Patents
Literature
60 results about "Rodent model" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
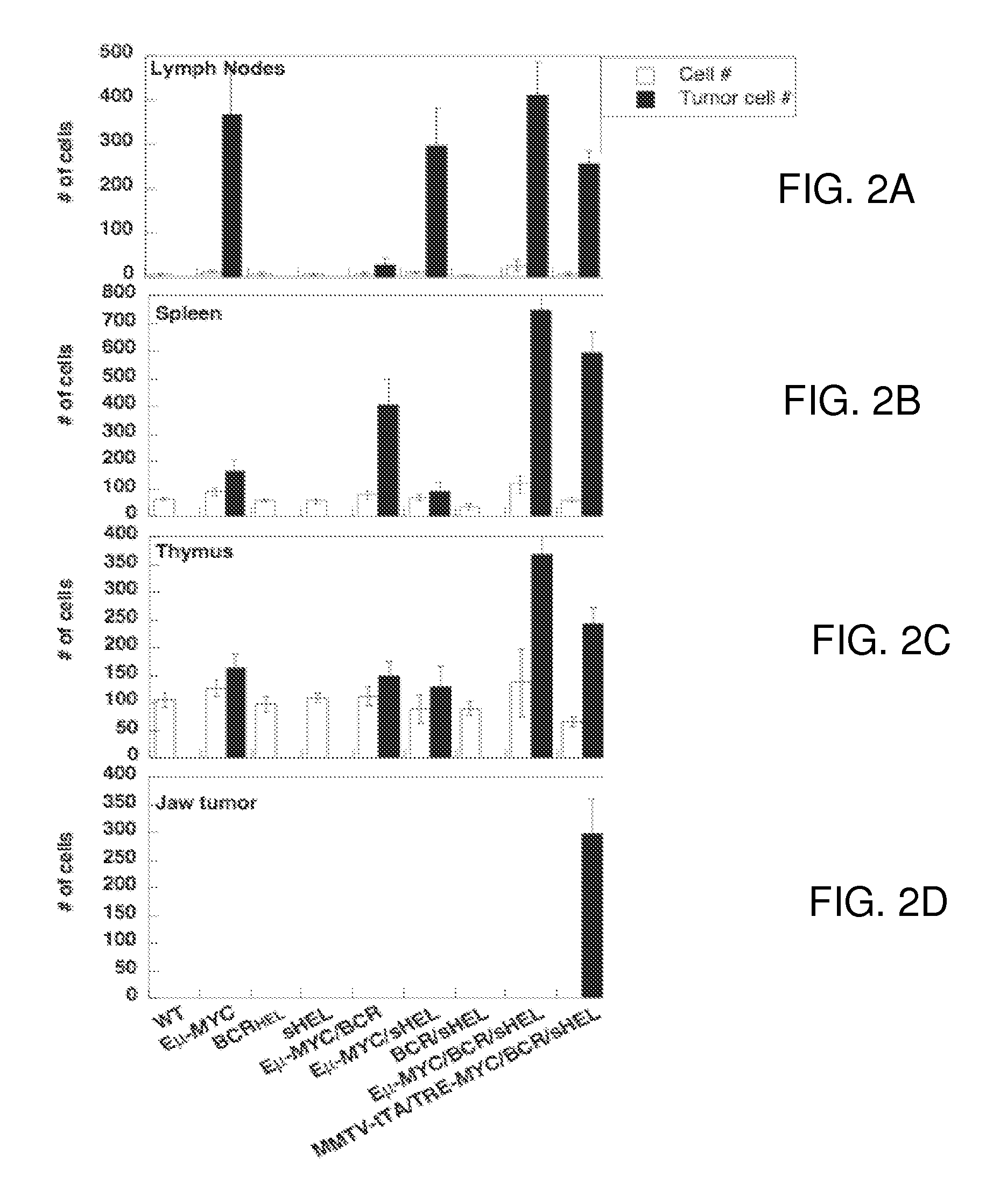
