Use of aminoglycoside analogs in the treatment of rett syndrome
a technology of aminoglycosides and rett syndrome, applied in the field of therapy, can solve the problems of reducing the suppression efficiency at subtoxic doses, compromising the clinical applicability of aminoglycosides of the gentamicin family,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
MECP2 Readthrough in Rett Human R294X Cells
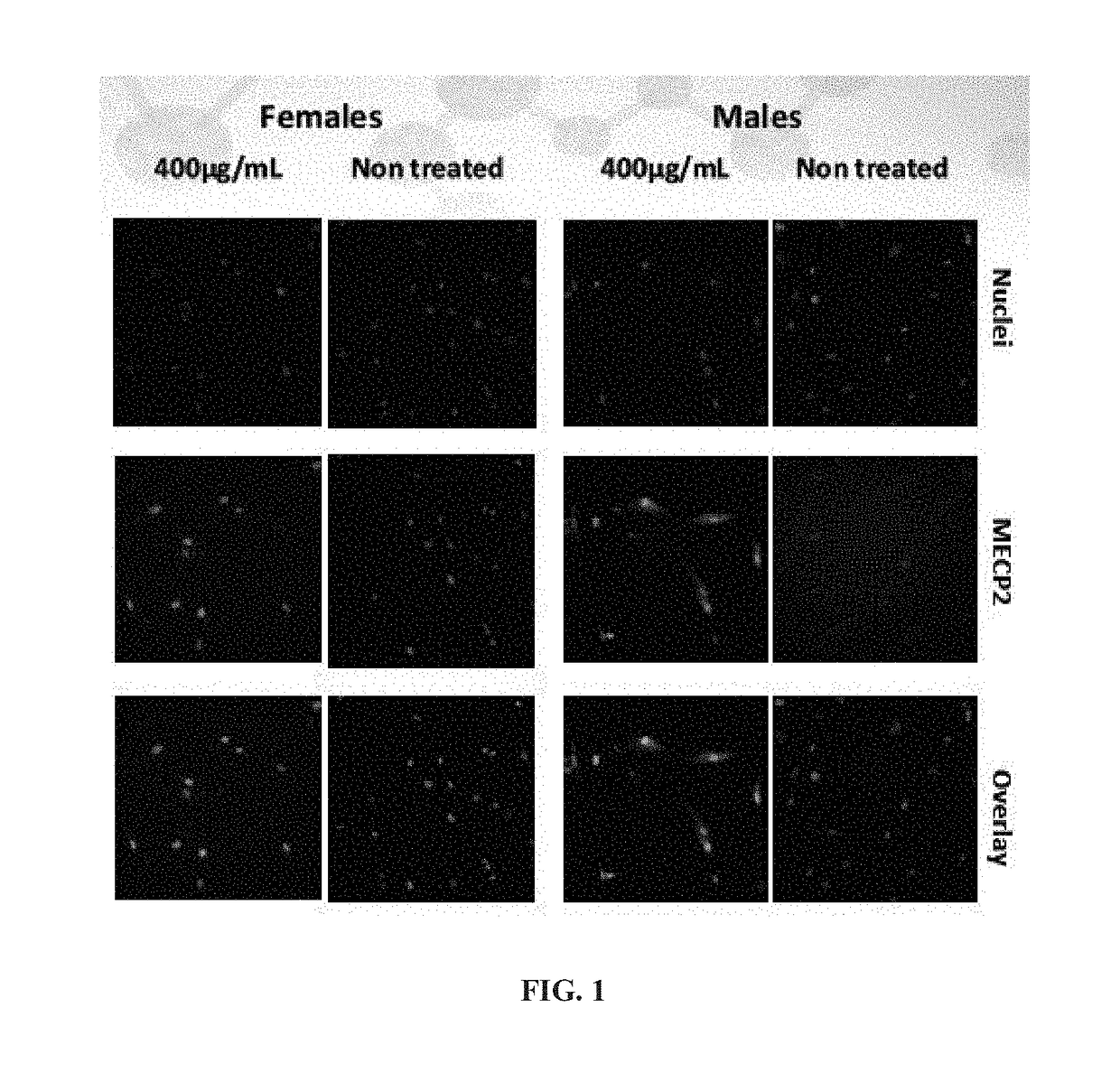
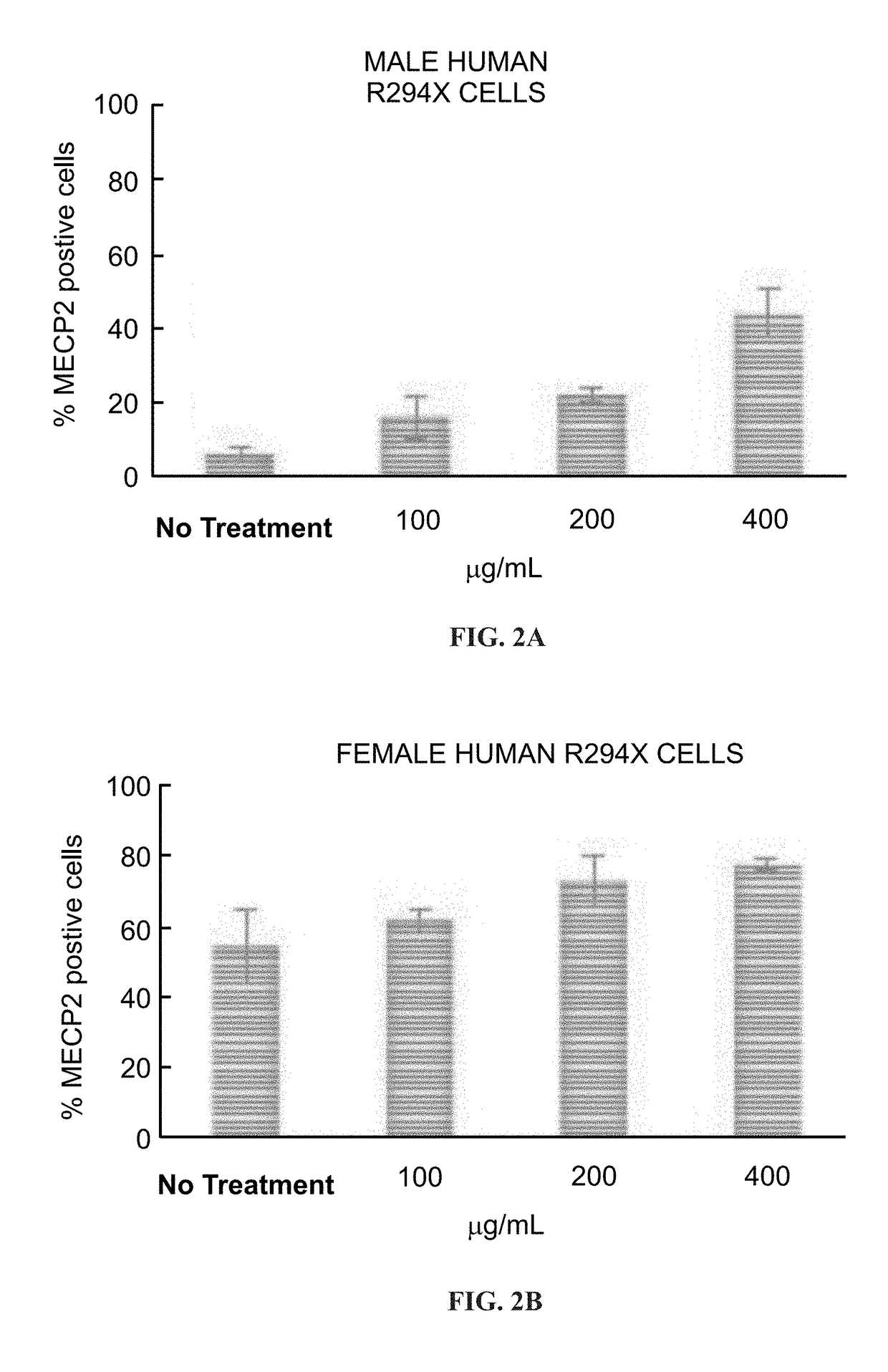
[0198]Fibroblasts derived from Human R294X Rett syndrome male and female patients were plated in a 384 black clear bottom plate at 750 cells / well. Cells were treated with 100, 200 and 400 μg / mL of NB124 for 3 days. Localization of endogenous MeCP2 protein was visualized by using a specific MeCP2 antibody detecting the C-terminal part of the protein and immunofluorescent microscopy.
[0199]FIG. 1 presents high content screening automated microscope pictures obtained for cells treated with 400 μg / mL of NB124 compared with non-treated cells, and show MECP2 nuclei localization and translation in all tested samples, and enhanced MECP2 translation in the treated samples.
[0200]The localization of MECP2 in the nuclai of the cell indicates that the protein retained is functionality and was able to return from the plasma to the nuclai.
[0201]The number of MECP2 positive cells was calculated using an automate algorithm and the obtained data is presented ...
