount of a repeat fragment of Reelin, or a construct formed from fragment repeats of Reelin to a patient or subject. Changes to Reelin levels can be used to treat various neurodegenerative diseases, neuronal insults, or stroke, such as fragile X syndrome, William's syndrome, Rett syndrome, Down's syndrome, Angelman syndrome, autism, ischemia, hypoxia, Alzheimer's disease, and schizophrenia. Reelin can also be used to alter dendritic spine density, diminished long-term potentiation, and diminished synaptic plasticity and associative learning deficits. Constructs formed from repeat region 3 of full length Reelin and repeat region 5 of full length Reelin, or repeat region 3 of full length Reelin and repeat region 6 of full length Reelin have been found particularly useful.
Owner:UNIV OF SOUTH FLORIDA
3-substituted-1,2,3-triazin-4-one's and 3 substituted 1,3-pyrimidinone's for enhancing glutamatergic synaptic responses
InactiveUS20100267728A1Increase synaptic responseIncreasing AMPA receptor functionBiocideNervous disorderSIDS - Sudden infant death syndromeCentral sleep apnea
This invention relates to compounds, pharmaceutical compositions and methods for use in the prevention and treatment of cerebral insufficiency, including enhancement of receptor functioning in synapses in brain networks responsible for basic and higher order behaviors. These brain networks, which are involved in regulation of breathing, and cognitive abilities related