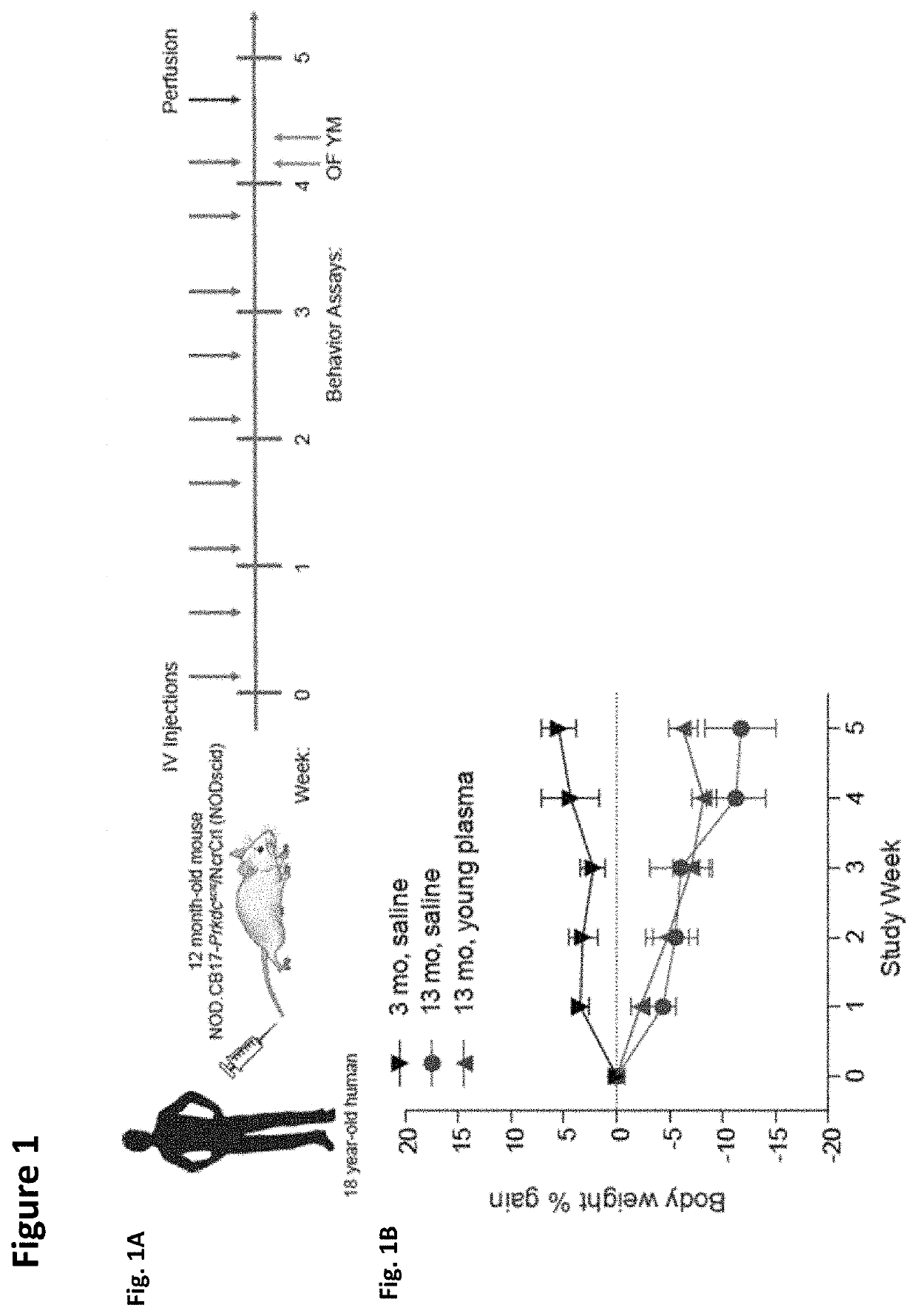
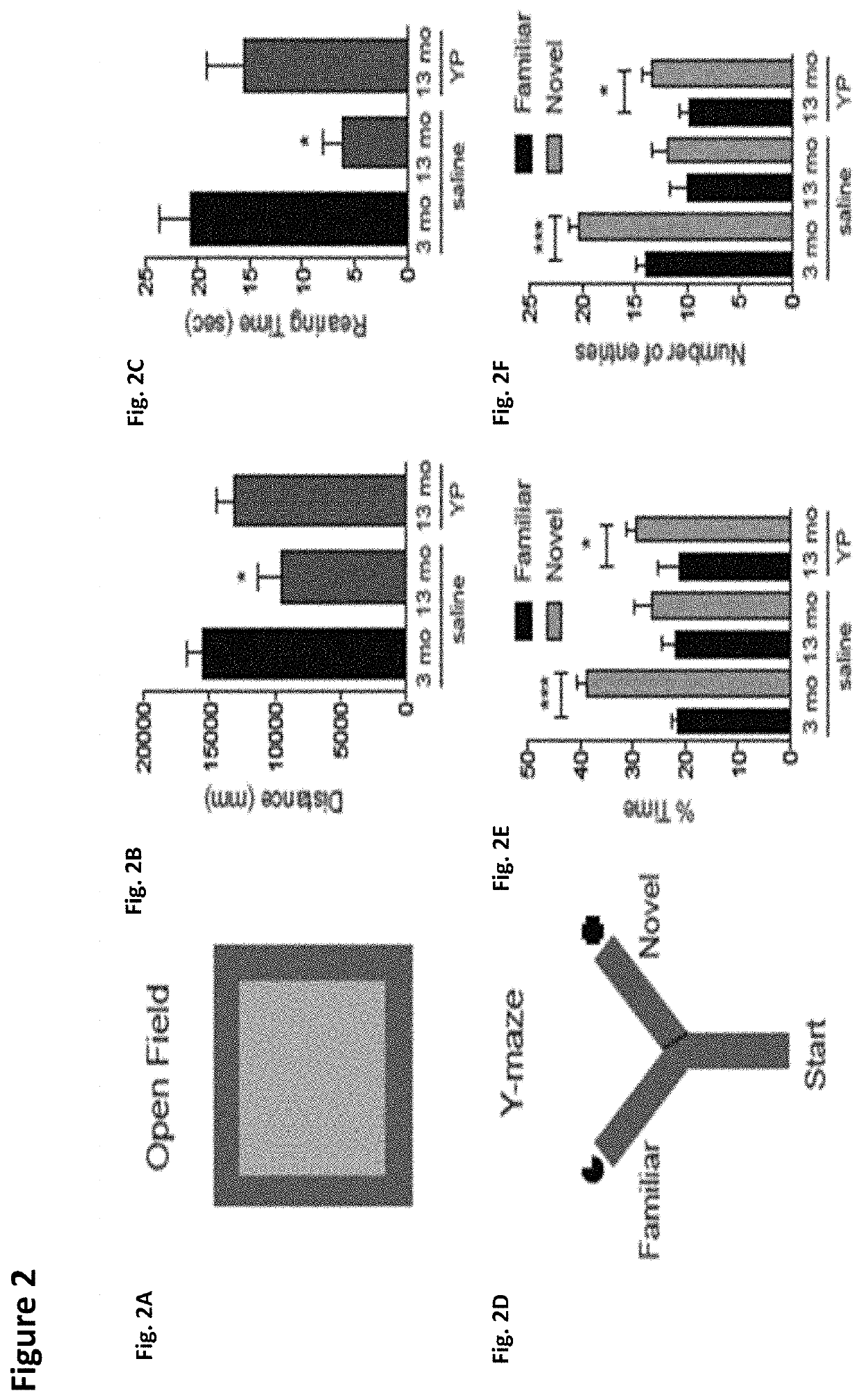
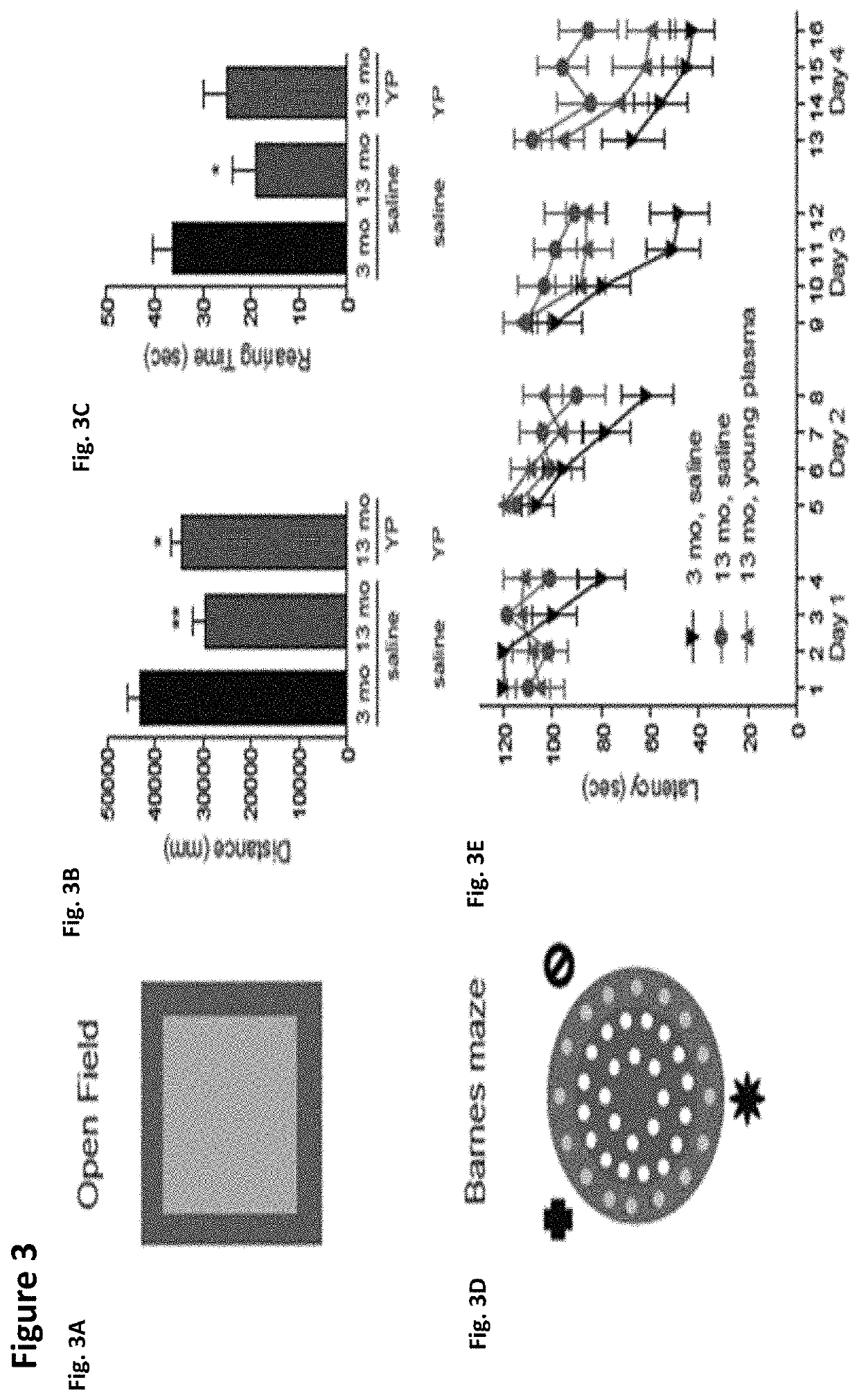
