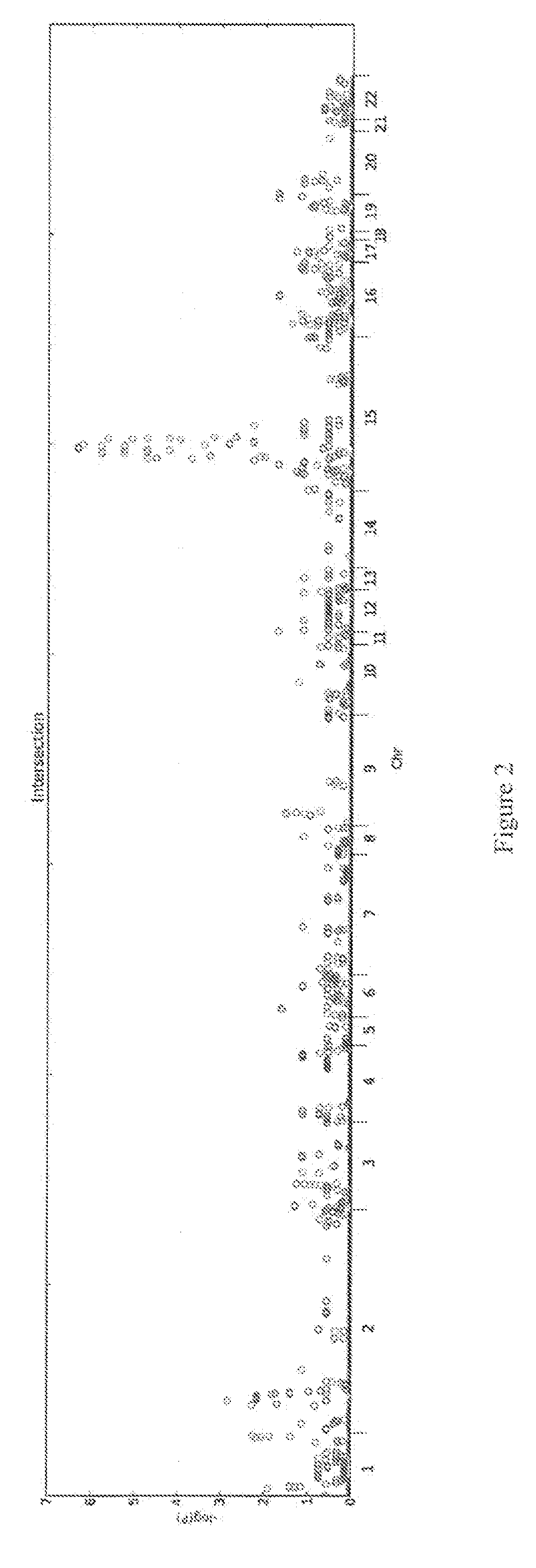
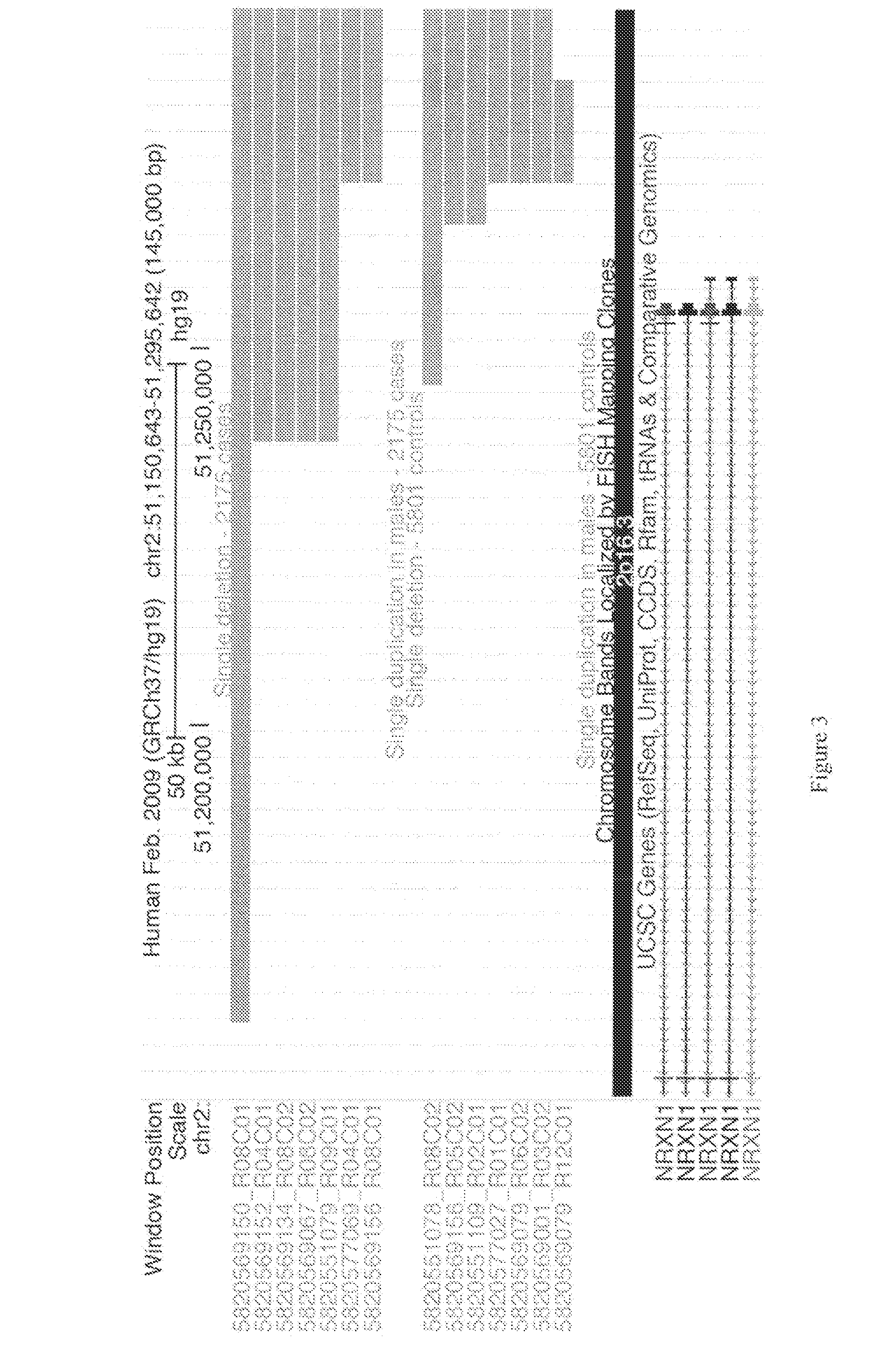
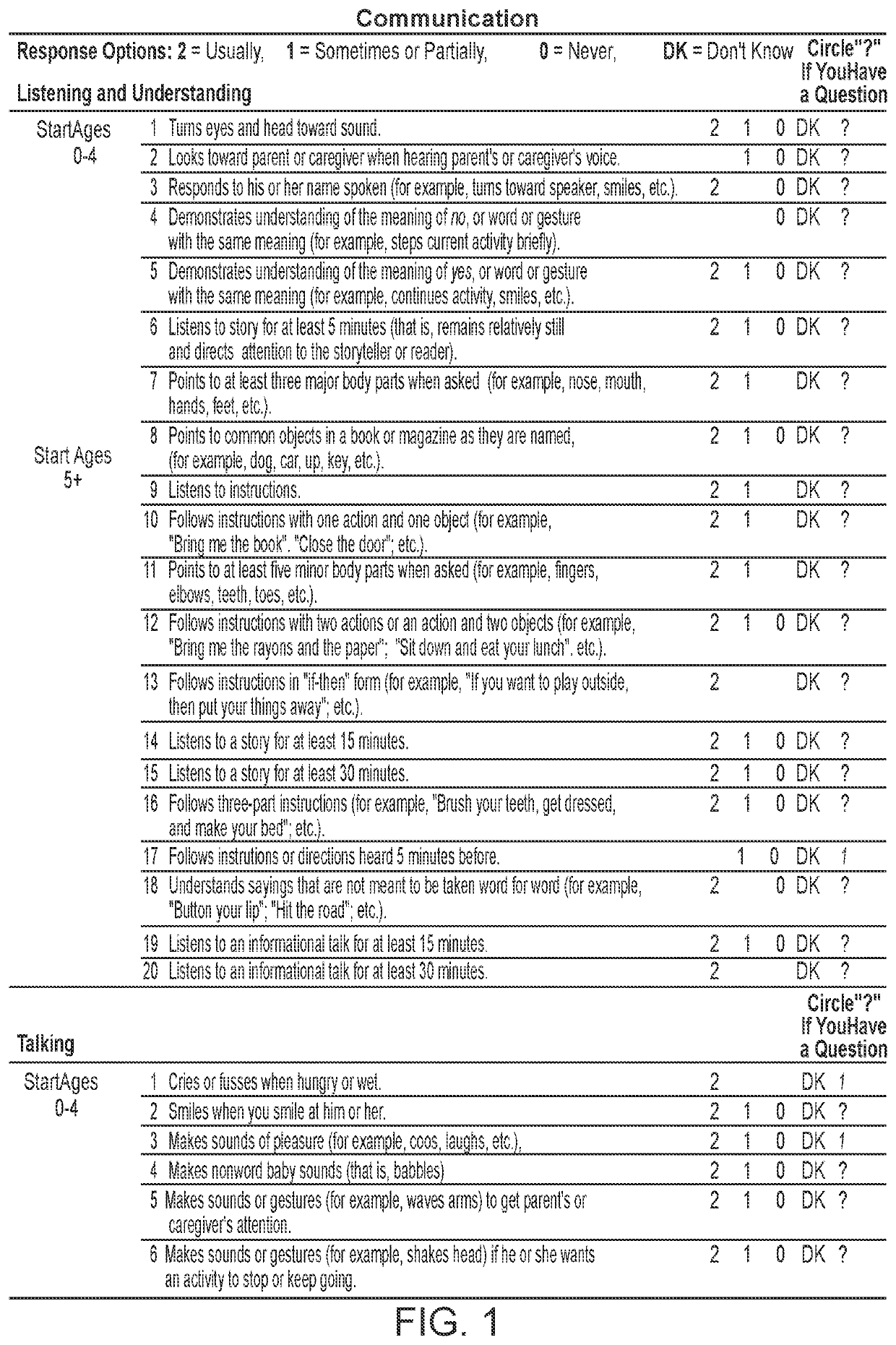
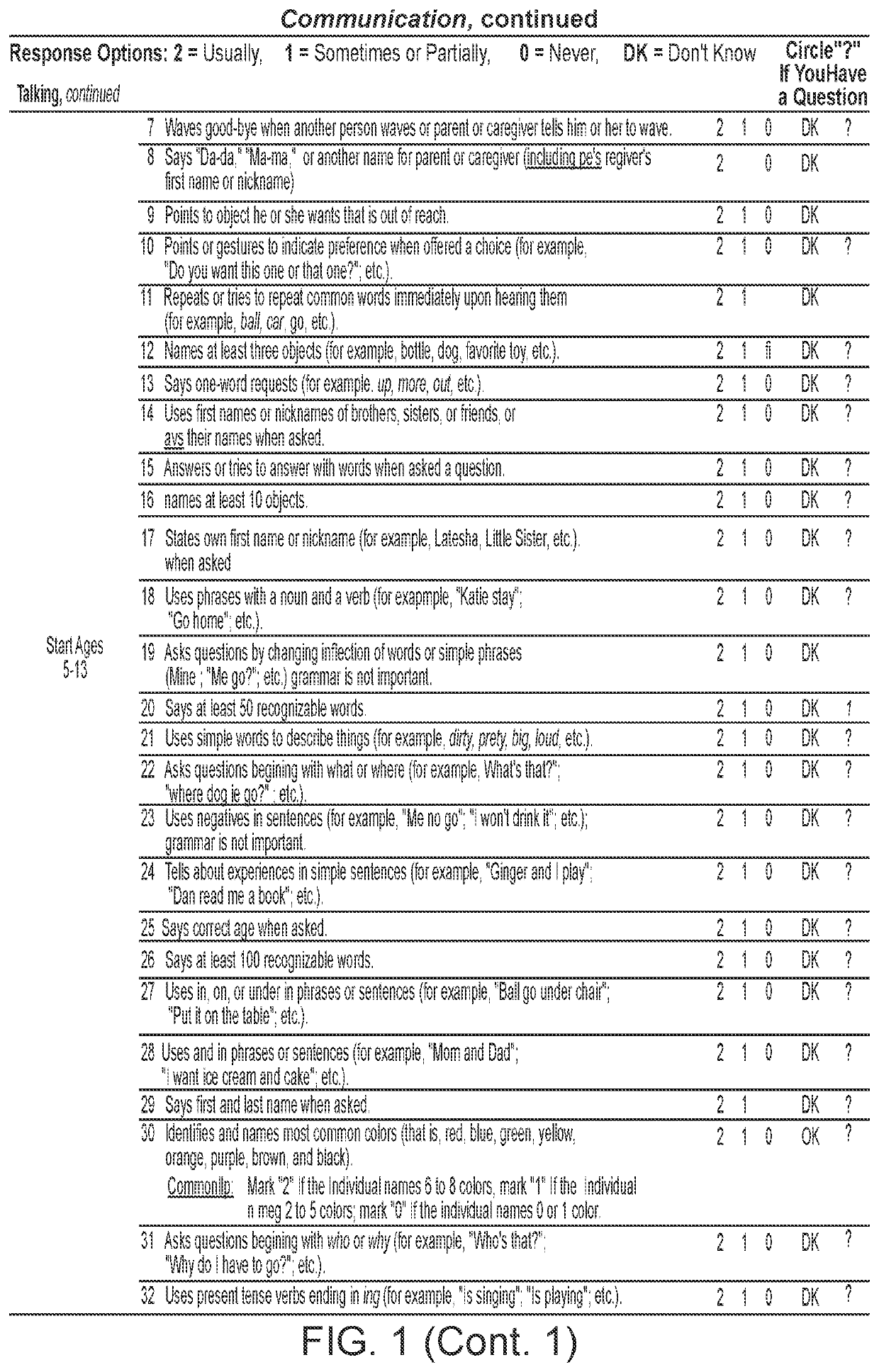
Patents
Literature
162 results about "Donohue's syndrome" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Combination therapy for effecting weight loss and treating obesity
InactiveUS7056890B2Efficient and effective treatmentReduce effectBiocideCarbohydrate active ingredientsSYMPATHOMIMETIC AGENTSDrug
The present invention features a novel therapy for effecting weight loss which involves treating a subject with a sympathomimetic agent (e.g., phentermine or a phentermine-like drug) in combination with an anticonvulsant sulfamate derivative (e.g., topiramate) such that the subject experiences weight loss.The combination methods of the present invention also are effective against symptoms associated with Syndrome X. The invention also features pharmaceutical compositions and kits for use in the practice of these novel therapies.
Owner:VIVUS
Stable amorphous ambroxol hydrochloride compound
The invention belongs to the technical field of medicine, and particularly relates to an amorphous ambroxol hydrochloride compound and a preparation method thereof. The amorphous ambroxol hydrochloride compound has high purity and stability, does not have obvious moisture absorption and weight increase even under the condition of high humidity, ensures that relative substances do not grow, and has higher dissolution rate than crystalline ambroxol hydrochloride. The invention also relates to an application of the amorphous ambroxol hydrochloride compound in preparation of medicine for the treatment of acute and chronic pulmonary diseases with the symptom of ropy sputum and difficult expectoration, phlegm eliminating treatment of acute exacerbation of chronic bronchitis, asthmatic bronchitis and bronchial asthma, prophylactic treatment of lung complication after an operation, and treatment of infant respiratory distress syndrome (IRDS) of premature infants and neonates.
Owner:天津梅花生物医药科技有限公司
Methods for diagnosing irritable bowel syndrome
InactiveUS20080166719A1Accurate diagnostic predictionImprove forecast accuracyDigital data processing detailsMicrobiological testing/measurementSAPS IIStatistical algorithm
The present invention provides methods, systems, and code for accurately classifying whether a sample from an individual is associated with irritable bowel syndrome (IBS). In particular, the present invention is useful for classifying a sample from an individual as an IBS sample using a statistical algorithm and / or empirical data. The present invention is also useful for ruling out one or more diseases or disorders that present with IBS-like symptoms and ruling in IBS using a combination of statistical algorithms and / or empirical data. Thus, the present invention provides an accurate diagnostic prediction of IBS and prognostic information useful for guiding treatment decisions.
Owner:PROMETHEUS BIOSCI INC
Methods for diagnosing irritable bowel syndrome
InactiveUS20100094560A1Accurate classificationAccurate predictionMicrobiological testing/measurementDigital computer detailsStatistical algorithmDisease cause
The present invention provides methods, systems, and code for accurately classifying whether a sample from an individual is associated with irritable bowel syndrome (IBS). In particular, the present invention is useful for classifying a sample from an individual as an IBS sample using a statistical algorithm and / or empirical data. The present invention is also useful for ruling out one or more diseases or disorders that present with IBS-like symptoms and ruling in IBS using a combination of statistical algorithms and / or empirical data. Thus, the present invention provides an accurate diagnostic prediction of IBS and prognostic information useful for guiding treatment decisions.