to memory impairment, such as is observed in a variety of dementias, in imbalances in neuronal activity between different brain regions, as is suggested in disorders such as Parkinson's disease, schizophrenia, respiratory depression, sleep apneas, attention deficit hyperactivity disorder and affective or mood disorders, and in disorders wherein a deficiency in neurotrophic factors is implicated, as well as in disorders of respiration such as overdose of an alcohol, an opiate, an opioid, a barbiturate, an anesthetic, or a nerve toxin, or where the respiratory depression results form a medical condition such as central sleep apnea, stroke-induced central sleep apnea, obstructive sleep apnea, congenital hypoventilation syndrome, obesity hypoventilation syndrome, sudden infant death syndrome, Rett syndrome, spinal cord injury, traumatic brain injury, Cheney-Stokes respiration, Ondines curse, Prader-Willi's syndrome and drowning. In a particular aspect, the invention relates to compounds useful for treatment of such conditions, and methods of using these compounds for such treatment.
Owner:CORTEX PHARMA
Compositions and methods for reducing tactile dysfunction, anxiety, and social impairment
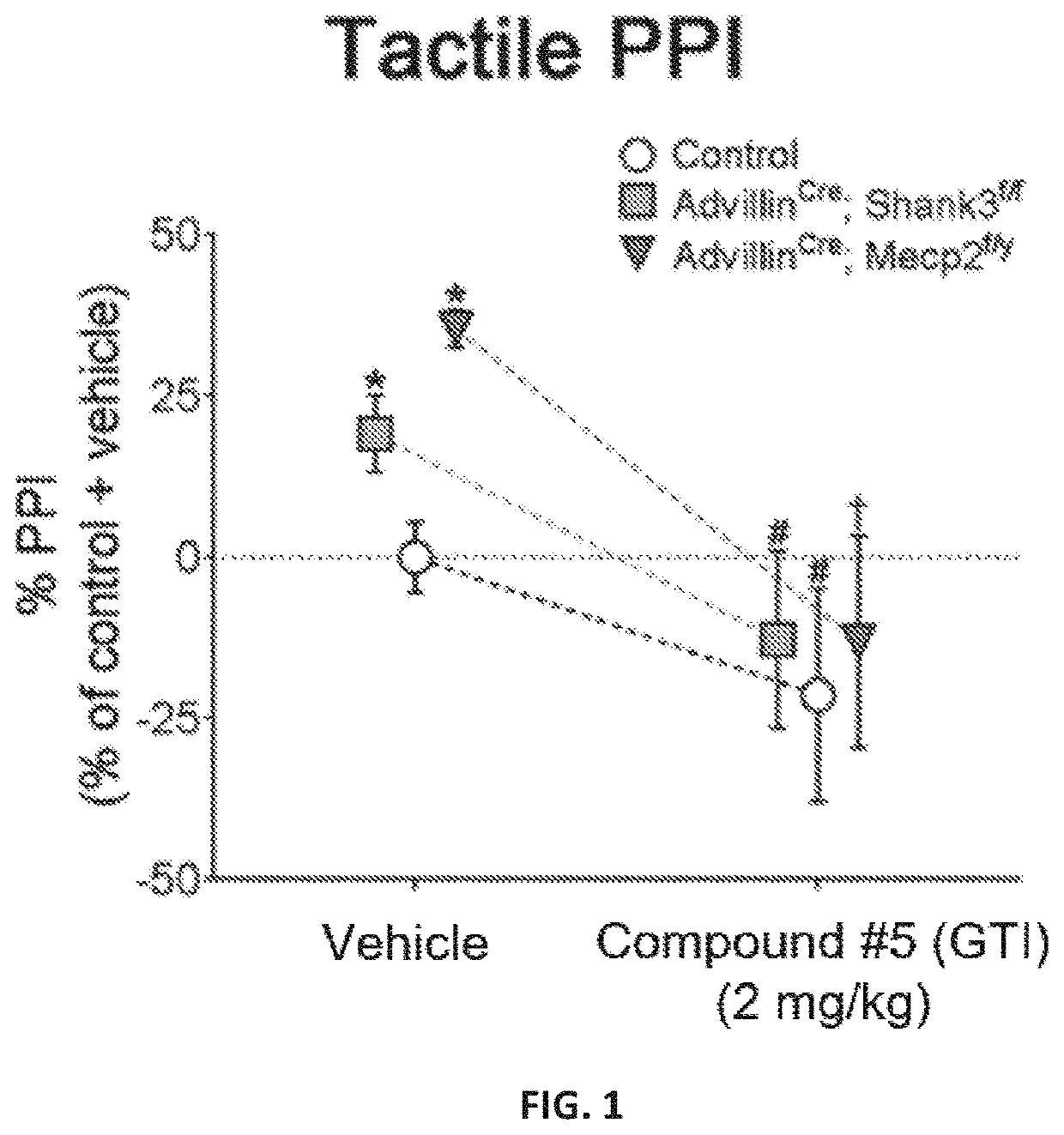
PendingUS20210206714A1Reducing tactile dysfunctionReduced dysfunctionNervous disorderOrganic chemistryRett syndromePhelan-McDermid syndrome