example 2
In Vitro Studies of Readthrough Efficacy
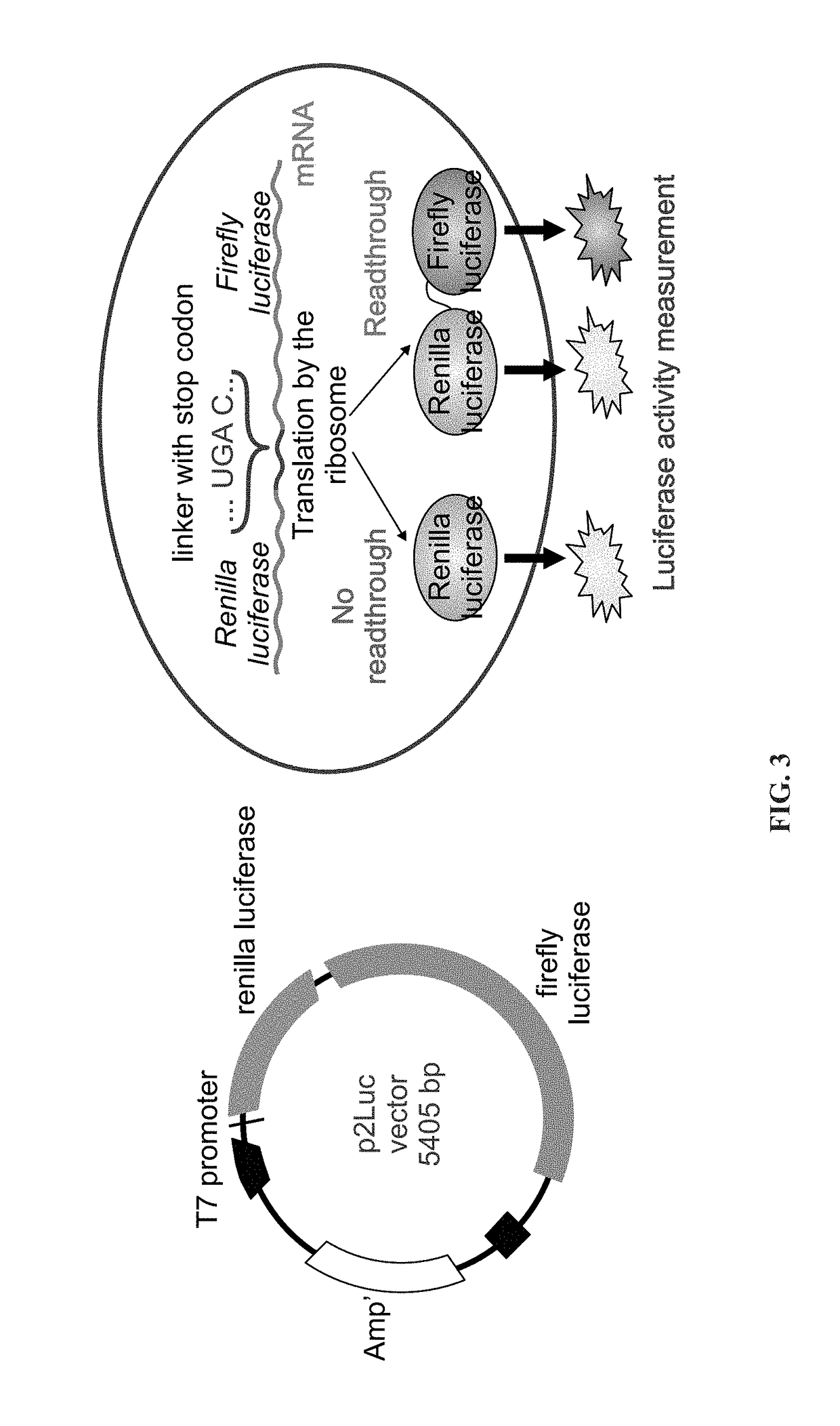
[0202]Readthrough activity was assayed using TNT reticulocyte lysate (i-vitro),and the nucleic acid constructs: Rett R168X, R270X & R294X for the nonsense mutations tested, as depicted in FIG. 3.
[0203]The obtained plasmids in the presence of 0-50 μM of the tested aminoglycoside transcribed and translated using the TNT reticulocyte lysate quick-coupled transcription / translation system. Luciferase activity was determined 90 minutes post incubation at 30° C., using Dual luciferase reporter assay system, and read-through was calculated as shown in the follow equation:
%Readthrough=Luminescence(FFmut) / Luminescence(Renillamut)Luminescence(FFwt) / Luminescence(Renillawt)
[0204]The obtained data is presented in Table 1 below.
TABLE 1Translational InhibitionIC50 [μm]2,3(TI) [μm]2,3TI / IC503Compound1R168XR270XR294XR168XR270XR294XR168XR270XR294XRT %4NB1220.400.160.224.103.755.021023235.3%NB1231.661.391.7522.2314.4860.841310353.8%NB1240.880.430.766.675.325.6581...
example 3
Ex-Vivo Readthrough Efficacy and Toxicity Assays
[0207]Nucleic acid constructs harboring Rett R168X, R270X, and R294X mutations were inserted into HEK-293 (human embryonic kidney) cells using Calcium Phosphate method. Six hours post-transfection, the tested aminoglycosides, at a concentration of 0.3 or 1 mM were added. The cells were harvested following 16 hours incubation with the tested aminoglycoside using passive lysis buffer. Readthrough activity was calculated as described hereinabove (see, Example 2).
[0208]For the cytotoxicity assays, HEK-293 cells were grown in 96-well plates, the tested synthetic aminoglycosides, at various concentrations, were thereafter added (10 μL, per well), and the cells were incubated for additional 24 hours. A cell proliferation assay (wst-1 based calorimetric assay), was performed by using 3-hour incubation. Optical density was measured using an ELISA plate reader.
[0209]The obtained data are presented in Table 3 (readthrough activity) and Table 4 (t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com