Owner:NESTEC SA
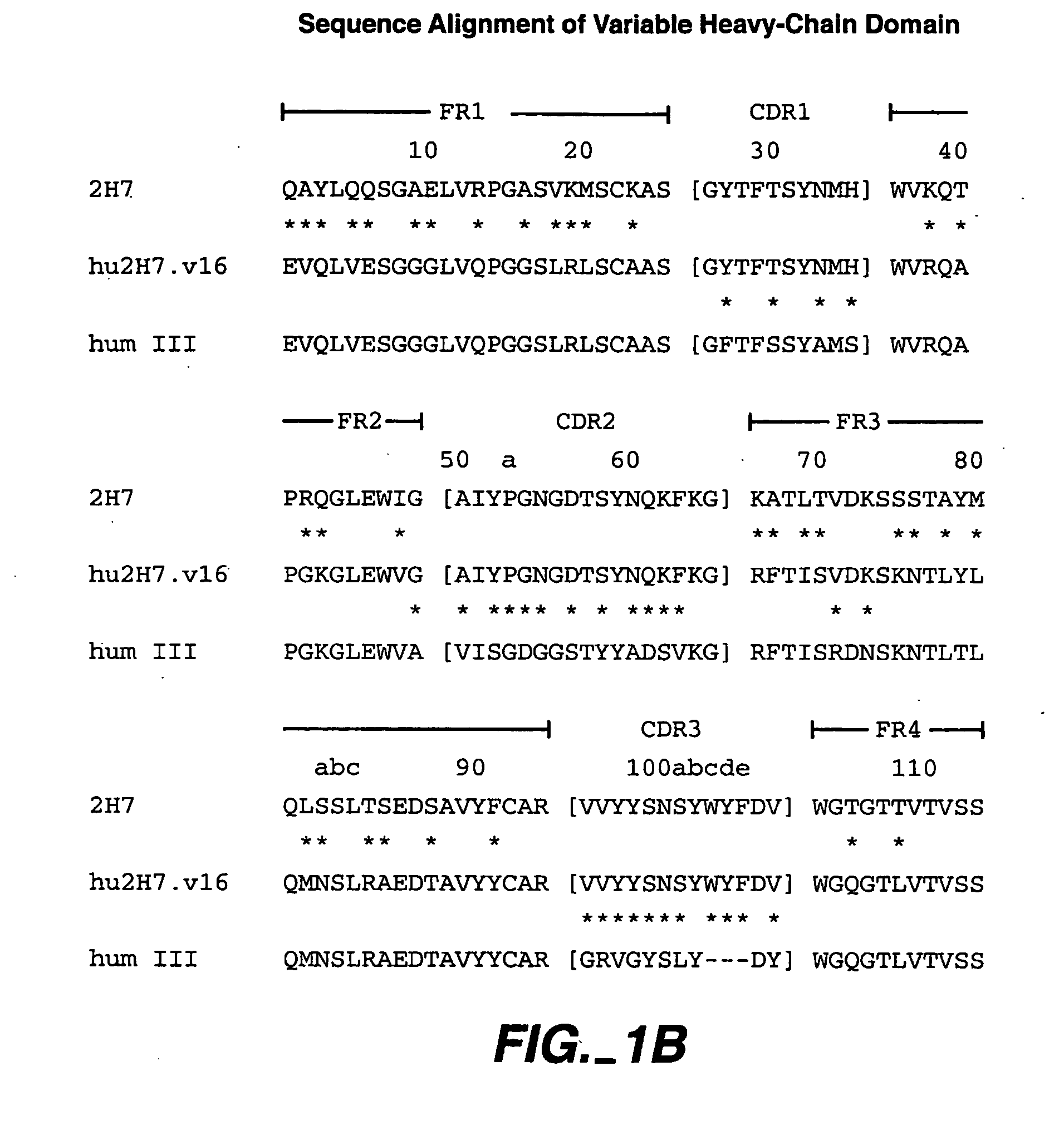
Method for treating Sjogren's syndrome
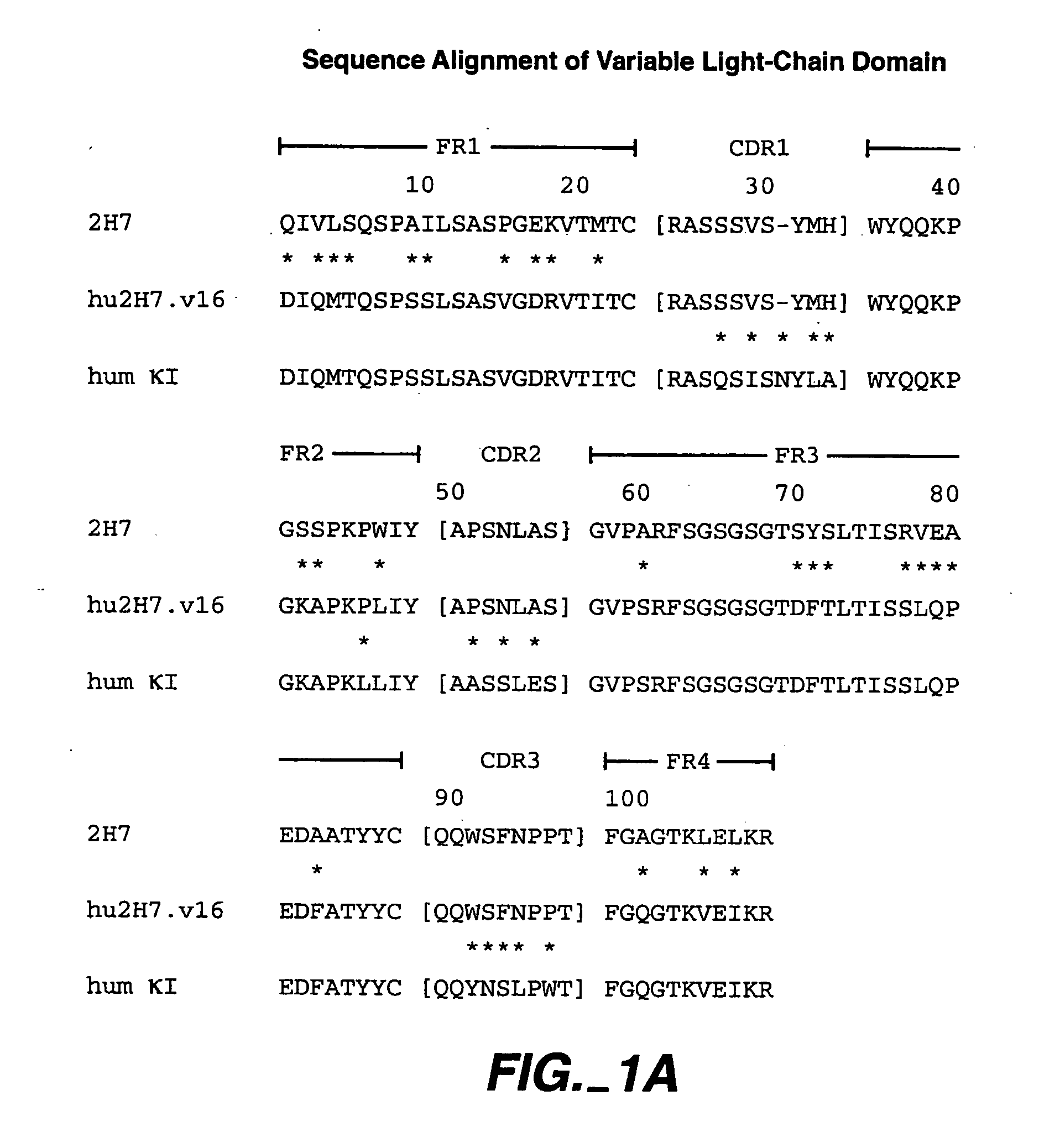
A method of treating Sjögren's syndrome in a patient eligible for treatment is provided involving administering an effective amount of an antagonist that binds to a B-cell surface marker to the patient to provide significant improvement over baseline in two or more of dryness, fatigue, and joint pain on a Visual Analogue Scale, and an article of manufacture therefor. Methods and articles are also provided involving treating Sjögren's syndrome in a subject eligible for treatment is provided involving administering an effective amount of an antibody that binds to a B-cell surface marker to the subject to provide an initial exposure and a subsequent exposure to the antibody within certain dosing regimens and an article of manufacture therefor.
Owner:GENENTECH INC
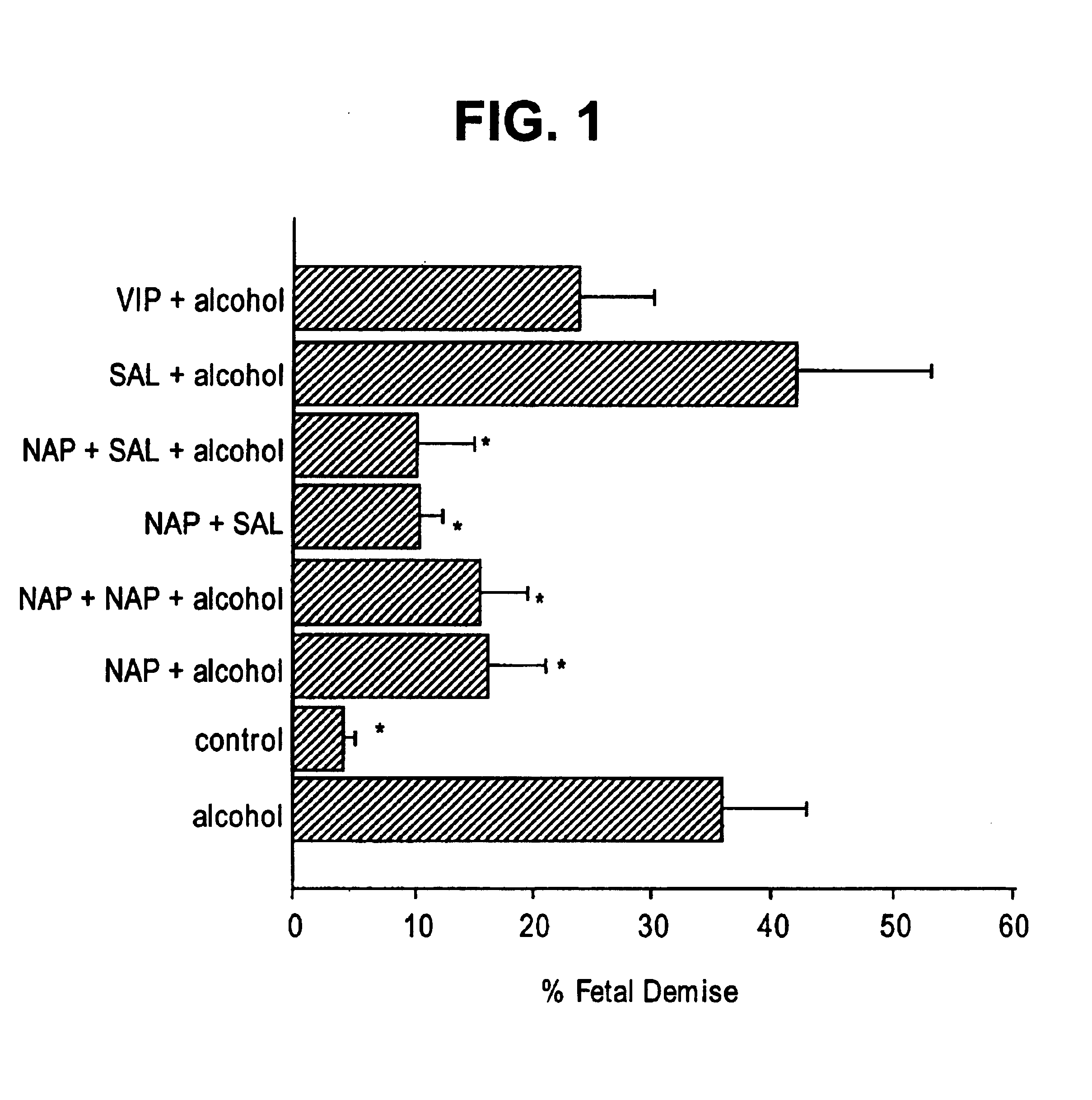
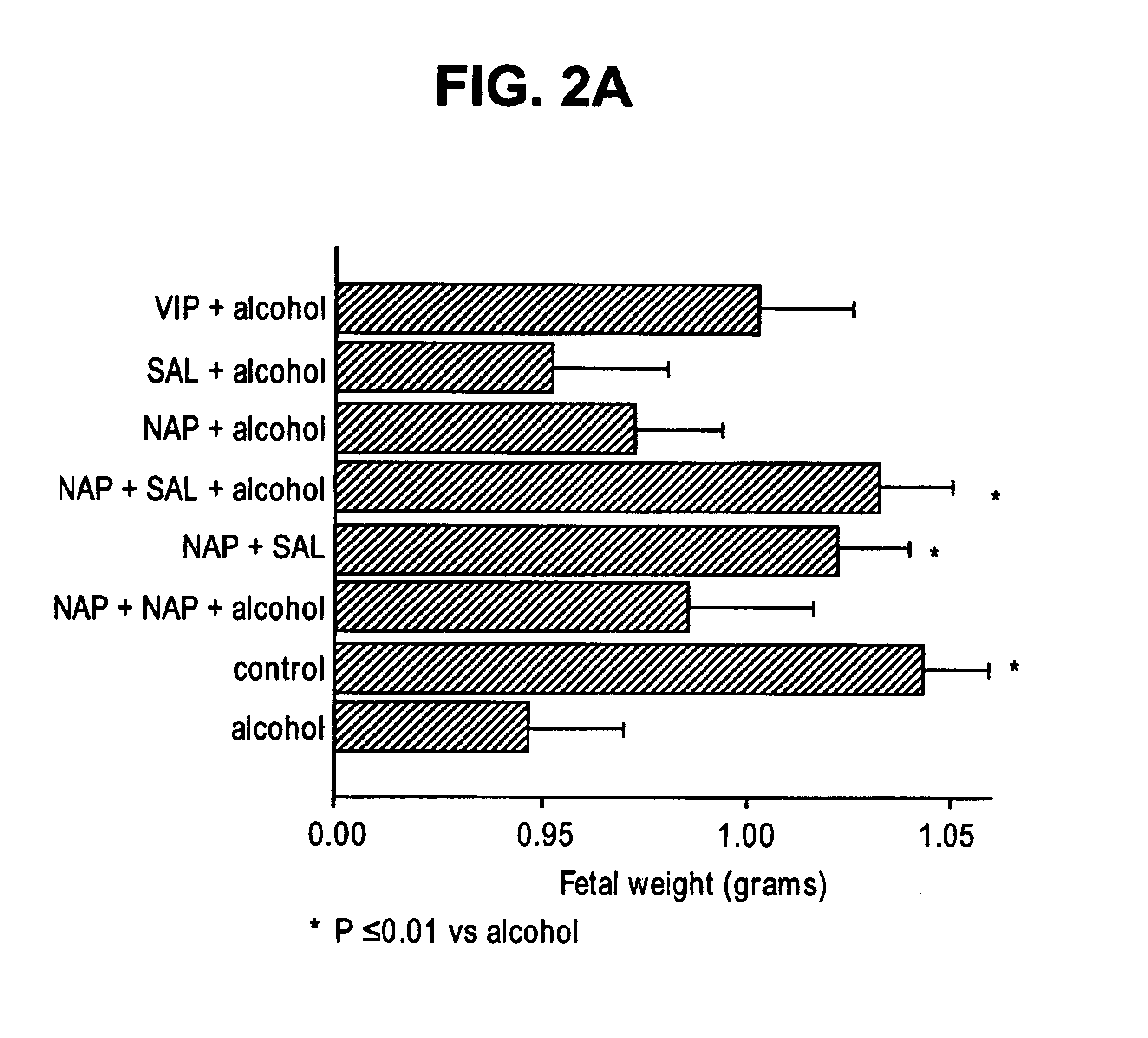
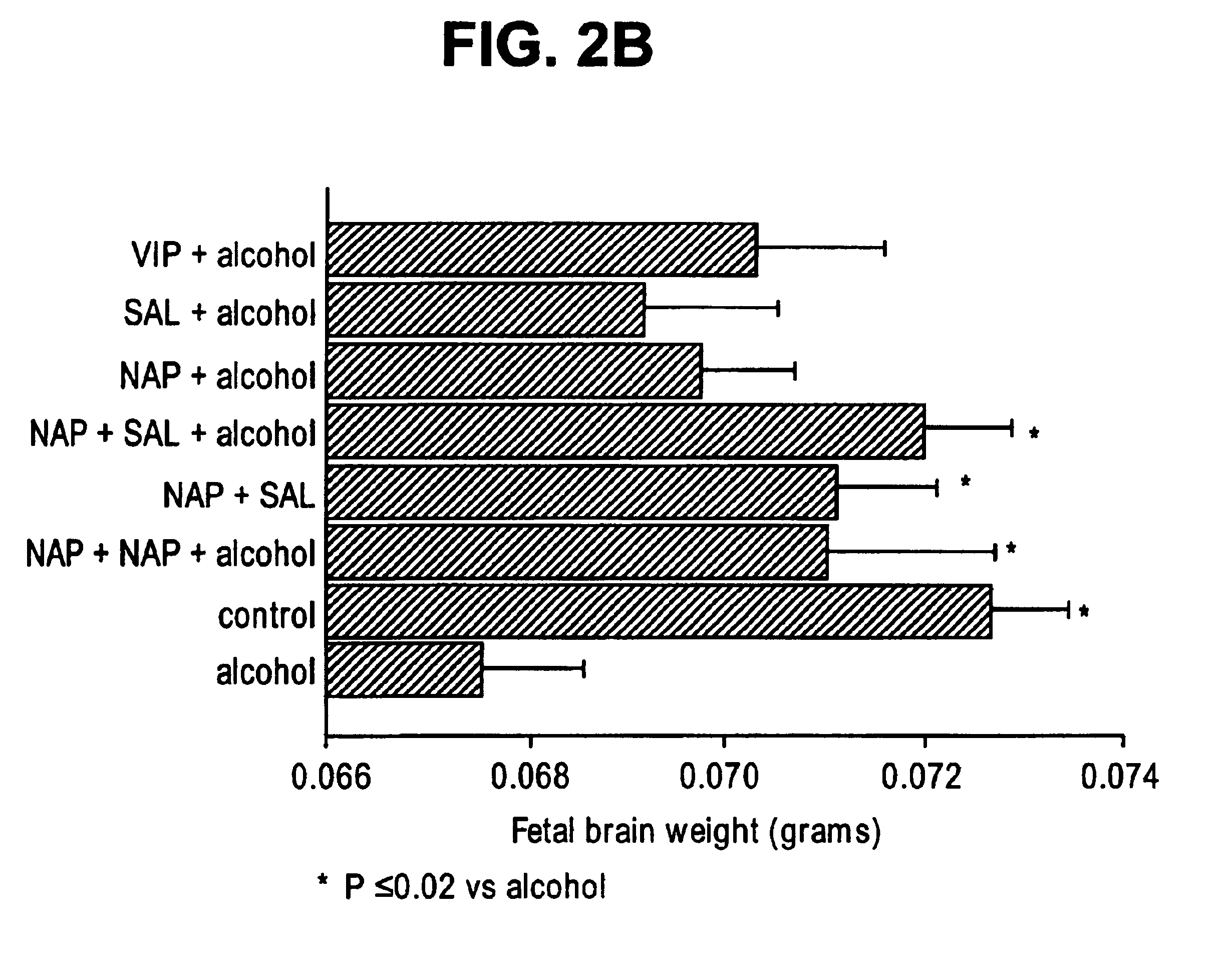
Prevention of fetal alcohol syndrome and neuronal cell death with ADNF polypeptides
InactiveUS6933277B2Reducing neuronal cell deathRemissionBiocideNervous disorderFetal alcoholMedicine
This invention relates to a method for reducing a condition associated with fetal alcohol syndrome in a subject who is exposed to alcohol in utero with an ADNF polypeptide. In particular, the present invention relates to a method of reducing a condition associated with fetal alcohol syndrome in a subject who is exposed to alcohol in utero with a combination of ADNF I and ADNF III polypeptides. The present invention further relates to a method for reducing neuronal cell death by contacting neuronal cells with a combination of ADNF I and ADNF III polypeptides. Still further, the present invention relates to a pharmaceutical composition comprising a combination of ADNF I and ADNF III polypeptides.
Owner:DEPT OF HUMAN SERVICES GOVERNMENT OF THE UNITED STATES THE AS REPRESENTED BY THE SEC OF THE +1
Probe set and reagent kit used for detecting pathopoiesia/susceptibility genes of congenital megacolon and relative syndromes
ActiveCN105256051AStrong targetingReduce or avoid birthsMicrobiological testing/measurementDNA/RNA fragmentationDiseaseMass survey
The invention provides a probe set and a reagent kit used for detecting pathopoiesia / susceptibility genes of congenital megacolon and relative syndromes. At most 172 pathopoiesia / susceptibility genes related to diseases can be detected at the same time, and reference can be provided for affected individual molecular genetics diagnosis, mass survey of congenital megacolon high-incidence areas, screening of high-risk affected groups in congenital megacolon family members and corresponding genetic counseling or prenatal intervention.