The present invention features novel peripherally-restricted isoguvacine analogs with reduced blood brain barrier permeability and methods of use thereof for reducing tactile dysfunction, social impairment, and anxiety in a subject diagnosed with Autism Spectrum Disorder, Rett syndrome, Phelan McDermid syndrome, or Fragile X syndrome.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
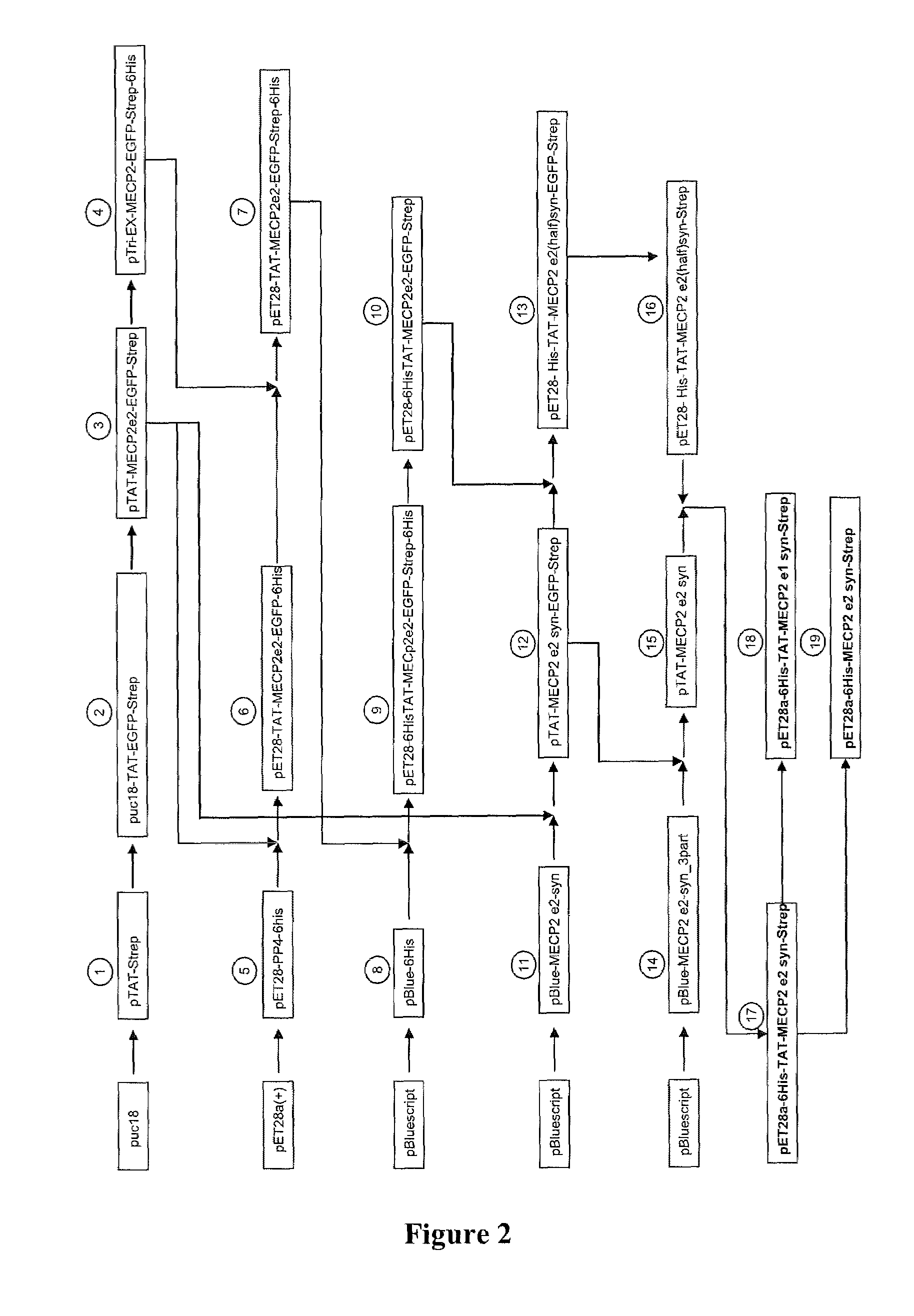
Synthetic mecp2 sequence for protein substitution therapy
ActiveUS20090233856A1Improve purification effectHigh transduction efficiencyNervous disorderPeptide/protein ingredientsDiseaseNucleic acid sequencing
The invention relates to the MeCP2 protein and its use in protein substitution therapy. More specifically, the invention relates to condon-optimized nucleic acid sequences for the expression of MeCP2 proteins, methods for creating such a nucleic acid sequence and expressing such a protein, fusions of a protein of the invention to a transduction domain, and vectors and host cells comprising a protein of the invention. Further, the invention relates to uses of nucleic acids or proteins of the invention in medicine, pharmaceutical compositions comprising nucleic acid sequences and proteins of the invention, as well as methods for the treatment, prevention, and / or therapy of neurodegenerative or neurodevelopmental diseases including Rett syndrome.
Owner:GEORG AUGUST UNIVERSITAT GOTTINGEN STIFTUNG OFFENLICHEN RECHTS +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com