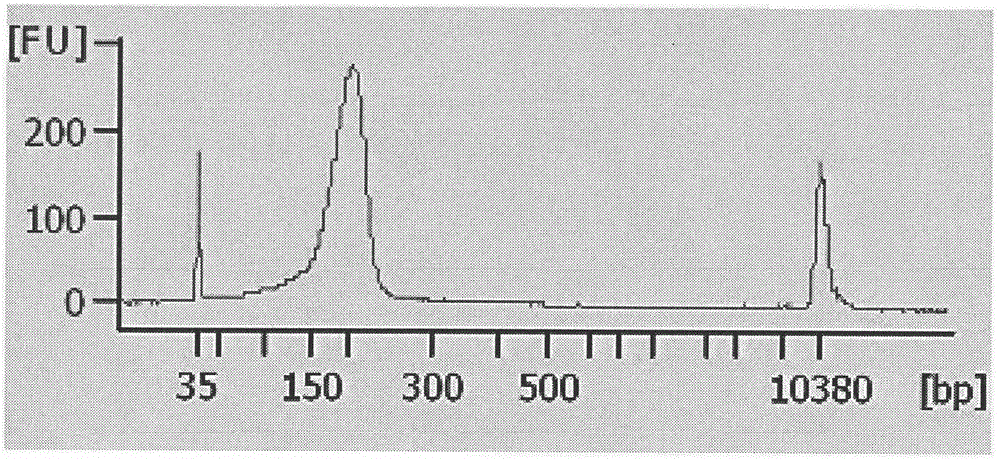
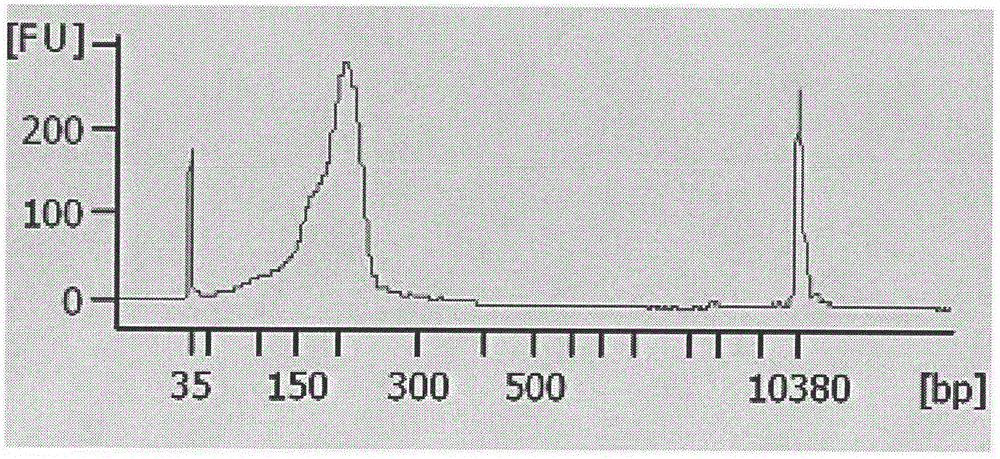
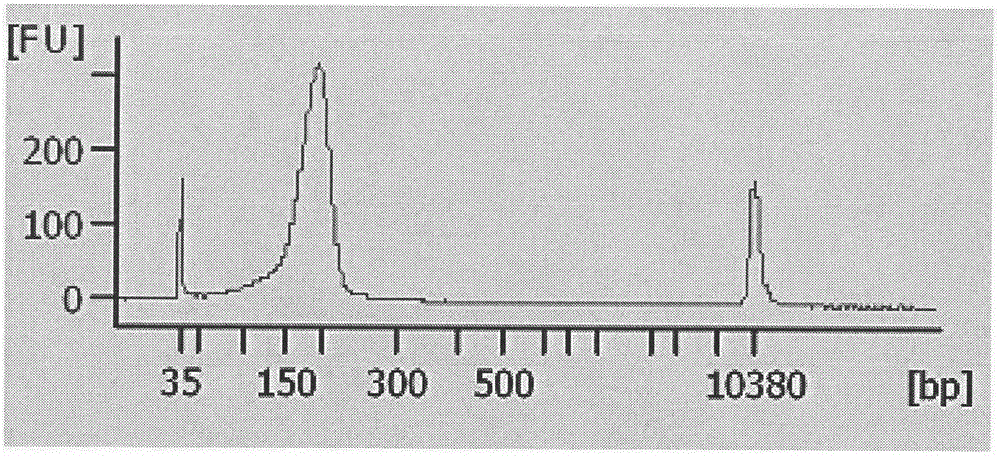
Noninvasive fetus 21, 18 and 13 trisomic syndrome antenatal detection positive quality control product and preparing method thereof
A technology of trisomy and positive quality control products, which is applied in the direction of biochemical equipment and methods, microbial determination/testing, etc., can solve problems affecting the accuracy and reliability of chromosomal diseases, and no positive quality control products are provided. Achieve the effect of easy storage and avoid deviation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: the preparation of positive quality control product
[0027] 1. Mixed plasma: Take 40 mL of plasma from 5 normal non-pregnant women and mix to obtain 200 mL of mixed plasma.
[0028] 2. Extract plasma free DNA and measure its concentration: Take 600 μL of mixed plasma for DNA extraction, set up 3 replicates, use Qubit for quantification, and calculate the concentration of free DNA in plasma. The results are shown in Table 1.
[0029] Table 1 Plasma free DNA concentration
[0030]
[0031] 3. Trisomy 21, Trisomy 18, and Trisomy 13 fetal abortion tissue DNA extraction and concentration determination: Qiagen 69504 DNeasy Blood&Tissue Kit was used to extract the DNA of each abortion tissue, and Qubit 2.0 and dsDNA HS Assay Kit were used to determine the concentration and Nanodrop200C to determine the OD 260 / OD 280 , the results are shown in Table 2.
[0032] Table 2 Tissue DNA Extraction and Measurement Results
[0033]
[0034] 4. DNA interruption:...
Embodiment 2
[0046] Embodiment 2: The impact of the initial amount of positive quality control product detection on detection accuracy and stability
[0047] The preparation method of the positive quality control product is the same as in Example 1.