Owner:首都儿科研究所 +1
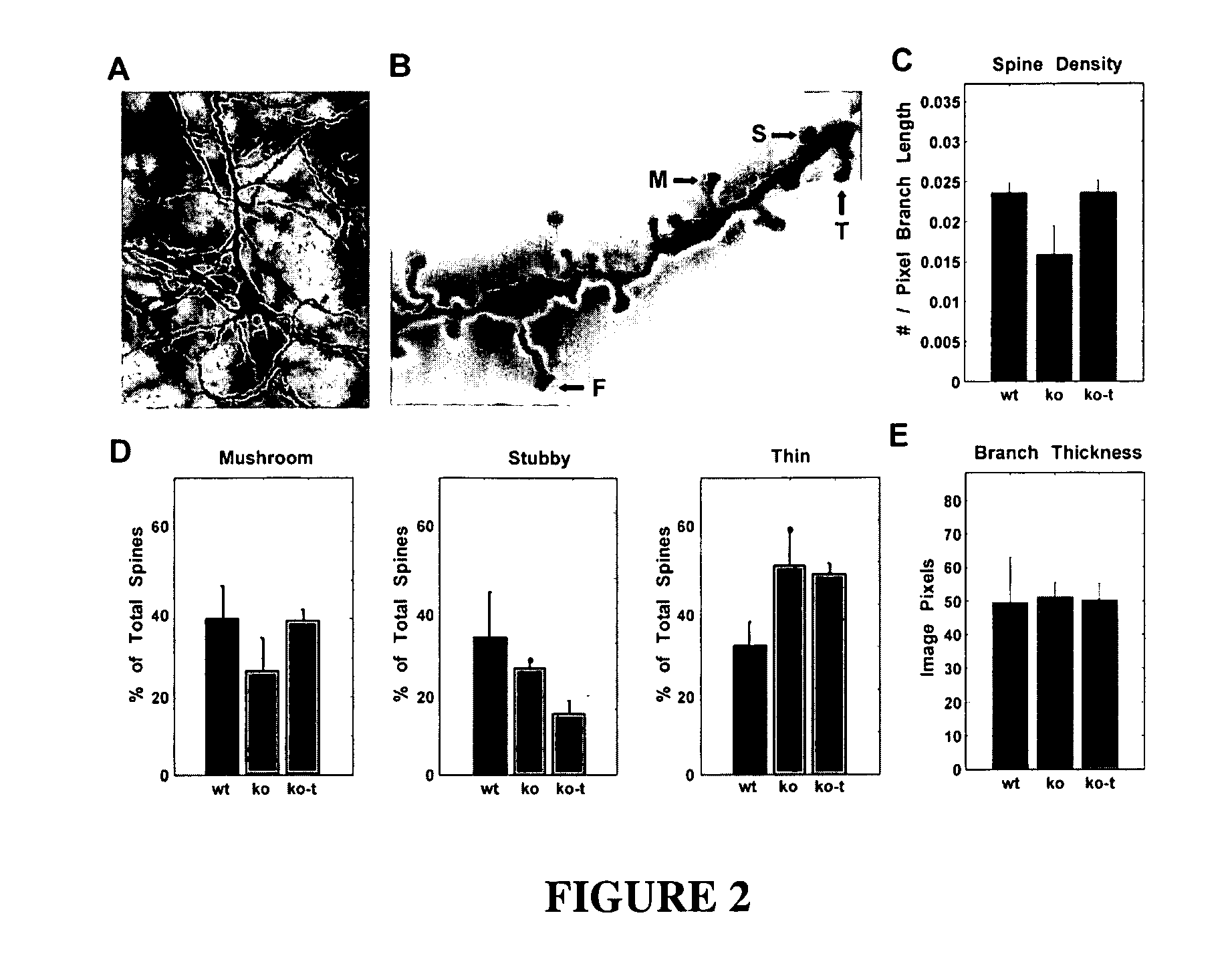
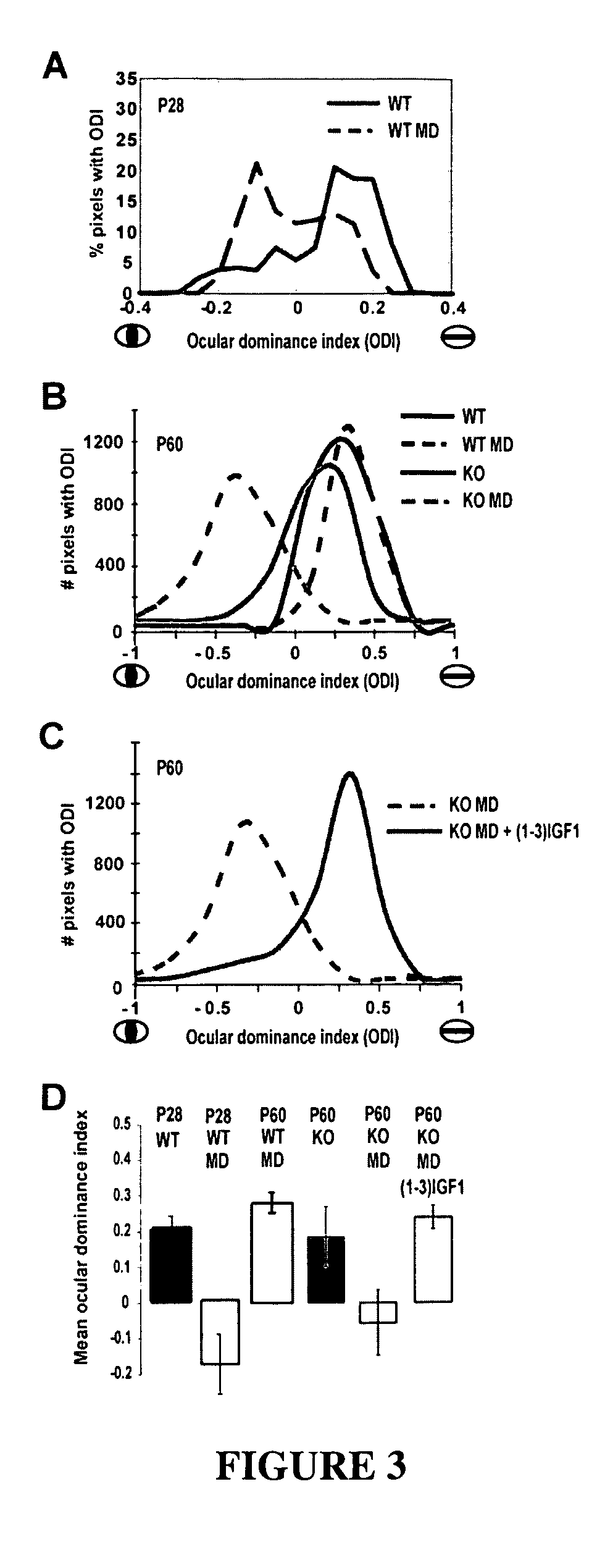
Treatment of rett syndrome and other disorders
ActiveUS20090099077A1Recovery functionRestore maturationNervous disorderDipeptide ingredientsDiseaseMedicine
The invention relates to methods for treatment of Rett Syndrome and other disorders of synaptic function and maturation using IGF1, (1-3)IGF-1, (1-3)IGF-1 analog(s) and / or related therapeutic molecules.
Owner:WHITEHEAD INST FOR BIOMEDICAL RES +1
Combination therapy for effecting weight loss and treating obesity
InactiveUS7659256B2Improve side effectsAmeliorating sleep apneaBiocideCarbohydrate active ingredientsSYMPATHOMIMETIC AGENTSSympatholytic Drugs
Owner:VIVUS
Combination therapy for effecting weight loss and treating obesity
InactiveUS20060234952A1Ameliorating sleep apneaLowering blood blood glucose blood levelBiocideMetabolism disorderSYMPATHOMIMETIC AGENTSPhentermine
The present invention features a novel therapy for effecting weight loss which involves treating a subject with a sympathomimetic agent (e.g., phentermine or a phentermine-like drug) in combination with an anticonvulsant sulfamate derivative (e.g., topiramate) such that the subject experiences weight loss. The combination methods of the present invention also are effective against symptoms associated with Syndrome X. The invention also features pharmaceutical compositions and kits for use in the practice of these novel therapies.
Owner:VIVUS
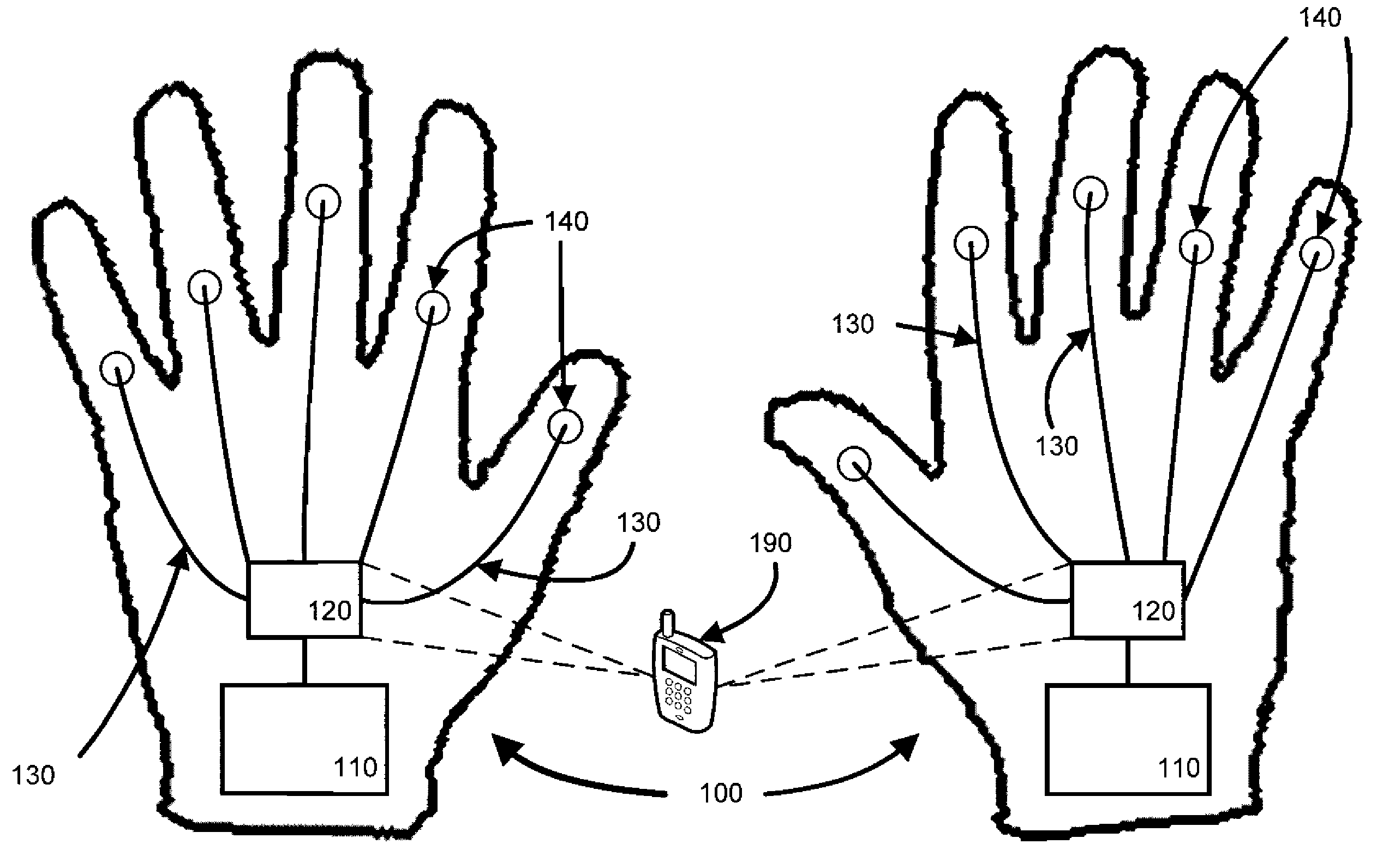
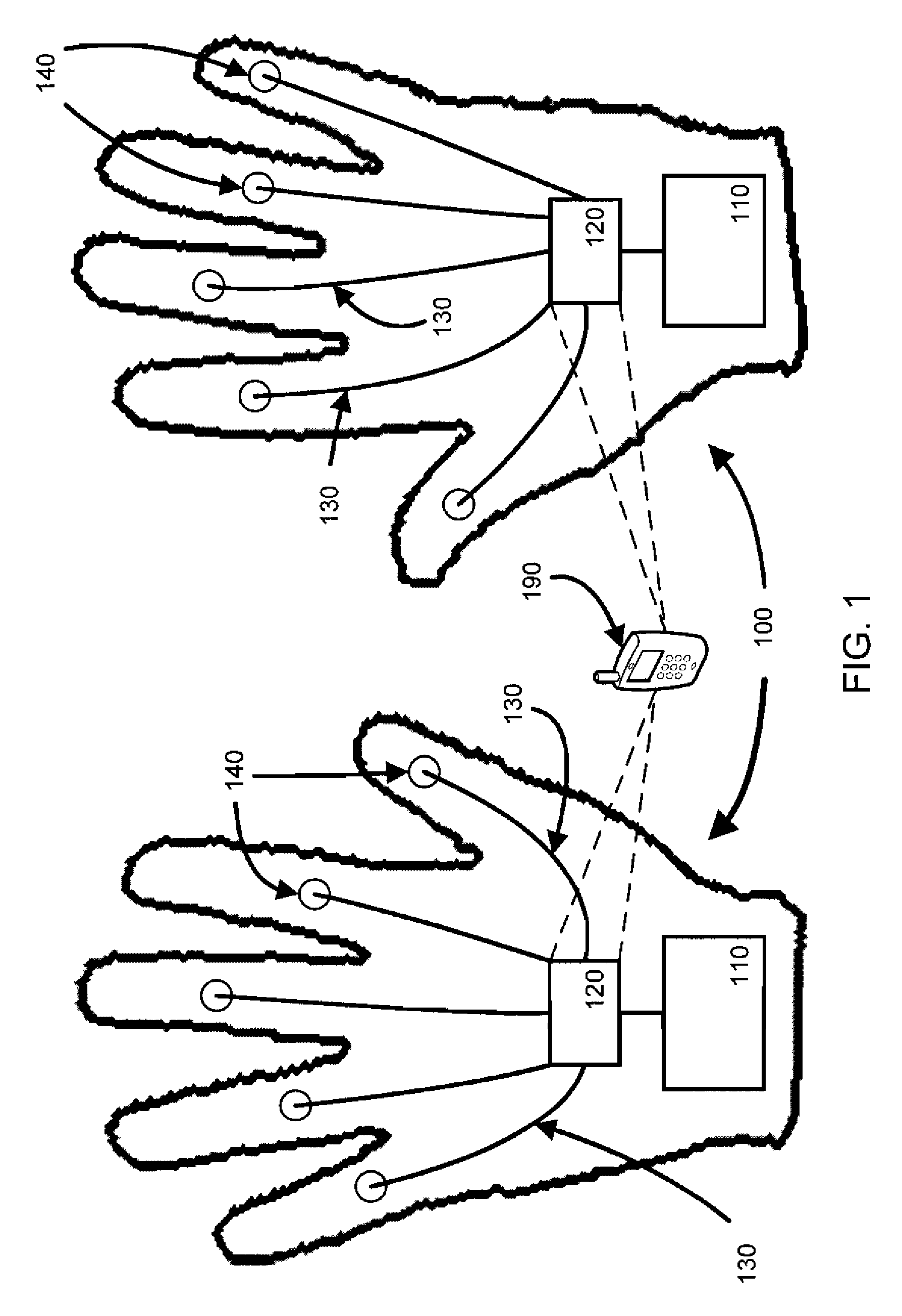
Raynaud's Conditions
This document provides methods and materials for treating Raynaud's conditions such as Raynaud's syndrome, Raynaud's phenomenon, and Raynaud's disease. For example, this document provides methods for using electrical stimulation to reduce the severity of a symptom of a Raynaud's condition. Gloves and socks that can be used to reduce the severity of a symptom of a Raynaud's condition also are provided.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
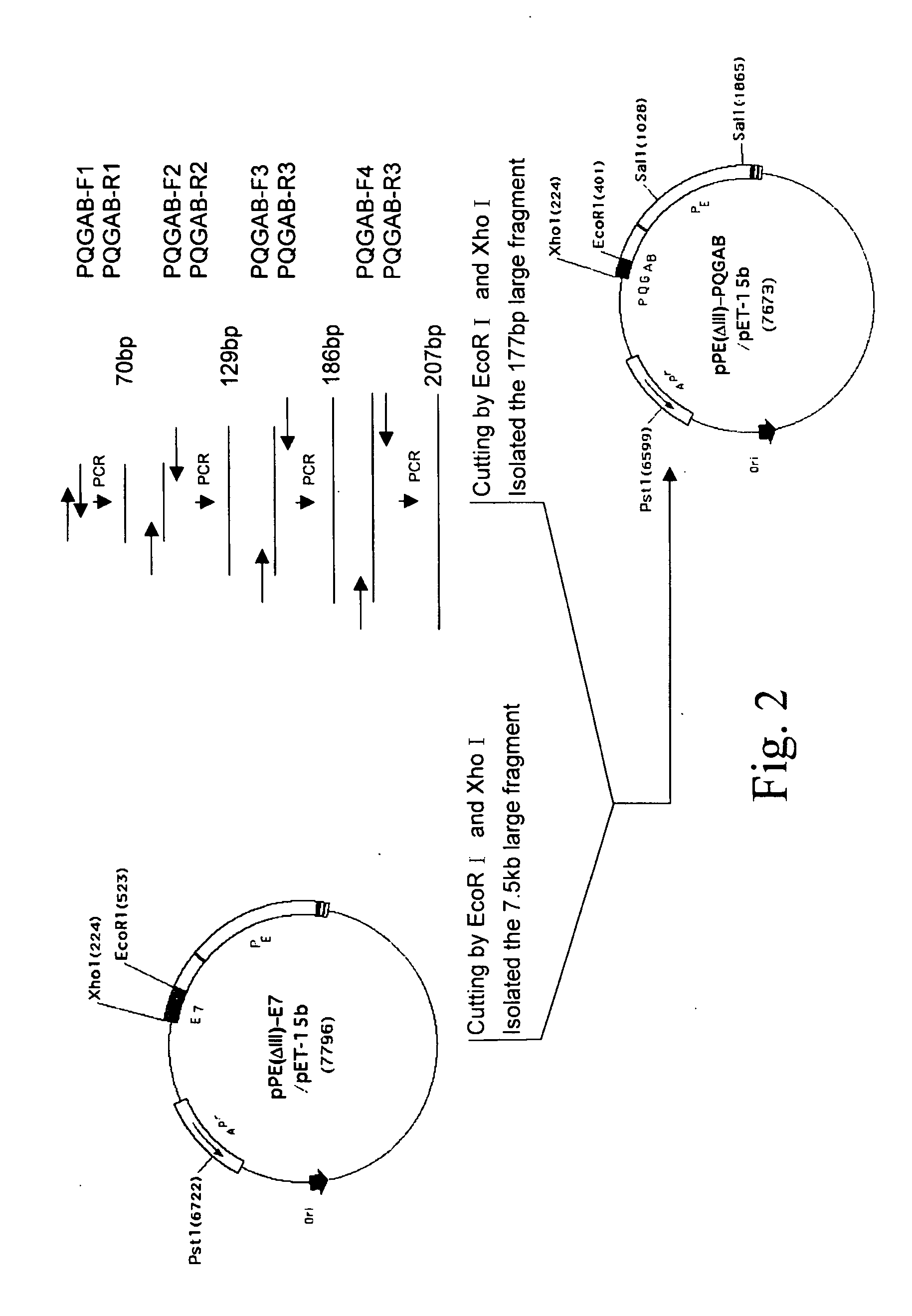
Fusion protein of porcine reproductive and respiratory syndrome virus as PRRS vaccine
ActiveUS20080008722A1SsRNA viruses positive-senseVirus peptidesPseudomonas aeruginosa exotoxin AAllergy
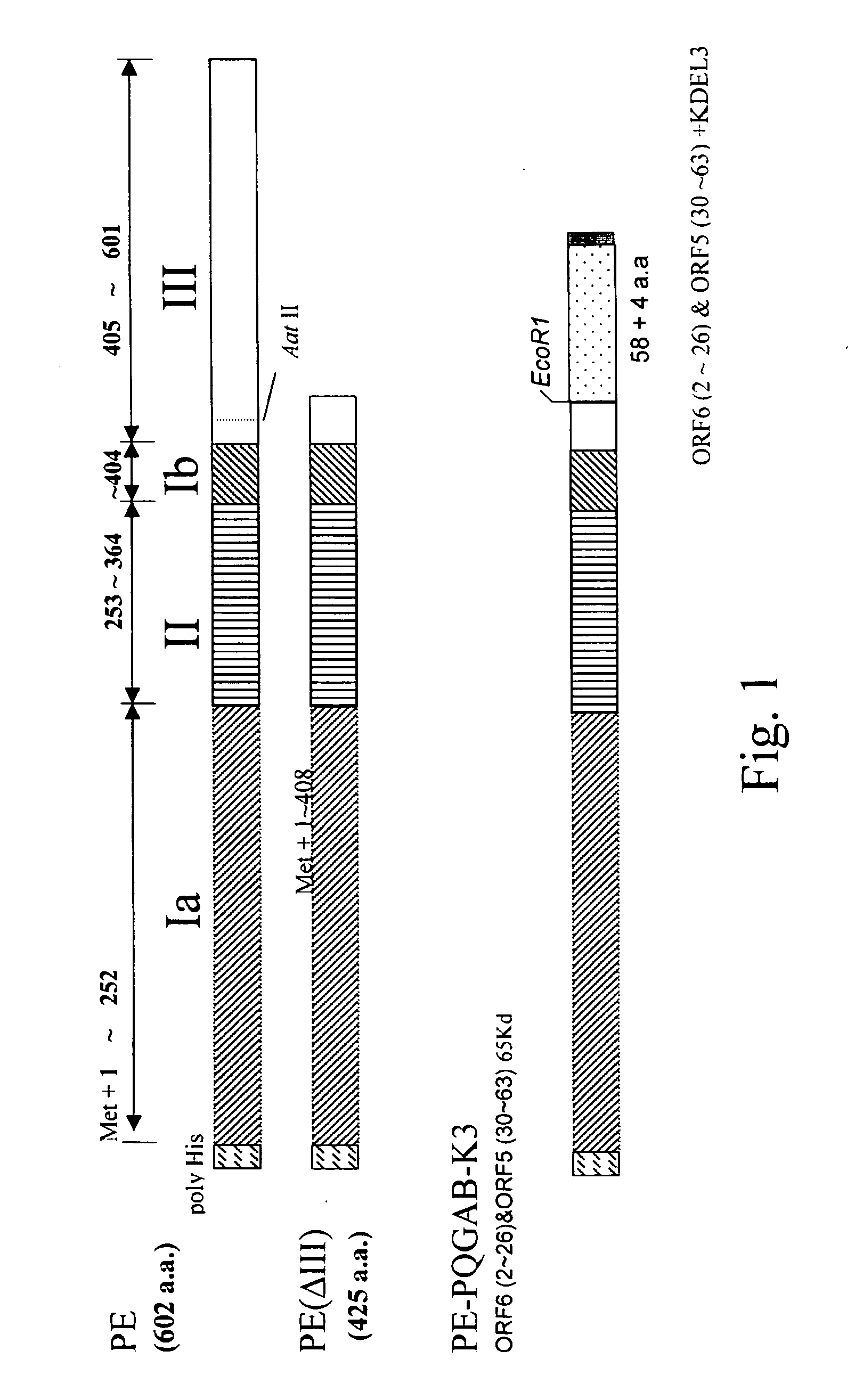
The present invention provides a PRRSV subunit vaccine comprising a fusion protein having neutralization titers evoked, PE-PQGAB-K3, which comprises a chimeric polypeptide containing N-terminal portions of PRRSV ORF5 and ORF6 structure proteins; a portion of Pseudomonas exotoxin A binding and translocation domain; and a carboxyl terminal domain containing KDEL-KDEL-KDEL(K3) sequence. Less inflammation of PE-PQGAB-K3 vaccine group in their lungs post being PRRSV-challenged indicates that PQGAB without an antigen-specific allergy effect. Importantly, PE-PQGAB-K3 vaccine presents a good protection against PRRSV infection than control groups in pig challenged experiment.
Owner:AGRI TECH RES INST
Method of detecting chromosomal abnormalities
The invention relates to a method of detecting chromosomal abnormalities, in particular, the invention relates to the diagnosis of fetal chromosomal abnormalities such as trisomy 21 (Down's syndrome) which comprises sequence analysis of cell-free DNA molecules in plasma samples obtained from maternal blood during gestation of the fetus.
Owner:PREMAITHA LTD
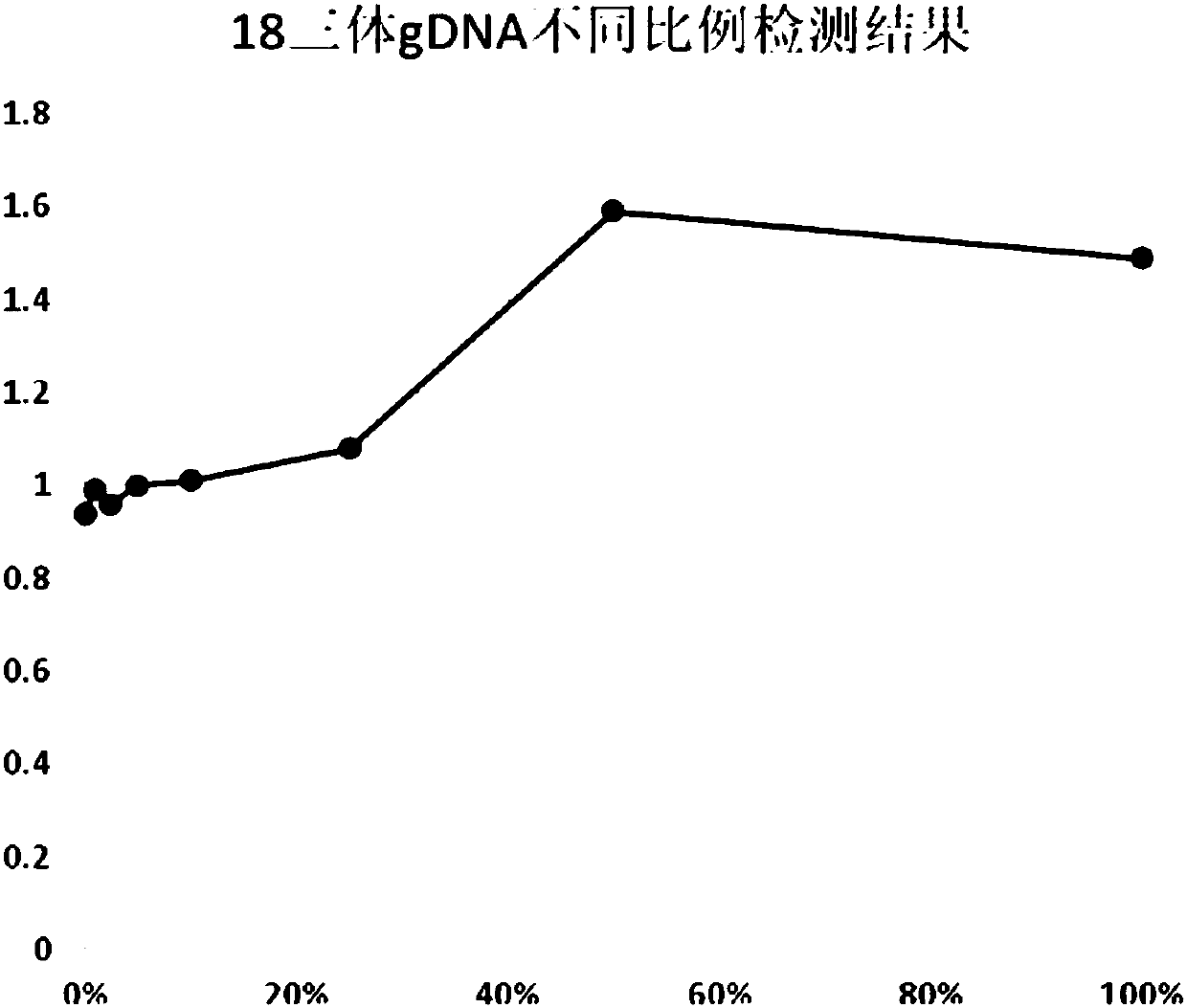
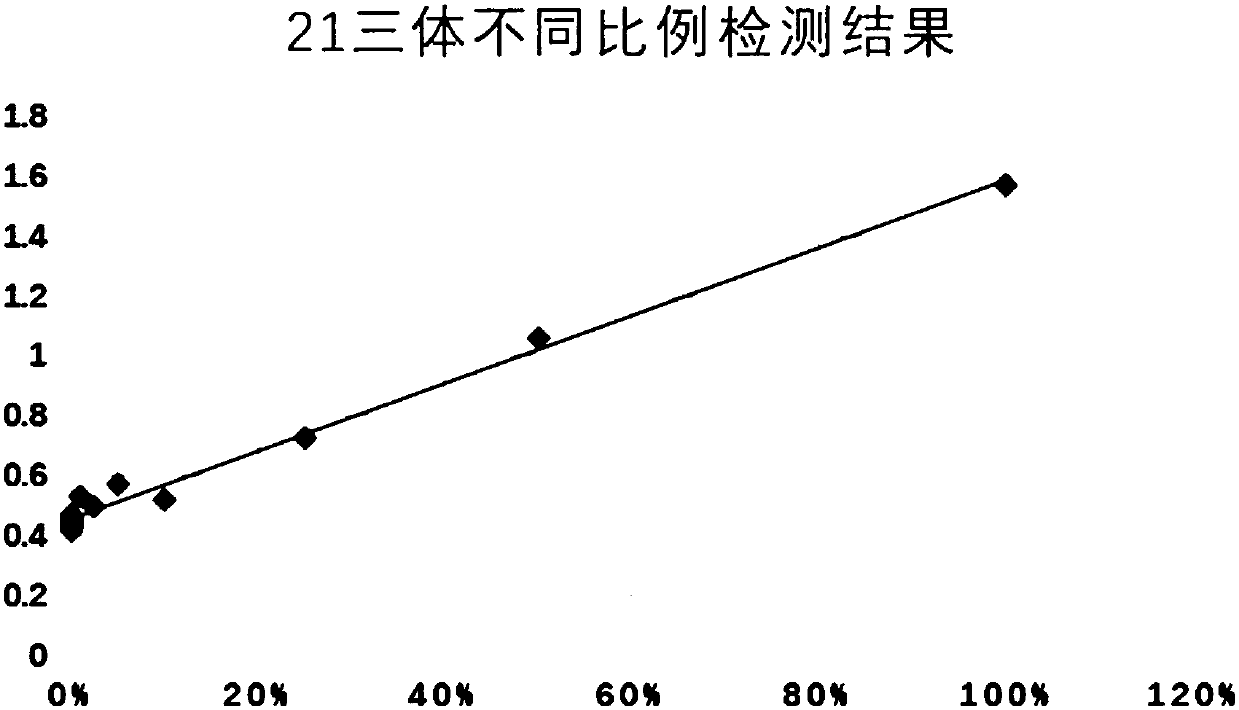
Noninvasive fetus 21, 18 and 13 trisomic syndrome antenatal detection positive quality control product and preparing method thereof
InactiveCN104531842AEasy to storePrevent deviationMicrobiological testing/measurementTrisomy 13 SyndromeTrisomy X syndrome
The invention provides a noninvasive fetus 21, 18 and 13 trisomic syndrome antenatal detection positive quality control product and a preparing method thereof, and belongs to the fetus chromosome aneuploid noninvasive antenatal detection range. The preparing method of the positive quality control product includes the steps that DNAs of abortion groups of fetuses suffering from 21, 18 and 13 trisomic syndromes are broken into DNA fragments with the length ranging 160 bp to 200 bp; the abortion group DNA fragments recycled after breaking are added into mixed plasma in proportion; and the total plasma free DNAs are finally extracted, and the positive quality control products with different concentrations are prepared after attenuation with ultrapure water is carried out. The positive quality control product is a white transparent DNA solution, and has the beneficial effects of being stable, easy to store, convenient and rapid to use, non-toxic and the like; in addition, deviations of detection results of other samples in the same batch can be effectively avoided; and the accuracy and the reliability of the 21, 18 and 13 trisomic syndrome noninvasive antenatal detection are improved.
Owner:DAAN GENE CO LTD
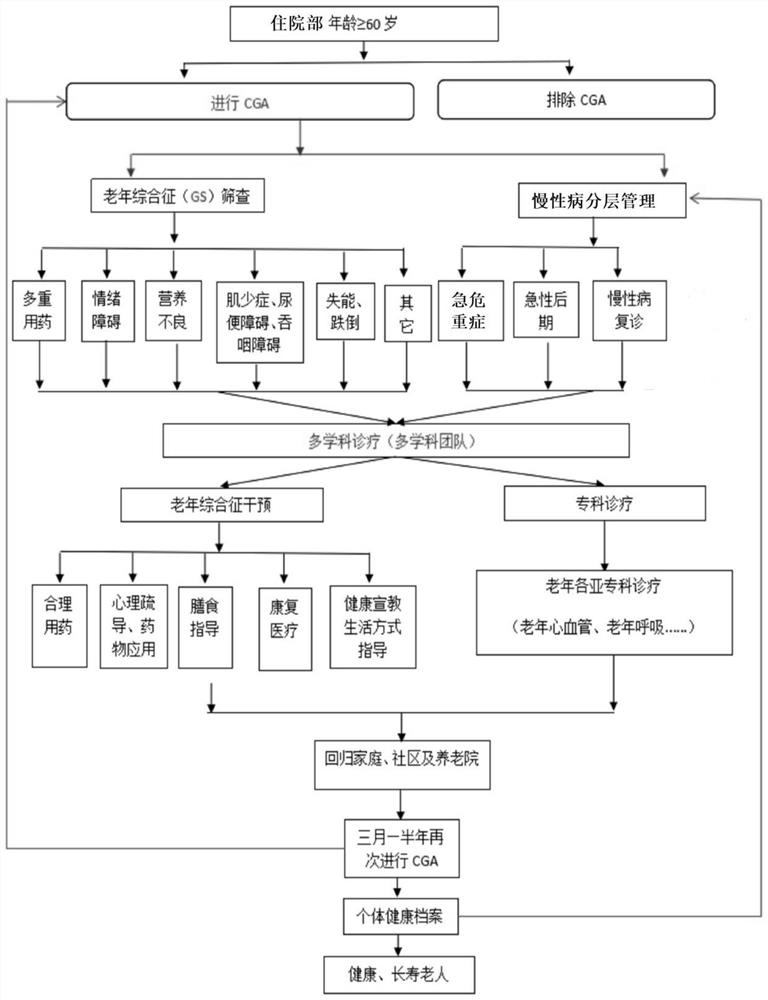
Method for screening, evaluating and intervening senile syndromes of hospitalized elderly patients
PendingCN112614555AImprovement of geriatric syndromeReduce adverse eventsEpidemiological alert systemsMedical reportsElderly healthPressure sore risk
The invention discloses a method for screening, evaluating and intervening senile syndromes of hospitalized senile patients, which comprises the following steps of: comprehensively screening the senile syndromes of the senile patients; carrying out special assessments including pressure sore risk degree, disability degree, emotional disorder degree, sleep quality, cognitive disorder degree, malnutrition degree, dysphagia degree, defecation and urination, social support, pain, delirium, tumble, sarcopenia, weakness, body diseases and the like; and making individualized intervention suggestions according to the evaluation conclusion. According to the invention, elderly health management is associated with different states of diseases, an elderly syndrome intelligent management method is provided for elderly patients in a targeted manner, and disease hierarchical management and continuous service are realized. The method comprises a complete process of health information collection, disease and function evaluation, intervention opinions and follow-up visits, so that the method can be widely popularized as a special diagnosis and treatment technical method of the elderly medical inpatient department, and can enable the elderly to obtain better standard, effective and homogenized services.
Owner:FIRST PEOPLES HOSPITAL OF YUNNAN PROVINCE
Use of relaxin as adjuvant in the differentiation of stem cells for the reconstruction of tissues
InactiveUS20050143299A1Promote rapid maturationRapidly organizationBiocideNervous disorderAdjuvantSmell loss
On the basis of the experimental results it has emerged that relaxin has a pro-differentiating effect on stem cells. It is therefore suggested the use of this hormone as principal component of a drug for the treatment of all those situations that find or will find benefit from the use of stem cells for the reconstruction of tissues damaged by traumatic events or by ischemic-inflammatory-degenerative diseases. It is also suggested the use of relaxin for the treatment of syndromes deriving from the missing activation of stem cells during fetal development (at subsequent somatic-functional maturation), such as for example hypogonotropic hypogonadism with anosmia (Kallman's syndrome).
Owner:BIGAZZI MARIO
Methods of treatment for guillain-barre syndrome
InactiveUS20160326237A1Immunoglobulins against animals/humansAntibody ingredientsPhysiologyGuillain-Barre syndrome
Owner:THE UNIV COURT OF THE UNIV OF GLASGOW +1
Genetic markers associated with asd and other childhood developmental delay disorders
Owner:LINEAGEN INC
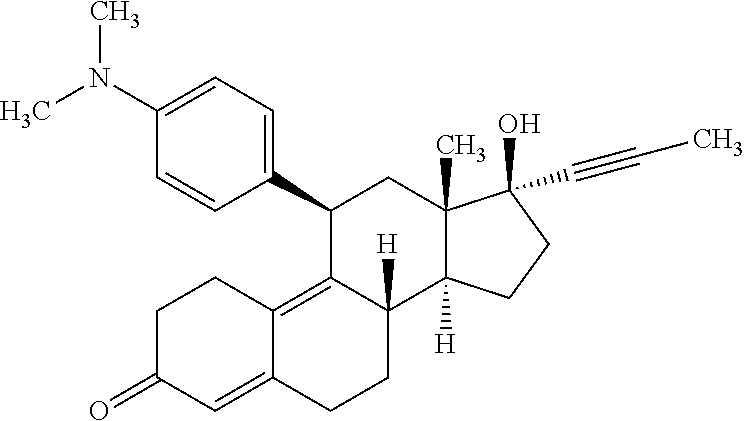
Method of treating patients with lennox-gastaut syndrome
A method of treating symptoms of Lennox-Gastaut syndrome in a patient diagnosed with Lennox-Gastaut syndrome, by administering an effective dose of fenfluramine to that patient over a period of time sufficient to reduce or completely eliminate seizures in the patient. The fenfluramine may be administering in an oral liquid formulation on a daily basis of 0.7 mg / kg / day, over a period of weeks until seizures are reduced by 25% or more, 50% or more, 75% or more.
Owner:ZOGENIX INT
Animal model for type II diabetes mellitus and Syndrome X and methods and uses thereof
InactiveUS20070271620A1Relieve stressNo pressureMetabolism disorderMedical devicesPhysiologyPancreatic A Cells
The invention provides a method for generating a type II diabetes mellitus and / or Syndrome X model in pigs. A method of the invention comprises partially destructing pancreatic beta-cells in pigs. From these pigs, a pig is preferably selected that comprises a fasting plasma glucose level higher than 6 mmol / L. The invention further provides a pig according to the invention and uses thereof.