[0048] Take 3ng, 30ng, and 300ng of positive quality control products, respectively, and use fetal chromosome aneuploidy trisomy 21, 18 and 13 detection kits (semiconductor sequencing method) to detect.
[0049] As can be seen from Table 6, all the Z values of the positive quality control products of the three gradient concentrations of 3ng, 30ng, and 300ng are greater than 3.00. Trisomy 13 is positive, indicating that the positive quality control products in the detection range of the three concentration gradients can be used for non-invasive prenatal detection of fetal chromosomal aneuploidy 21, 18, and 13 trisomy.
[0050] Table 6 Difference experiment between bottles
[0051]
[0052] Table 7 Matching kit detection coincidence rate...
Embodiment 3
[0054] Example 3: Accuracy of the non-invasive prenatal detection method for aneuploid chromosomes of positive quality control products
[0055] The preparation method of the positive quality control product is the same as in Example 1.
[0056] 20 cases of plasma samples from pregnant women were divided into two groups and tested with positive quality control products, the results are shown in Table 8.1 and 8.2 ( Figure 5 and Figure 6 ).
[0057] It can be seen from Table 8.1 and 8.2 that the actual test results of the 20 groups of samples are completely consistent with the control results, and the coincidence rate reaches 100%. This shows that the positive quality control product of the present invention can be used for the quality control of trisomy 21, trisomy 18 and trisomy 13 detection kits, and the detection results and methods are accurate and reliable.
[0058] Table 8.1 Positive quality control and plasma cell-free DNA test results of pregnant women
[0059] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com