Owner:STICHTING DLO
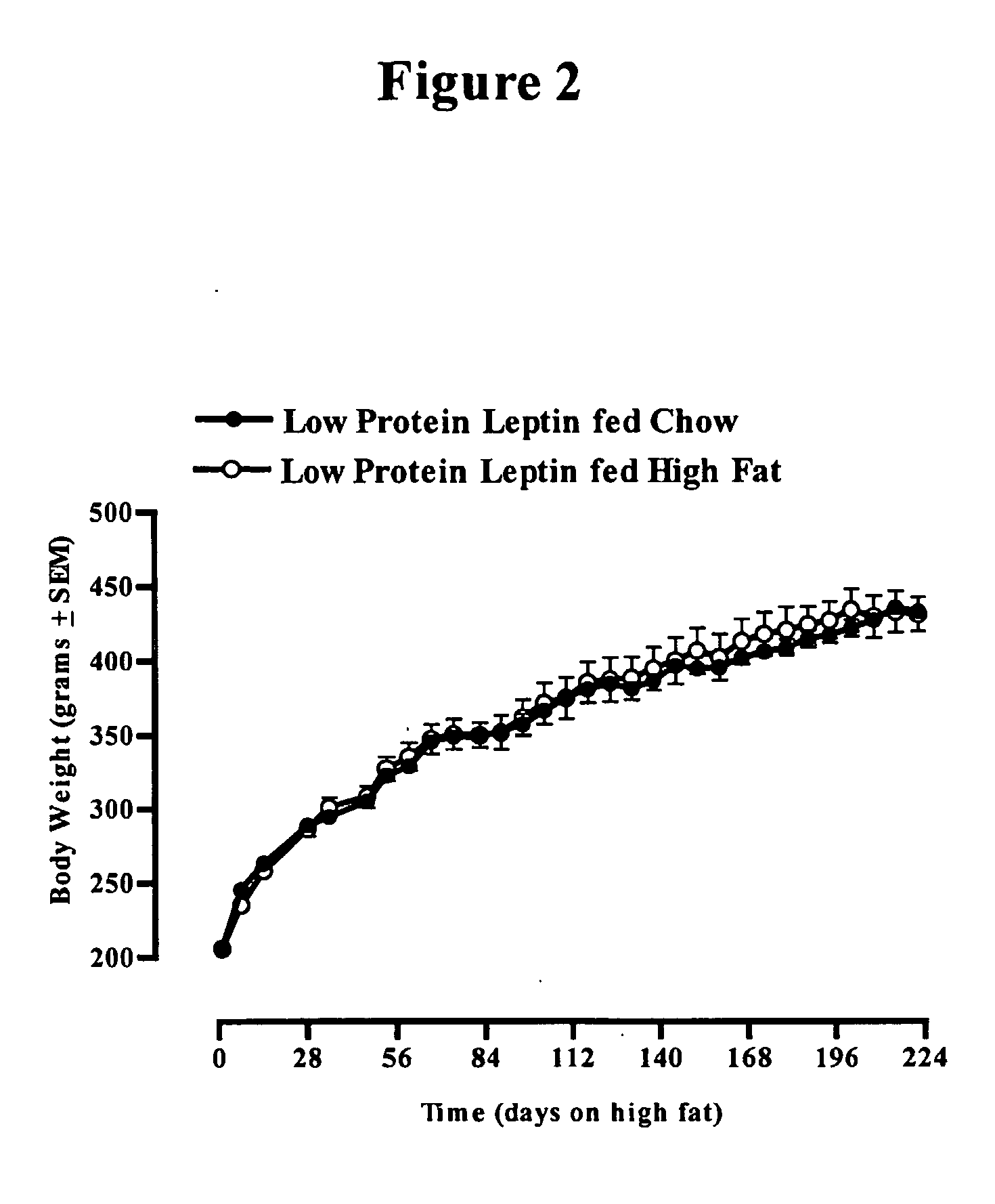
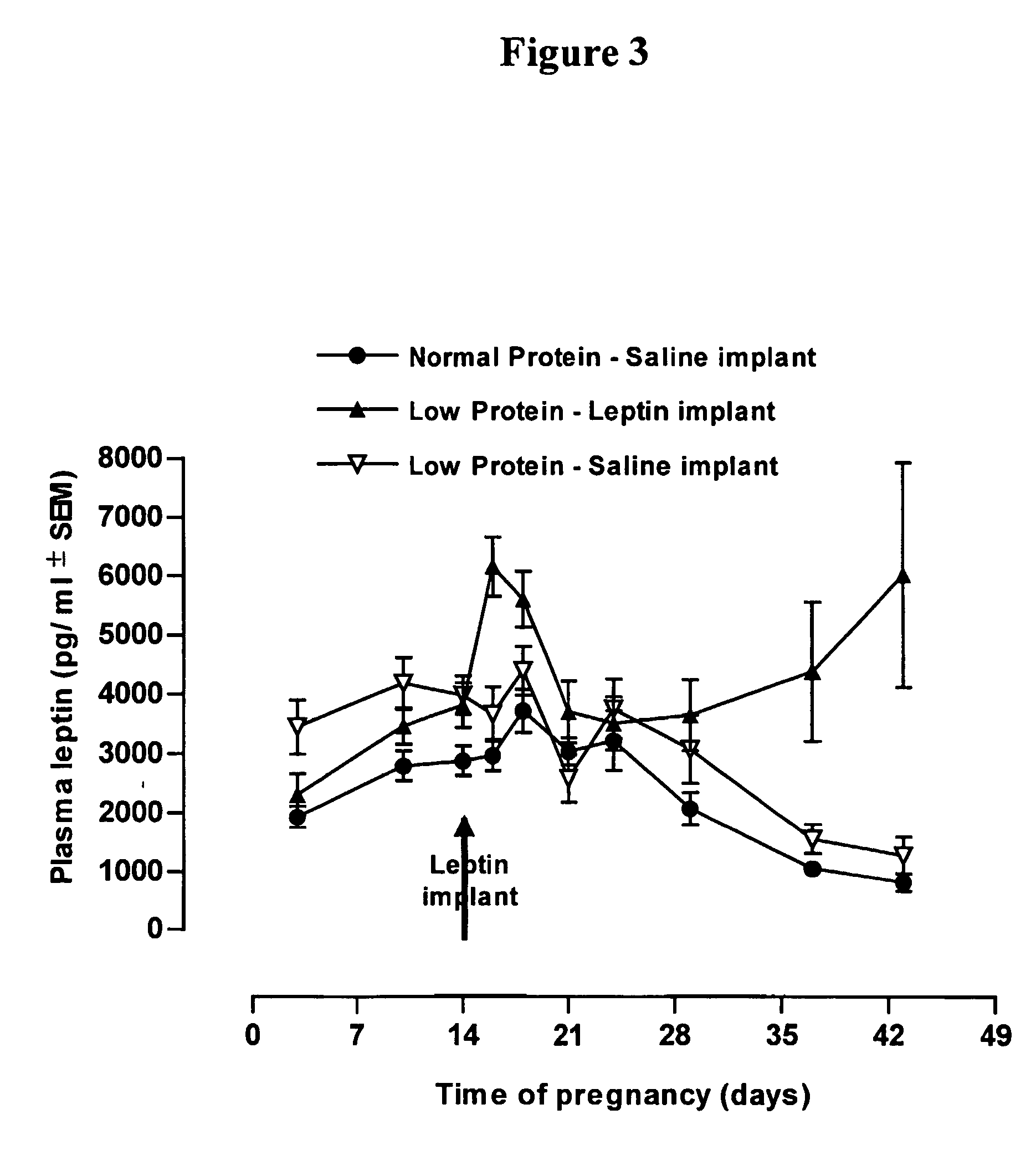
Use of leptin for infant with low birth weight for prevention of obesity
InactiveUS20050065078A1Avoid developmentEfficiently provideMilk preparationPeptide/protein ingredientsObesity preventionPediatrics
The invention discloses the therapeutic and prophylactic administration of leptin to (i) an infant of low birth weight for age; (ii) a nursing mother of an infant, the infant having low birth weight for age; or (iii) a pregnant female predisposed to giving birth to an infant of low birth weight for age; for the prevention or treatment, in later life of the infant, of a metabolic disorder or other condition associated with low birth weight, such as type 2 diabetes, obesity, cardiovascular disease, gestational diabetes, impaired glucose tolerance, insulin resistance, hypertension or syndrome X. It is thought that the beneficial properties of leptin may be due at least in part to an effect on glucocorticoid metabolism, in particular to an increase in type 2 11β-hydroxysteroid dehydrogenase activity.
Owner:CAWTHORNE ANTHONY MICHAEL +1
Optimizing mifepristone levels for cushing's patients
ActiveUS9943526B2Good curative effectOrganic active ingredientsDisease diagnosisBlood levelMifepristone
The present invention provides a method for optimizing levels of mifepristone in a patient suffering from Cushing's syndrome. The method comprises the steps of treating the patient with seven or more daily doses of mifepristone over a period of seven or more days; testing the serum levels of the patient to determine whether the blood levels of mifepristone are greater than 1631 ng / mL; and adjusting the daily dose of the patient to achieve mifepristone blood levels greater than 1631 ng / mL.
Owner:CORCEPT THERAPEUTICS INC
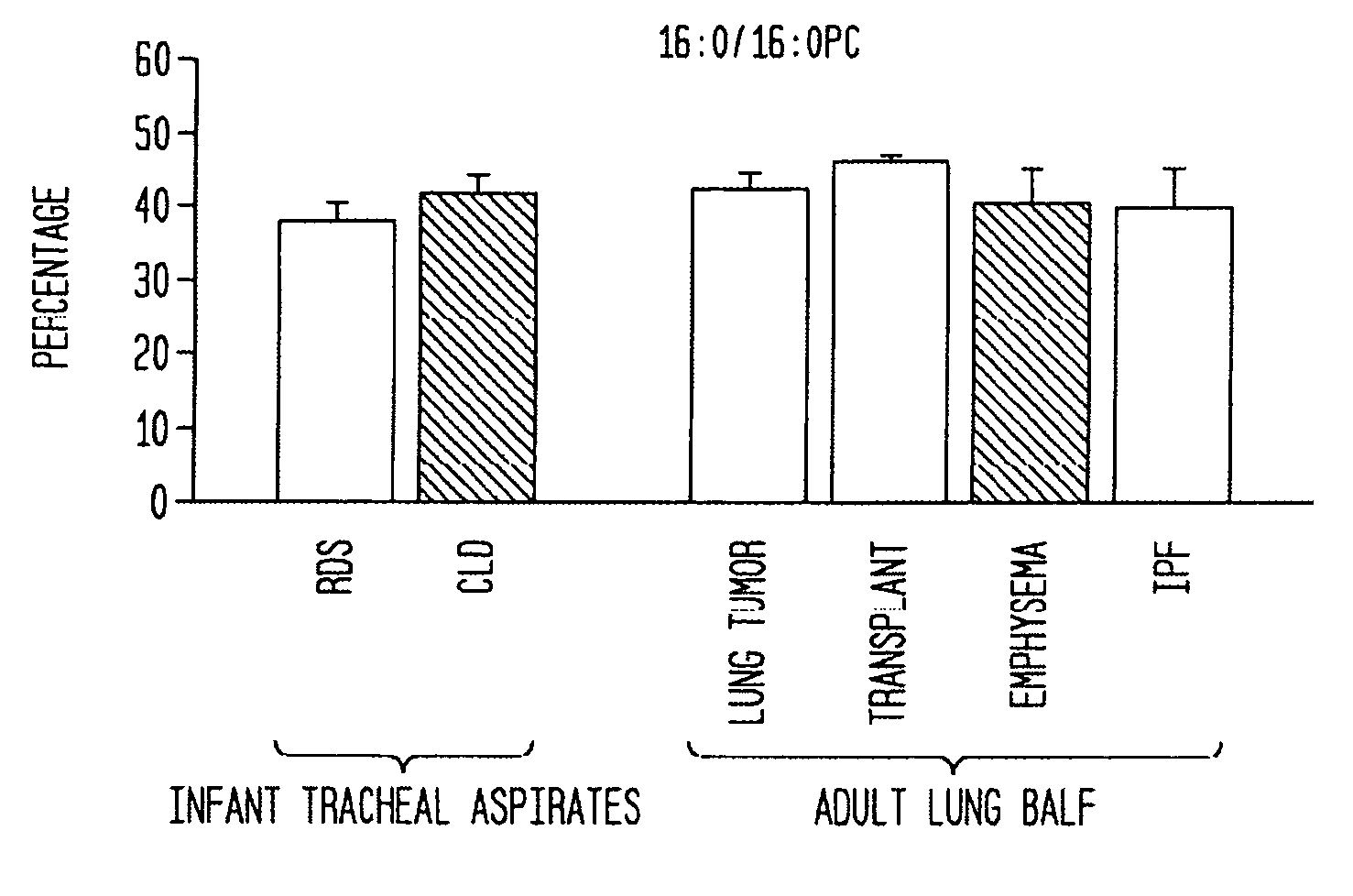
Phospholipid formulations and uses thereof in lung disease detection and treatment
InactiveUS20070010429A1Deter/inhibit damagePowder deliveryOrganic active ingredientsAir sacsSURFACTANT BLEND
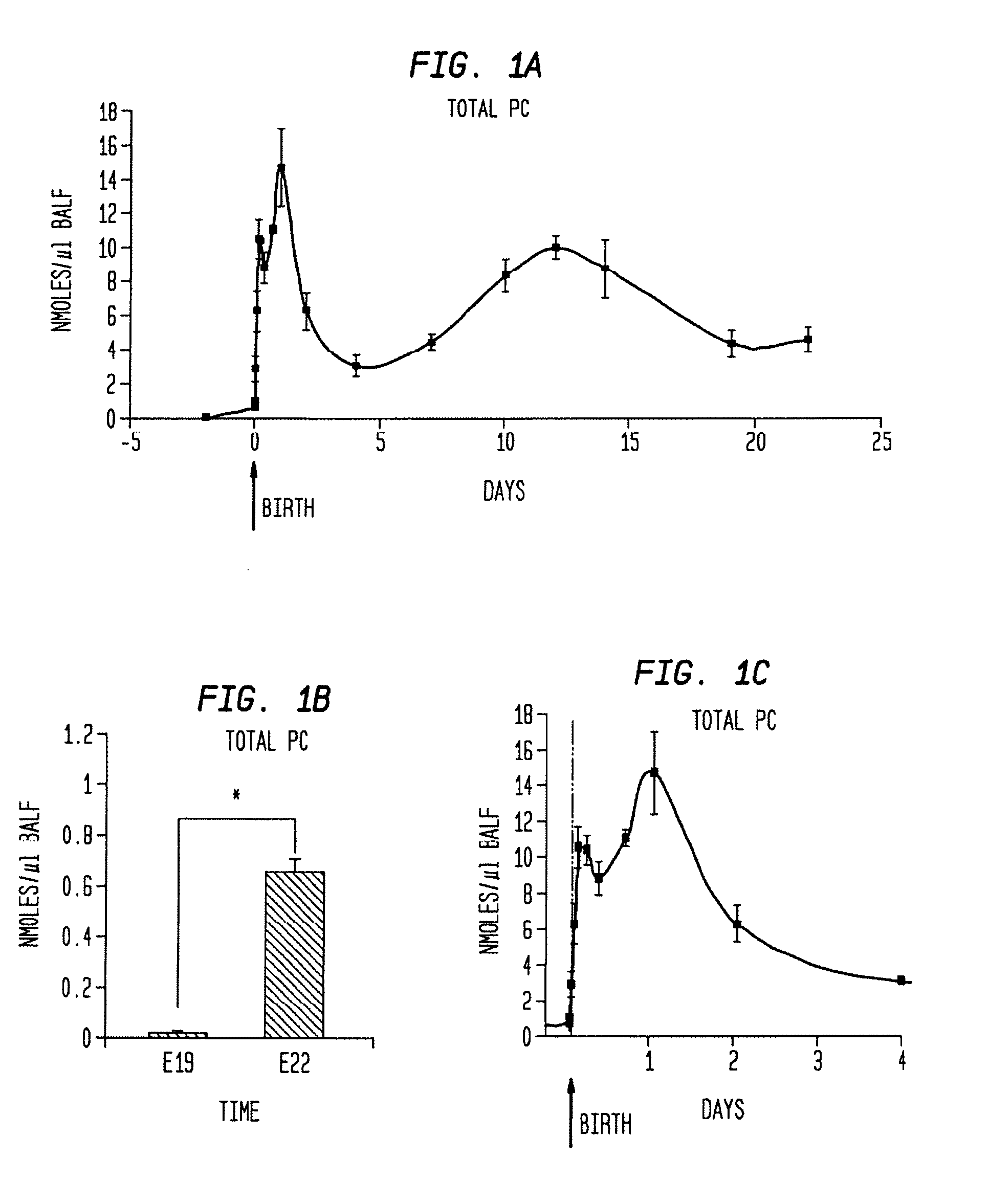
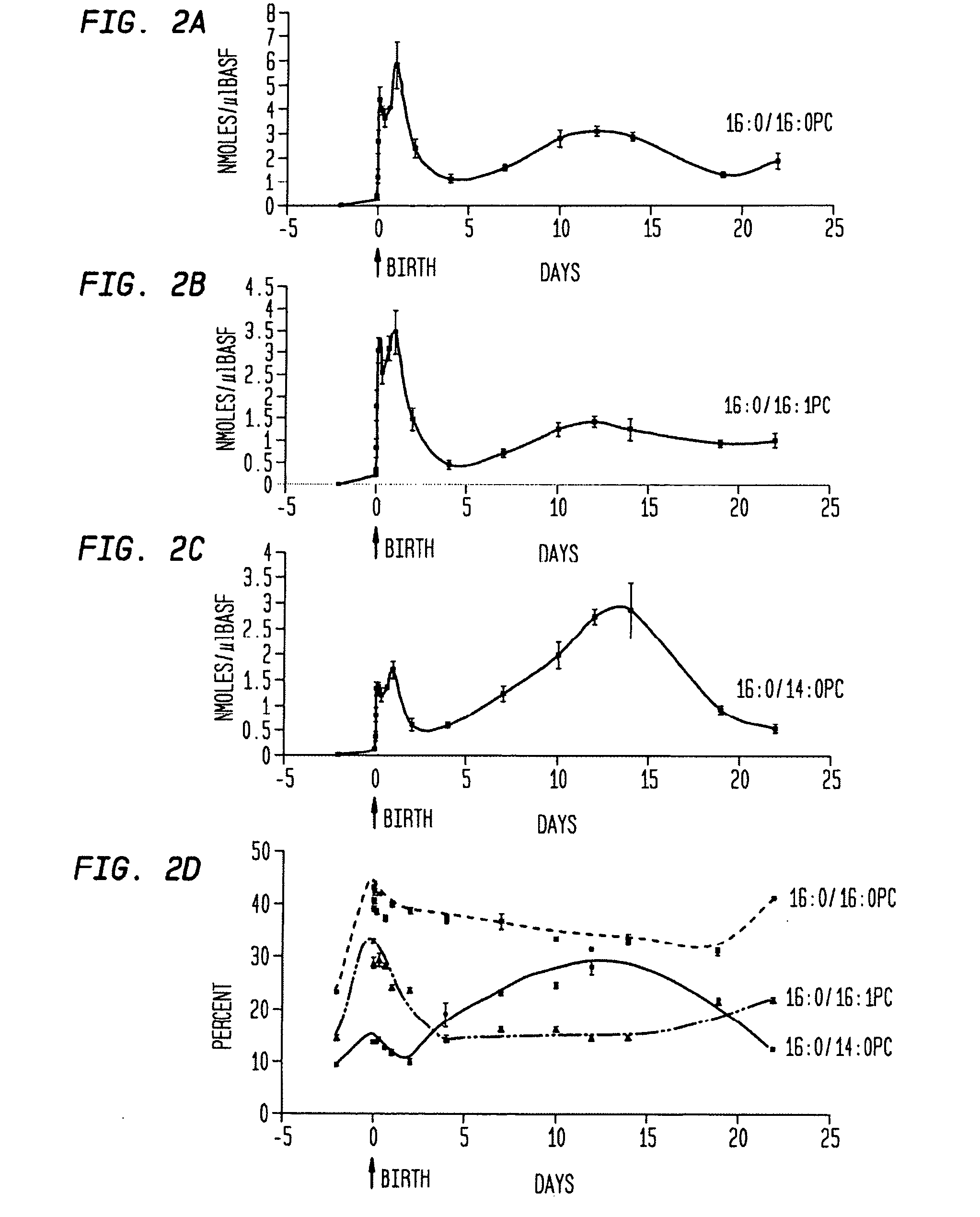
Disclosed are methods and compositions that are useful in the detection and therapy of diseases (e.g., emphysema) and damage that afflict the lungs. In some aspects, the compositions comprise a formulation enriched for a species of phosphatidylcholine, such as palmitoylmyristoyl phosphatidylcholine (16:0 / 14:0PC). The compositions may further be described as lung surfactant supplement preparations particularly useful in the treatment of pulmonary diseases and afflictions prevalent among premature infants, and in particular, Respiratory Distress Syndrome (RDS). A PC marker is also disclosed, 16:0 / 14:0PC, that may be used to detect pulmonary disease or reduced / compromised alveolar function in an animal. Phospholipid profiles of 16:0 / 14:0PC, 16:0 / 16:1PC and 16:0 / 16:0PC are also provided, and are correlated with particular pulmonary diseased states.
Owner:HOSPITAL FOR SICK CHILDREN
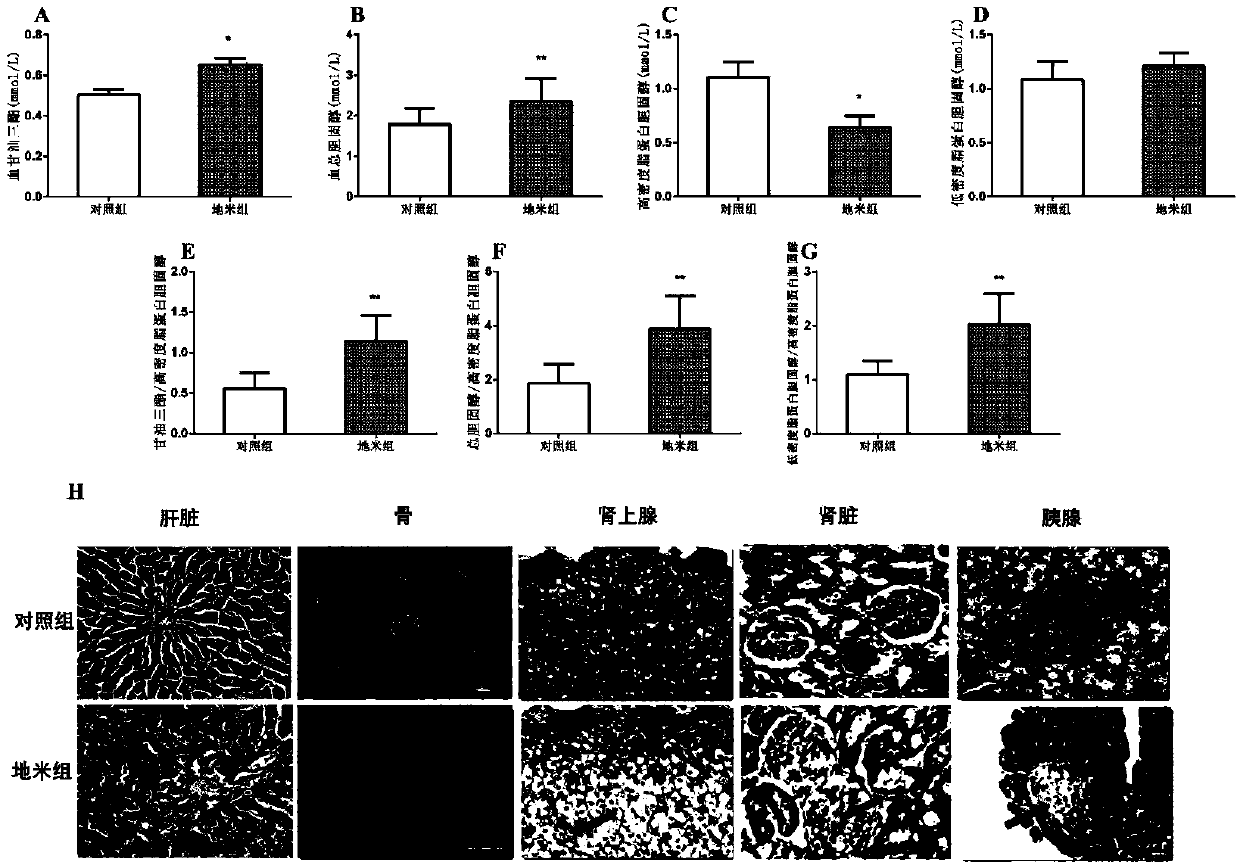
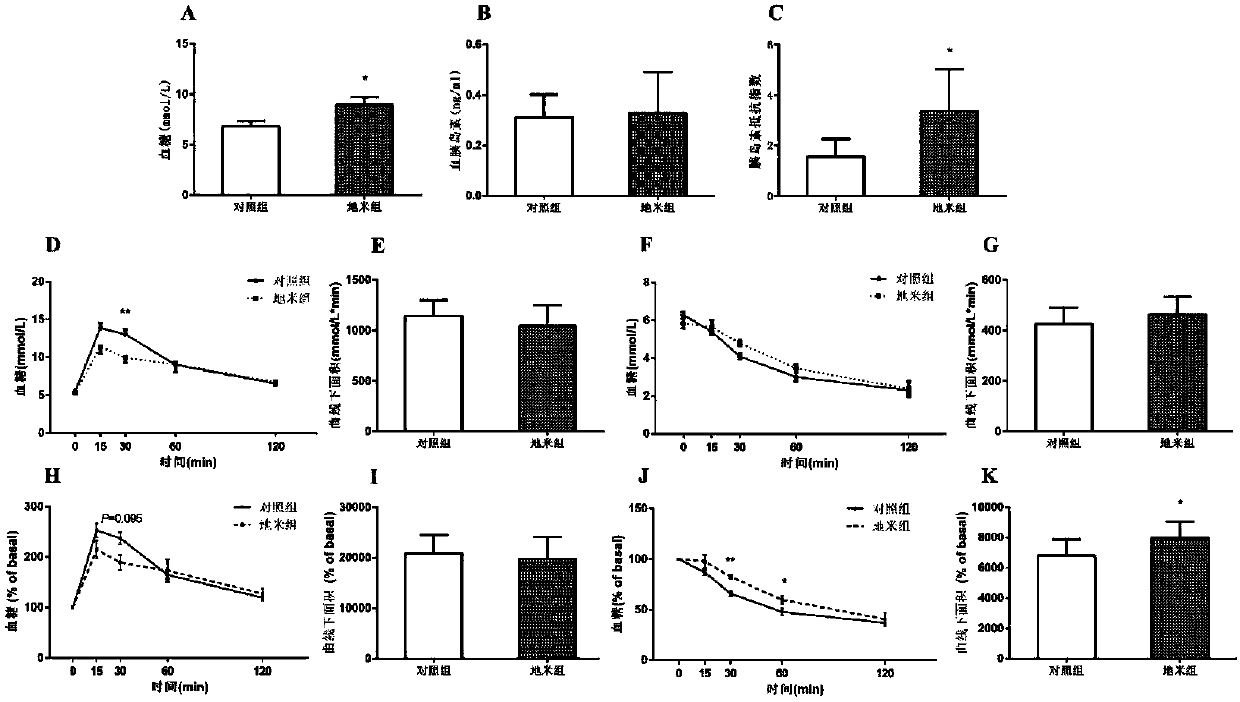
Fetal-origin metabolic syndrome animal model building method and application thereof
ActiveCN107773566AModeling method is simpleGood indicator stabilityOrganic active ingredientsMetabolism disorderDrugMolecular targets
The invention relates to a fetal-origin metabolic syndrome animal model building method and application thereof. A fetal-origin metabolic syndrome animal model is built by performing subcutaneous treatment of 0.1-2.0 mg / kg of hexamethasone per day on rodents 9-20 days after pregnancy and normally breeding baby rodents until 28-week-old and can present typical characteristics similar to those of metabolic syndromes of human. The built fetal-origin metabolic syndrome animal model is novel, reliable, simple and convenient and is significant to guiding reasonable clinical drug use during pregnancy, exploring action mechanism and molecular targets of fetal-origin metabolic syndrome, screening intrauterine environment disturbing chemicals and drugs and the like.
Owner:WUHAN UNIV
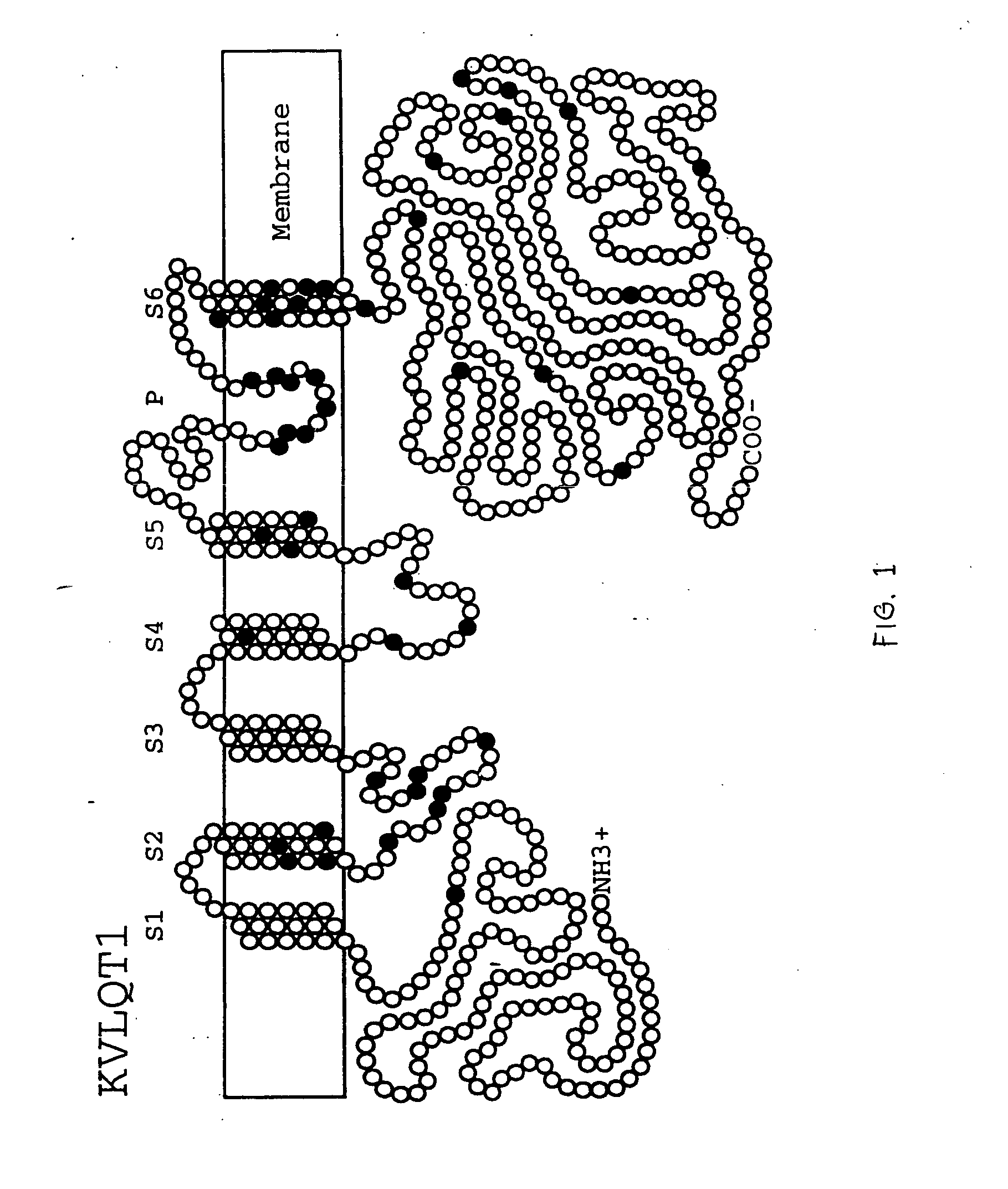
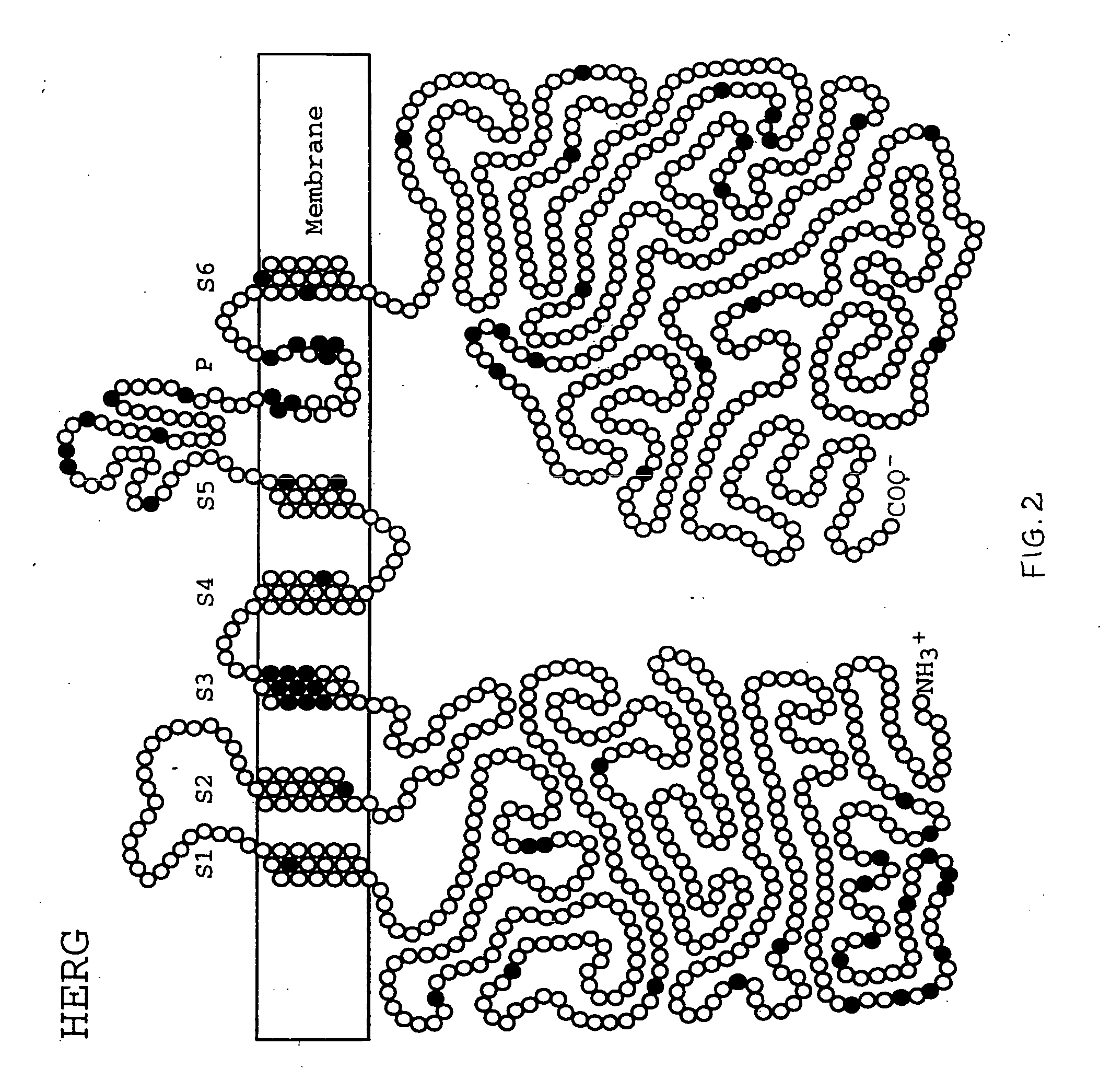
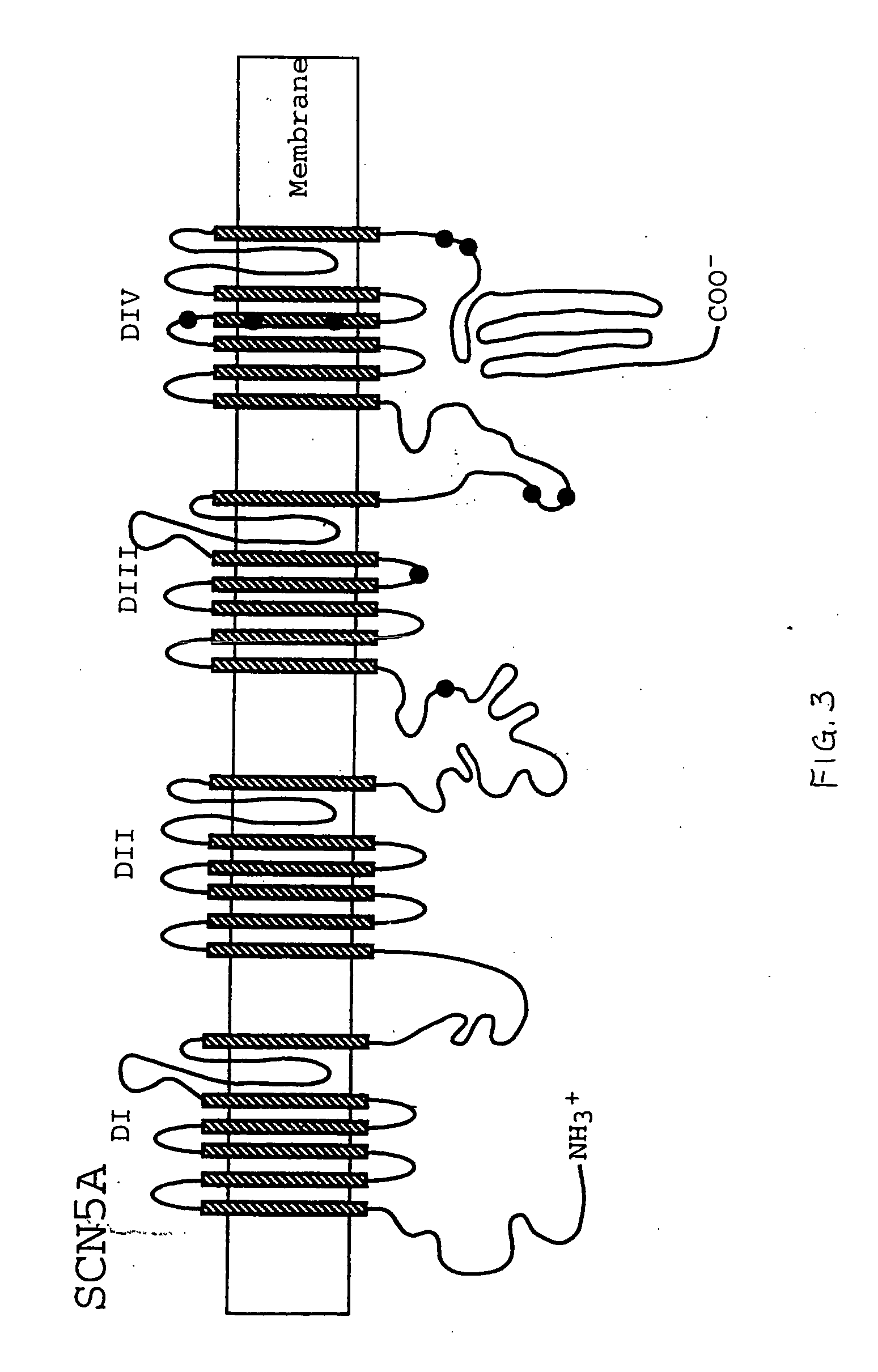
Alterations in the long QT syndrome genes KVLQT1 and SCN5A and methods for detecting same
Long QT Syndrome (LQTS) is a cardiovascular disorder characterized by prolongation of the QT interval on electrocardiogram and presence of syncope, seizures and sudden death. Five genes have been implicated in Romano-Ward syndrome, the autosomal dominant form of LQTS. These genes are KVLQT1, HERG, SCN5A, KCNE1 and KCNE2. Mutations in KVLQT1 and KCNE1 also cause the Jervell and Lange-Nielsen syndrome, a form of LQTS associated with deafness, a phenotypic abnormality inherited in an autosomal recessive fashion. Mutational analyses were used to screen 262 unrelated individuals with LQTS for mutations in the five defined genes. A total of 134 mutations were observed of which eighty were novel.
Owner:UNIV OF UTAH RES FOUND
Nutritional compositions for promoting gut barrier function and ameliorating visceral pain
ActiveUS20170128500A1Promote regenerationSupporting gut barrier resistancePeptide/protein ingredientsAntipyreticPresent methodVisceral pain
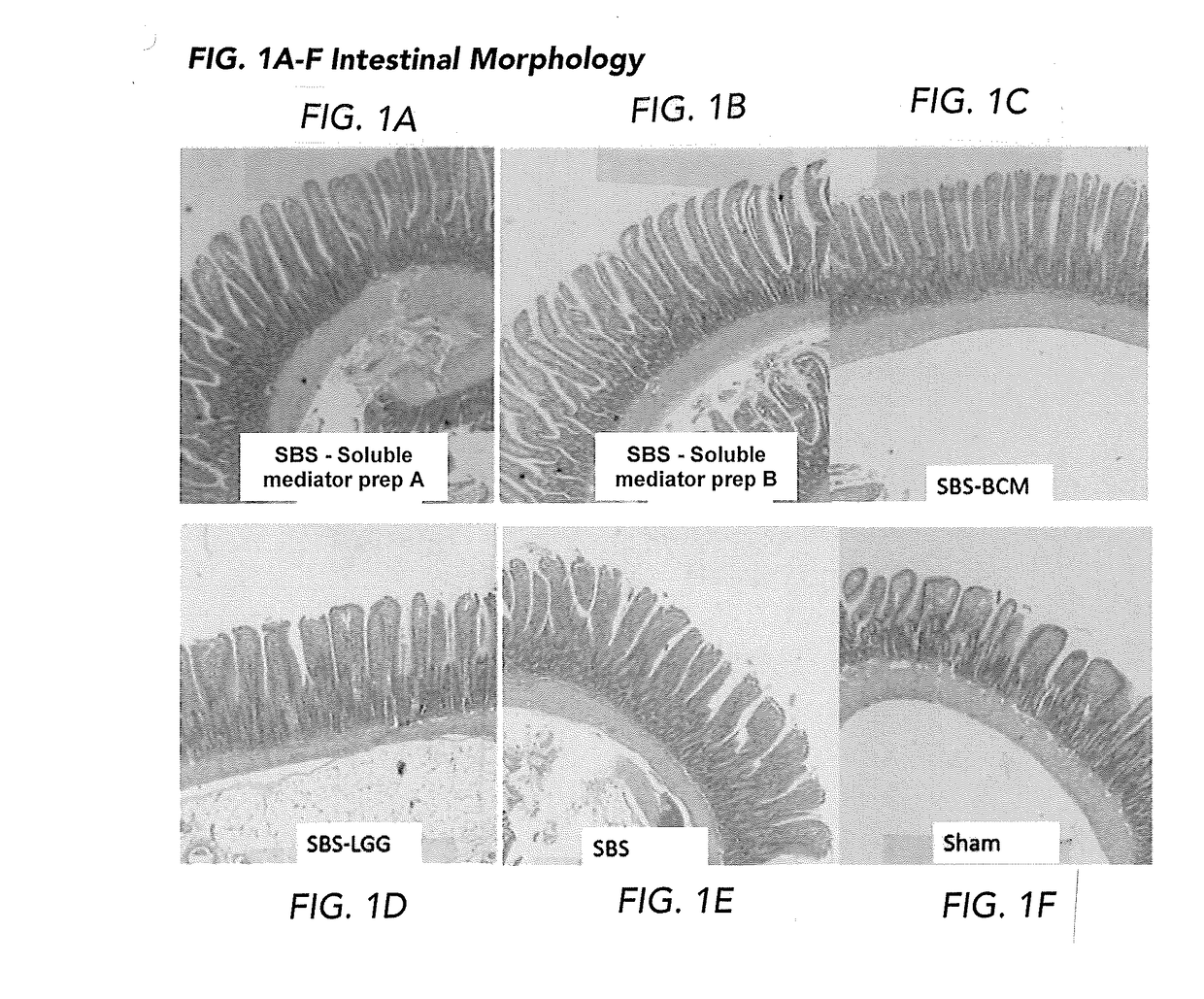
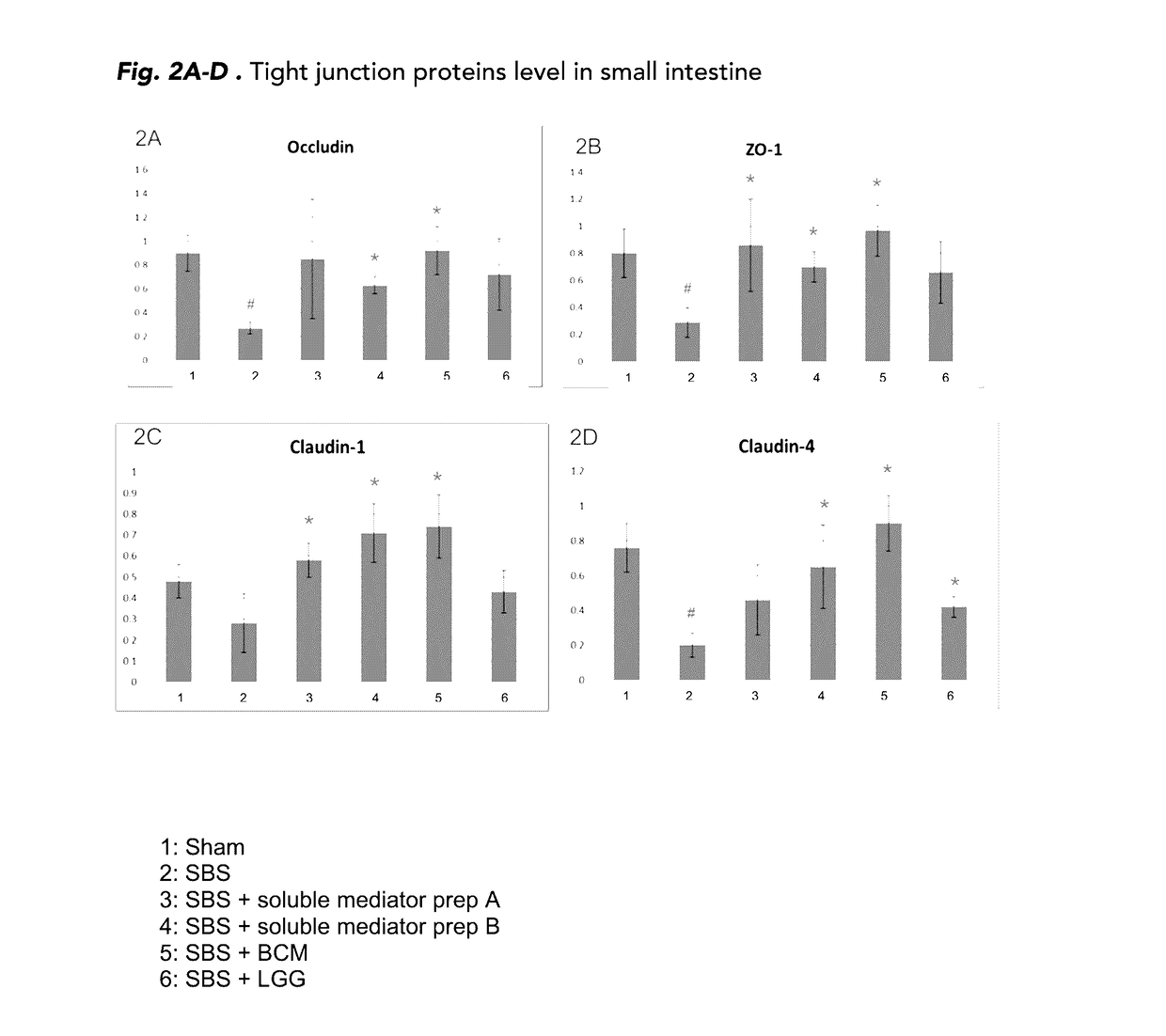
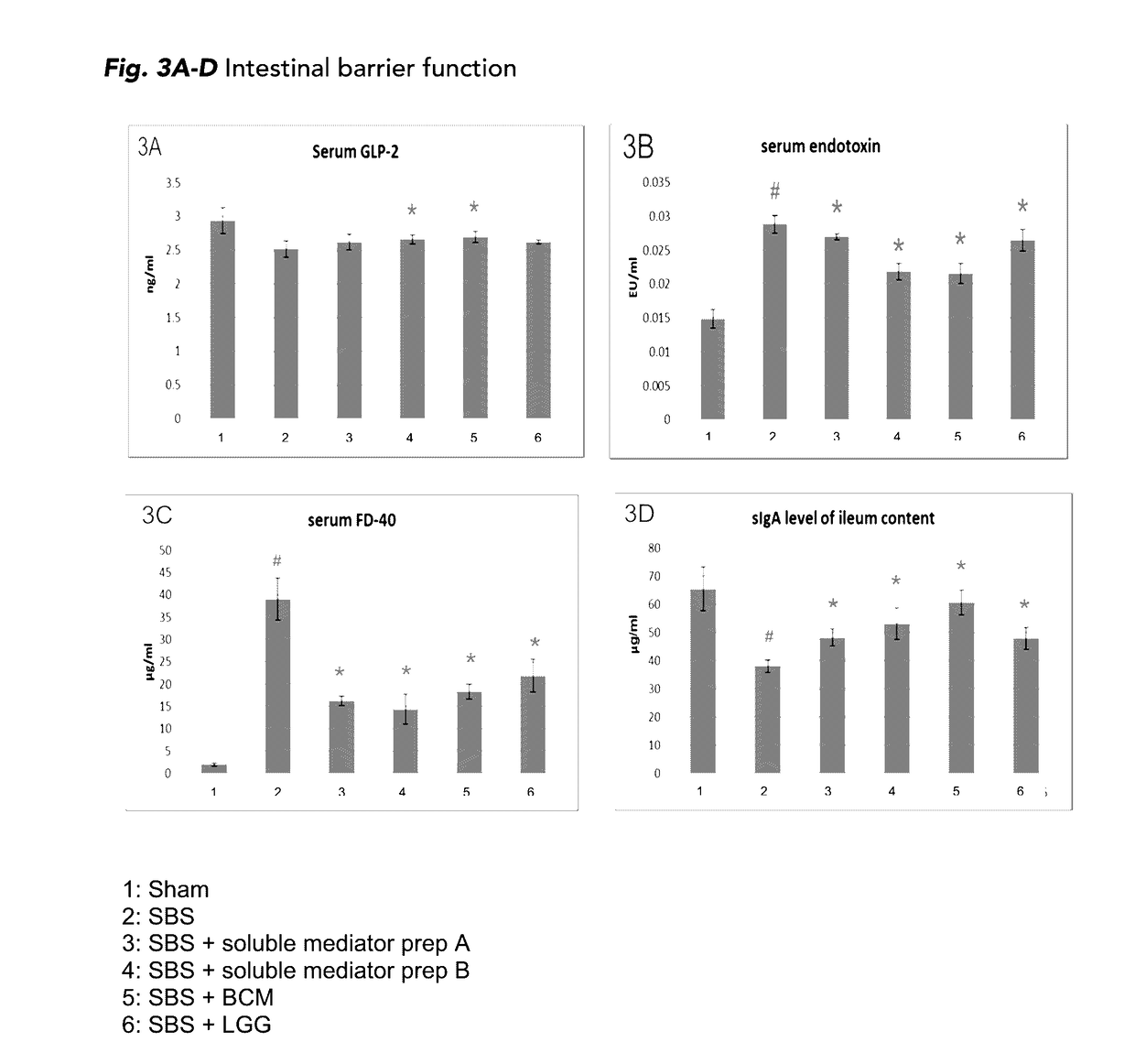
The present disclosure is directed to methods for i) promoting gut regeneration, ii) promoting gut maturation and / or adaptation, iii) supporting gut barrier resistance and / or iv) protecting gut barrier function in a pediatric subject comprising administering to the pediatric subject a composition comprising an effective amount of a soluble mediator preparation derived from a late-exponential growth phase of a probiotic batch-cultivation process, such as Lactobacillus rhamnosus GG (LGG). The present methods advantageously provide the gut-protection benefits of LGG soluble mediators without introducing live bacterial culture to individuals with impaired gut barrier function. In some embodiments, the pediatric subject has impaired gut barrier function and / or short bowel syndrome. The present disclosure in other embodiments, directed methods for reducing visceral pain in a pediatric subject comprising administering to the pediatric subject a composition comprising an effective amount of a soluble mediator preparation from a late-exponential growth phase of a probiotic batch-cultivation process, such as LGG.
Owner:MEAD JOHNSON NUTRITION
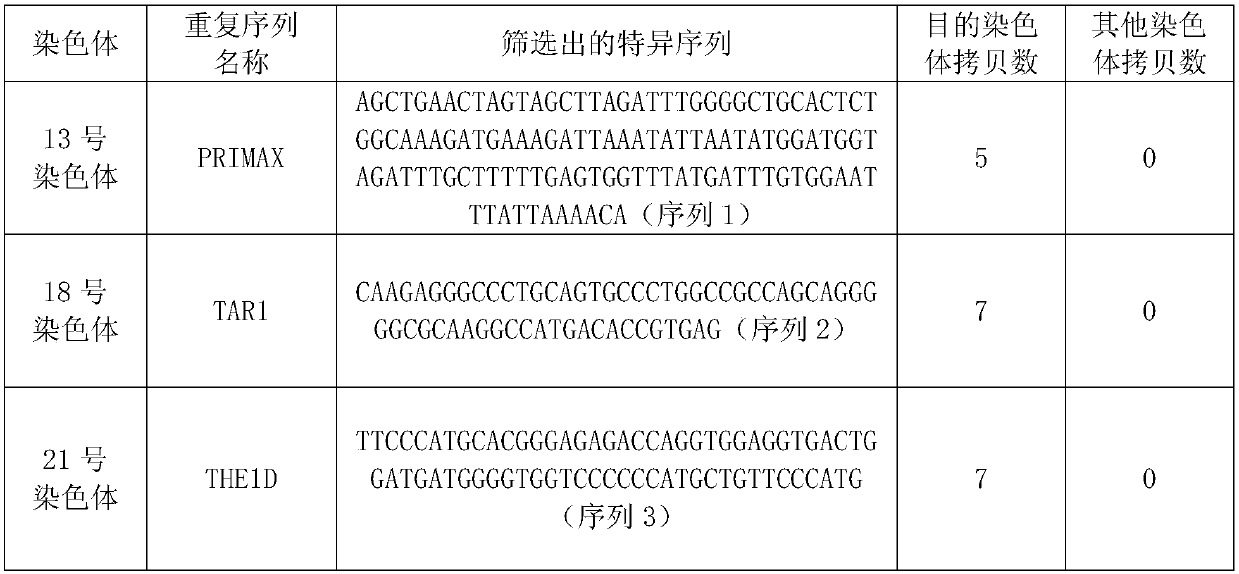
Kit for non-invasive prenatal screening for trisomy syndrome and application thereof
ActiveCN109694907ALow costShort detection cycleMicrobiological testing/measurementDNA/RNA fragmentationTrisomy E SyndromeTrisomy 13 Syndrome
The invention discloses a kit for non-invasive prenatal screening for trisomy syndrome and an application thereof. The kit of the present invention includes a primer pair and a probe for prenatal screening of trisomy 13 syndrome, a primer pair for prenatal screening of trisomy 18 syndrome, and a primer pair and a probe for prenatal screening of trisomy 21 syndrome. The invention also discloses a non-invasive prenatal screening method for trisomy syndrome. Compared with a second-generation sequencing method, the screening method has the advantages of low cost, short detection period, high detection sensitivity, convenience and absolute quantification.
Owner:SHENZHEN HUADA GENE INST
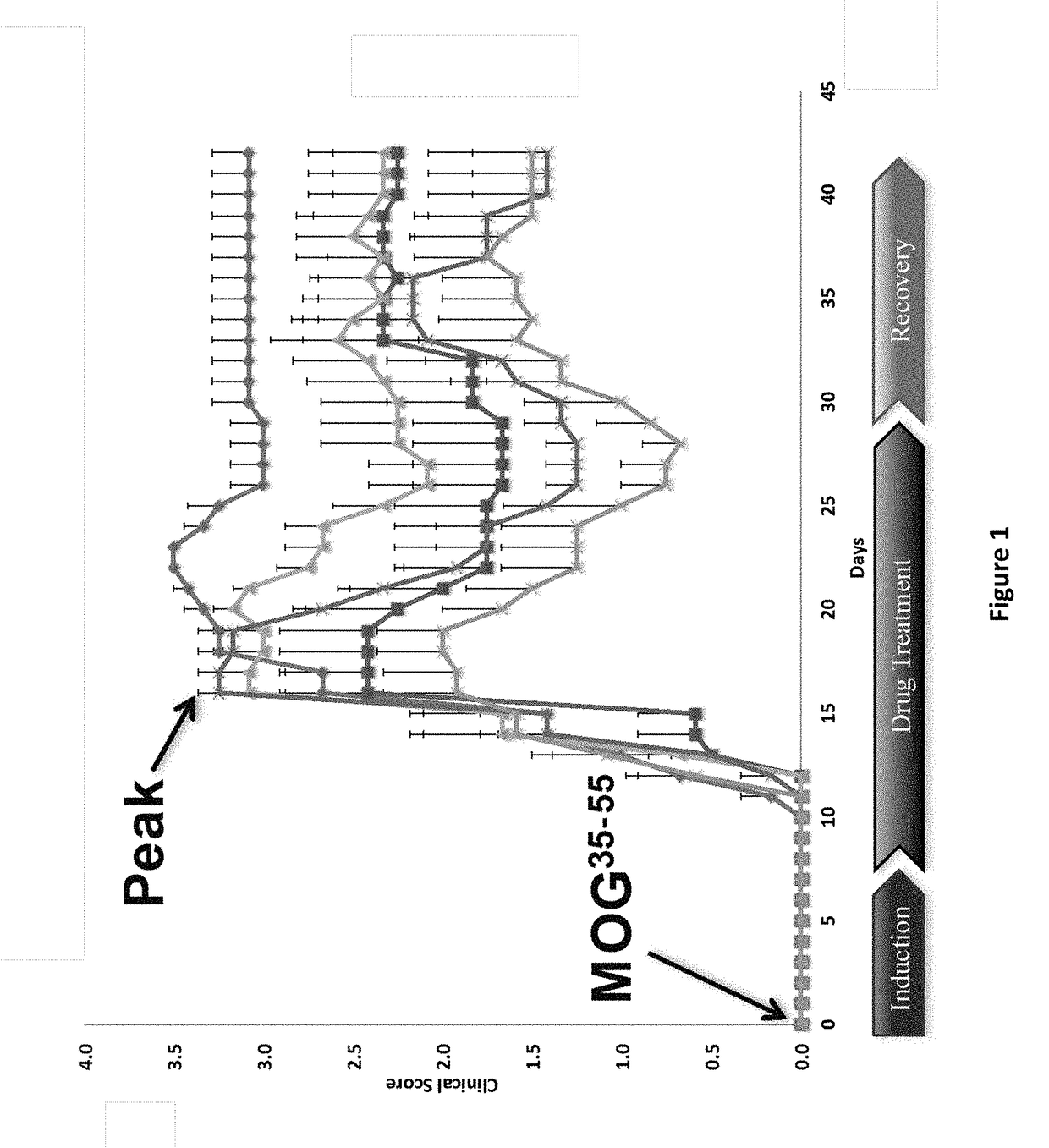
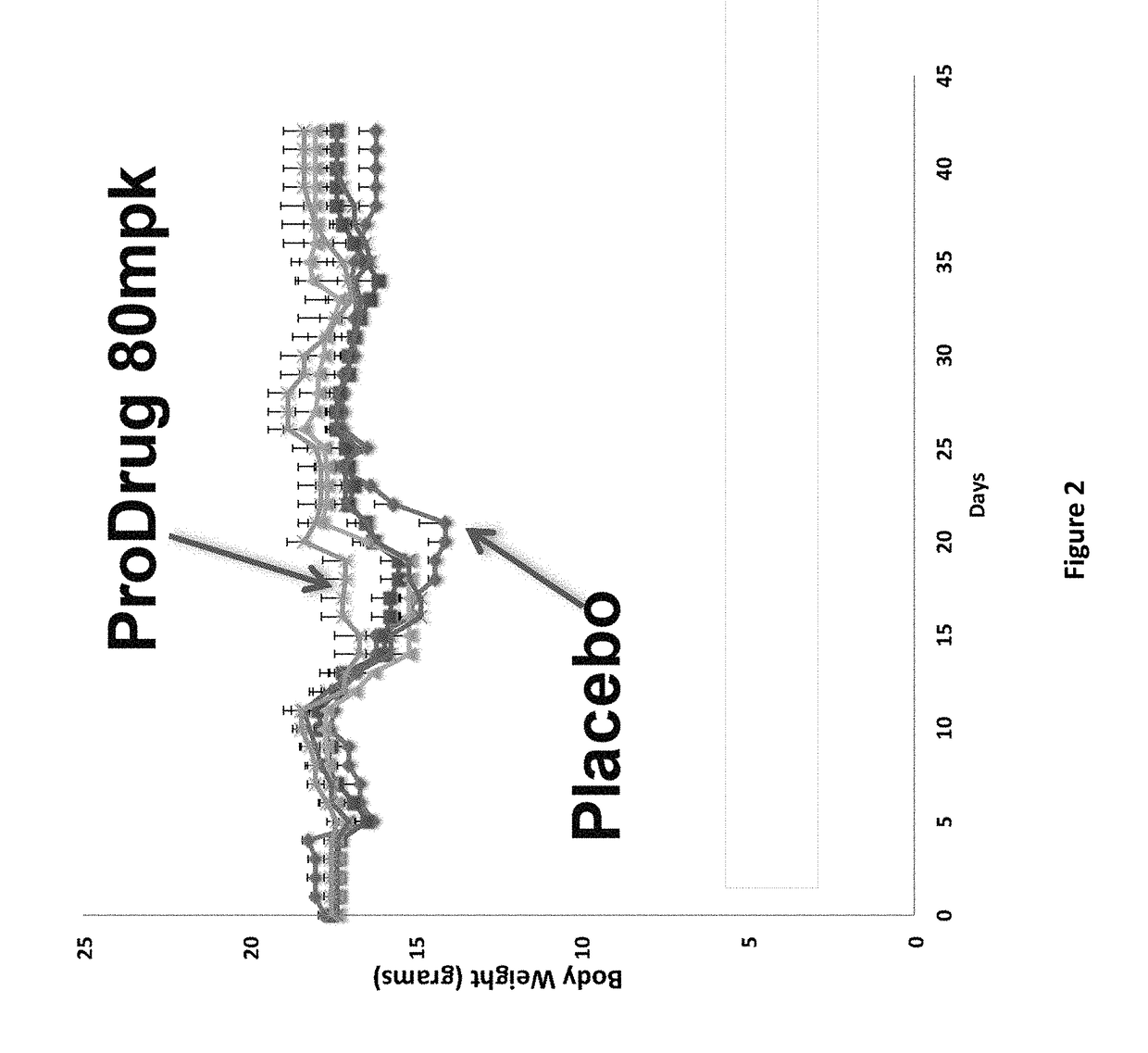
DNP and DNP Prodrug Treatment of Neuromuscular, Neurodegenerative, Autoimmune, Developmental, Traumatic Brain Injury, Concussion, Dry Eye Disease, Hearing Loss and/or Metabolic Diseases
ActiveUS20170252347A1Good blood pressureImprove blood sugar levelsSenses disorderNervous disorderHL - Hearing lossDepressant
A composition and method of treatment of neuromuscular, neuromuscular degenerative, neurodegenerative, autoimmune, developmental, traumatic, hearing loss related, and / or metabolic diseases, including spinal muscular atrophy (SMA) syndrome (SMA1, SMA2, SMA3, and SMA4, also called Type I, II, III and IV), traumatic brain injury (TBI), concussion, keratoconjunctivitis sicca (Dry Eye Disease), glaucoma, Sjogren's syndrome, rheumatoid arthritis, post-LASIK surgery, anti-depressants use, Wolfram Syndrome, and Wolcott-Rallison syndrome. The composition is selected from the group consisting of 2,3-DNP, 2,4-DNP, 2,5-DNP, 2,6-DNP, 3,4-DNP, or 3,5-DNP, bipartite 2,3-dinitrophenol, 2,4-dinitrophenol, 2,5-dinitrophenol, 2,6-dinitrophenol, 3,4-dinitrophenol, or 3,5-dinitrophenol (2,3-DNP, 2,4-DNP, 2,5-DNP, 2,6-DNP, 3,4-DNP, or 3,5-DNP) prodrugs; Gemini prodrugs, bioprecursor molecules, and combinations thereof. A dose of the composition for treatment of neurodegenerative diseases may be from about 0.01 mg / kg of body weight to about 50 mg / kg of body weight of the patient in need of treatment. A dose of the composition for treatment of metabolic diseases may be from about 1 mg / 70 kg of body weight to about 100 mg / 70 kg of body weight of the patient in need of treatment, and a maximum dose per day is about 200 mg / 70 kg of body weight of the patient in need of treatment.
Owner:MITOCHON PHARMA INC +1
Multi-site screening kit for Marfan syndrome
PendingCN109666734AReliable specificityBright futureMicrobiological testing/measurementDNA/RNA fragmentationBiologyMutation type
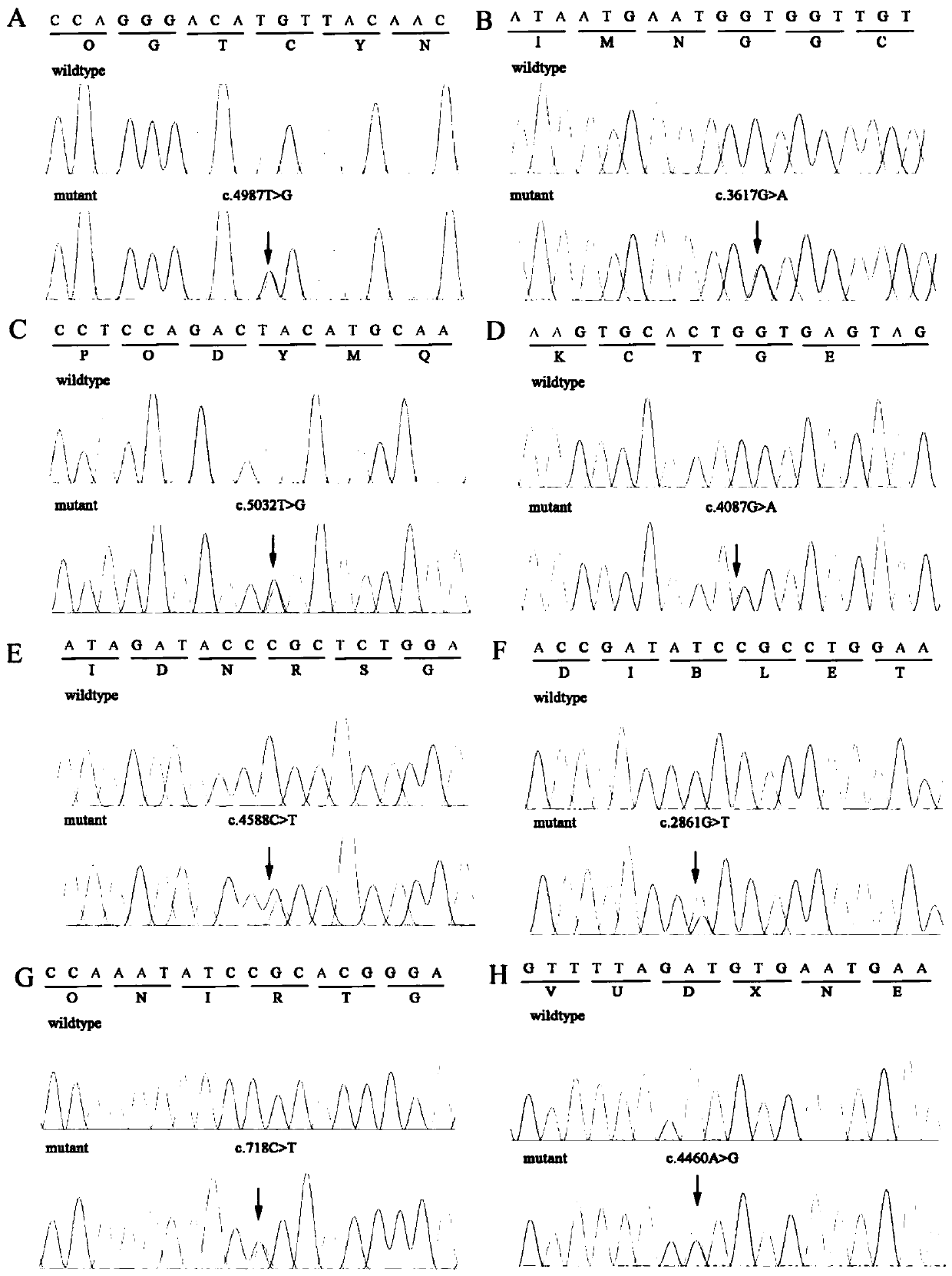
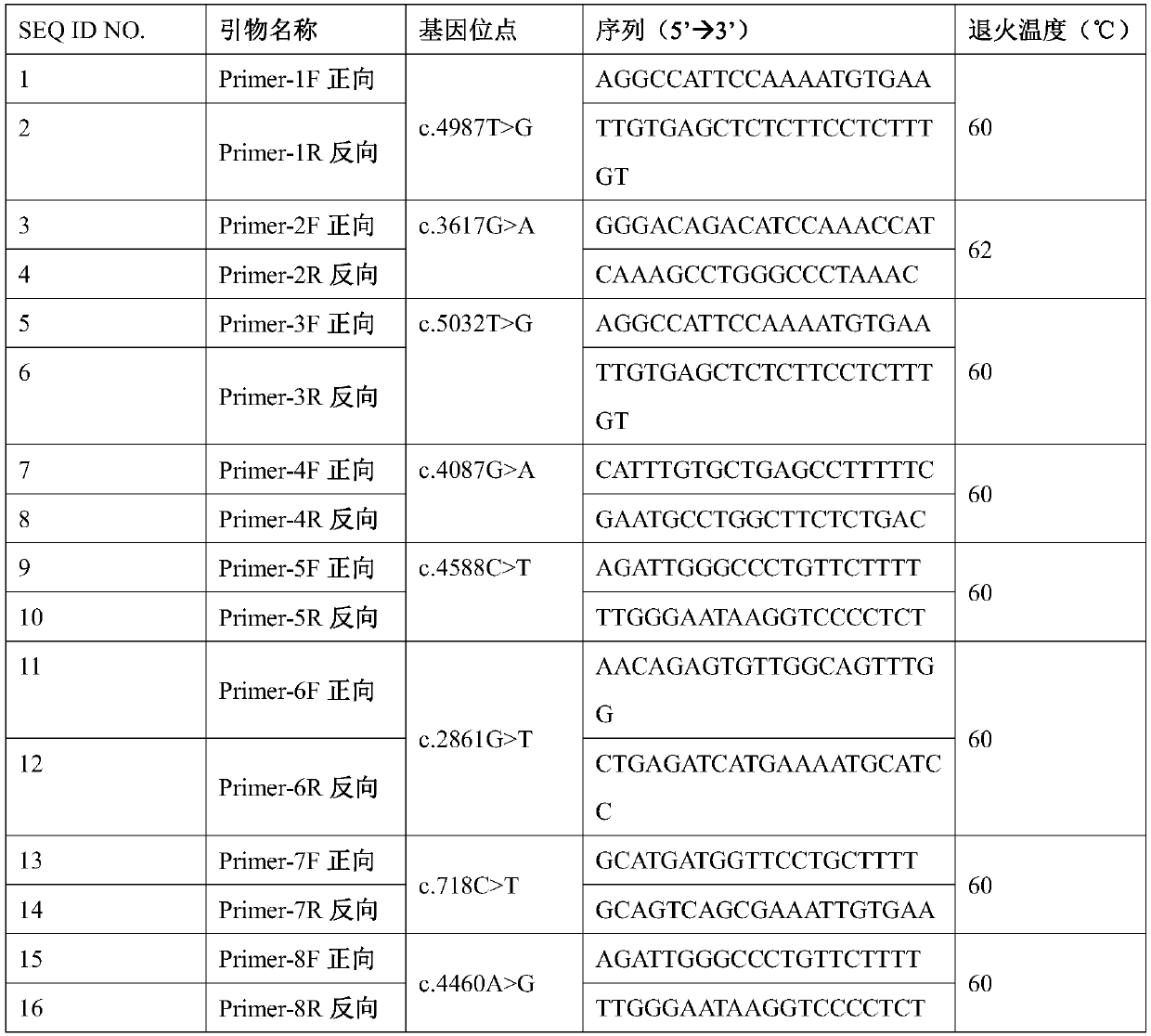
The invention provides a mutant gene. The mutant gene is characterized in that the mutant gene is obtained through at least one mutation type of the following eight types comprising c. 4987T>G, c. 3617G>A, c. 5032T>G, c. 4087G>A, c. 4588C>T, c. 2861G>T, c. 718C>T and c. 4460A>G based on a human FBN1 gene. The invention further provides application of a related reagent used for detecting the mutation sites of the c. 4987T>G, the c. 3617G>A, the c. 5032T>G, the c. 4087G>A, the c. 4588C>T, the c. 2861G>T, c. 718C>T and / or the c. 4460A>G of the human FBN1 gene in preparation of a screening reagentfor the Marfan syndrome. The mutation of the sites of the c. 4987T>G, the c. 3617G>A, the c. 5032T>G, the c. 4087G>A, the c. 4588C>T, the c. 2861G>T, c. 718C>T and / or the c. 4460A>G of the human FBN1gene is applied to preparation of the auxiliary diagnostic kit for the Marfan syndrome, and the screening purpose can be achieved.
Owner:SICHUAN PROVINCIAL PEOPLES HOSPITAL
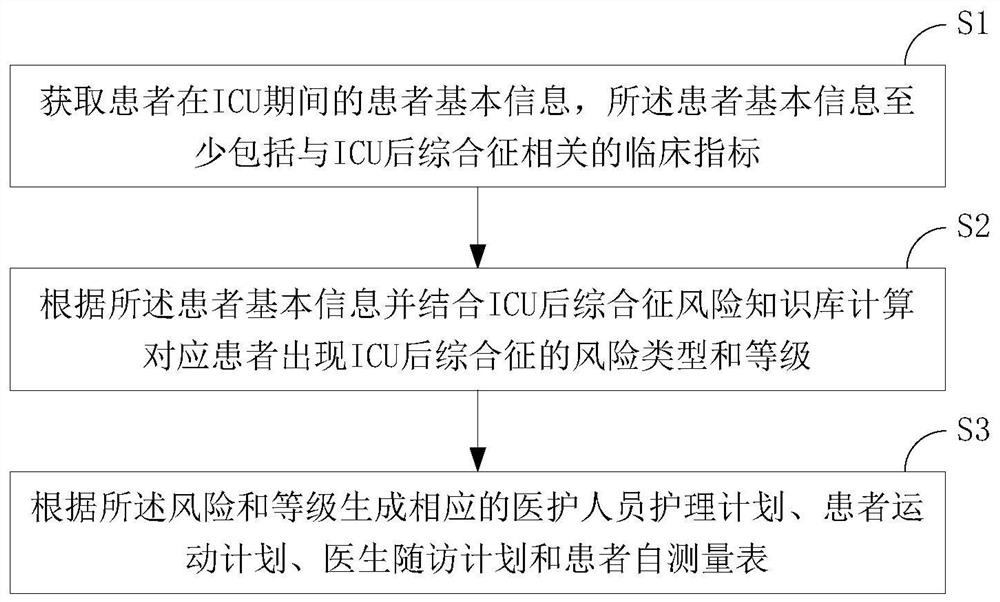
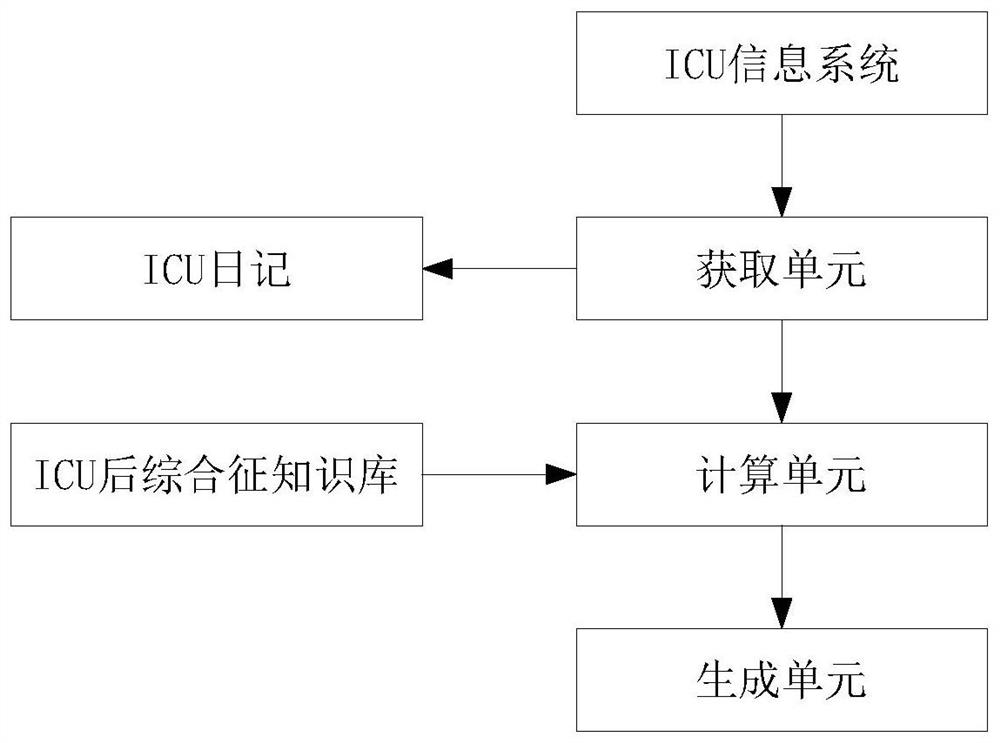
Follow-up visit method and system for post-ICU syndrome
PendingCN113035296ARaise attentionReduce the chance of occurrenceMedical communicationHealth-index calculationInformation processingNursing care
The invention relates to the technical field of medical information processing, aims to solve the problems of high human resource consumption and no pertinence in the existing post-ICU syndrome intervention guidance process, and provides a follow-up visit method and system for post-ICU syndrome, and the main scheme comprises the following steps: obtaining patient basic information of a patient during an ICU period, wherein the basic information of the patient at least comprises clinical indexes related to the post-ICU syndrome; according to the basic information of the patient and in combination with a post-ICU syndrome risk knowledge base, calculating the risk type and grade of the post-ICU syndrome of the corresponding patient, and according to the risk and grade, generating a corresponding medical staff nursing plan, a patient movement plan, a doctor follow-up plan and a patient self-measurement table. According to the invention, the investment of human resources is reduced, the pertinence of rehabilitation guidance is improved, and the method and system are suitable for follow-up visit of post-ICU syndromes.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com