Methods for screening human blood products comprising plasma using immunocompromised rodent models
ActiveUS20170232118A1Compounds screening/testingMammal material medical ingredientsNeurogenesisBlood plasma
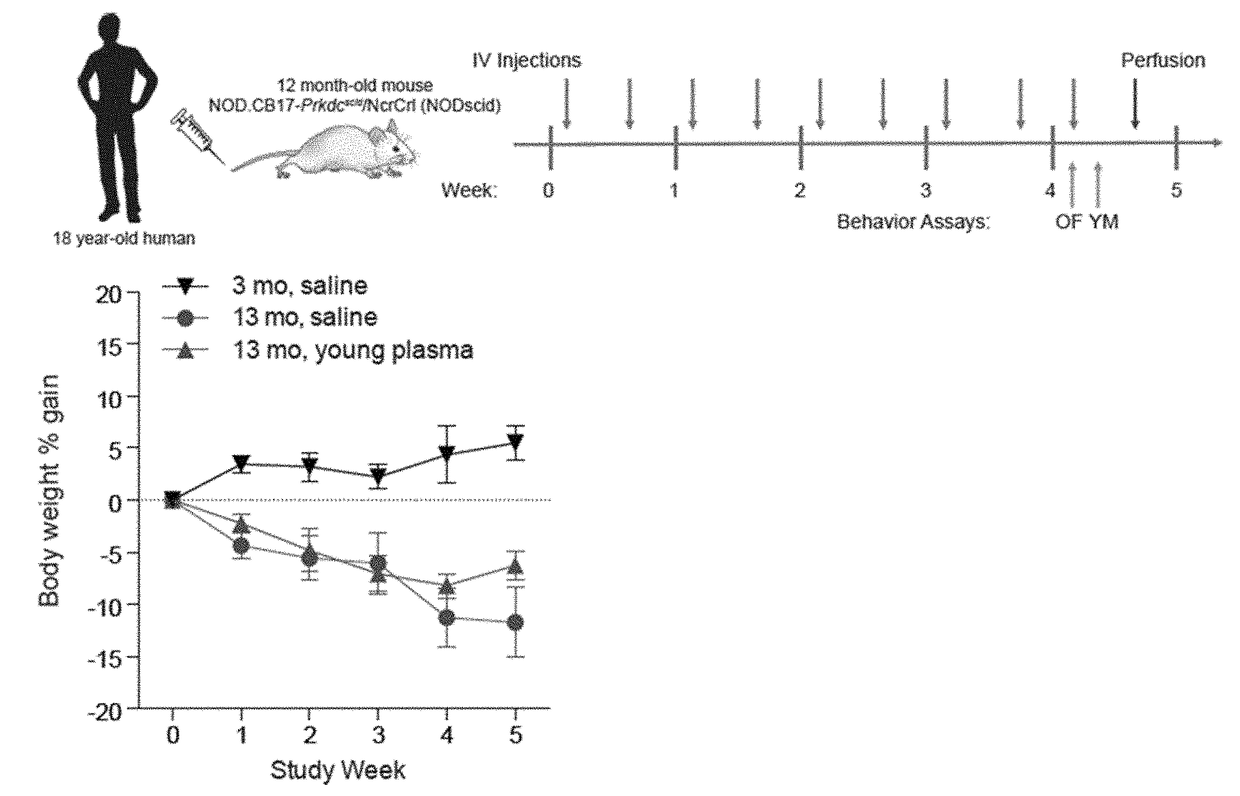
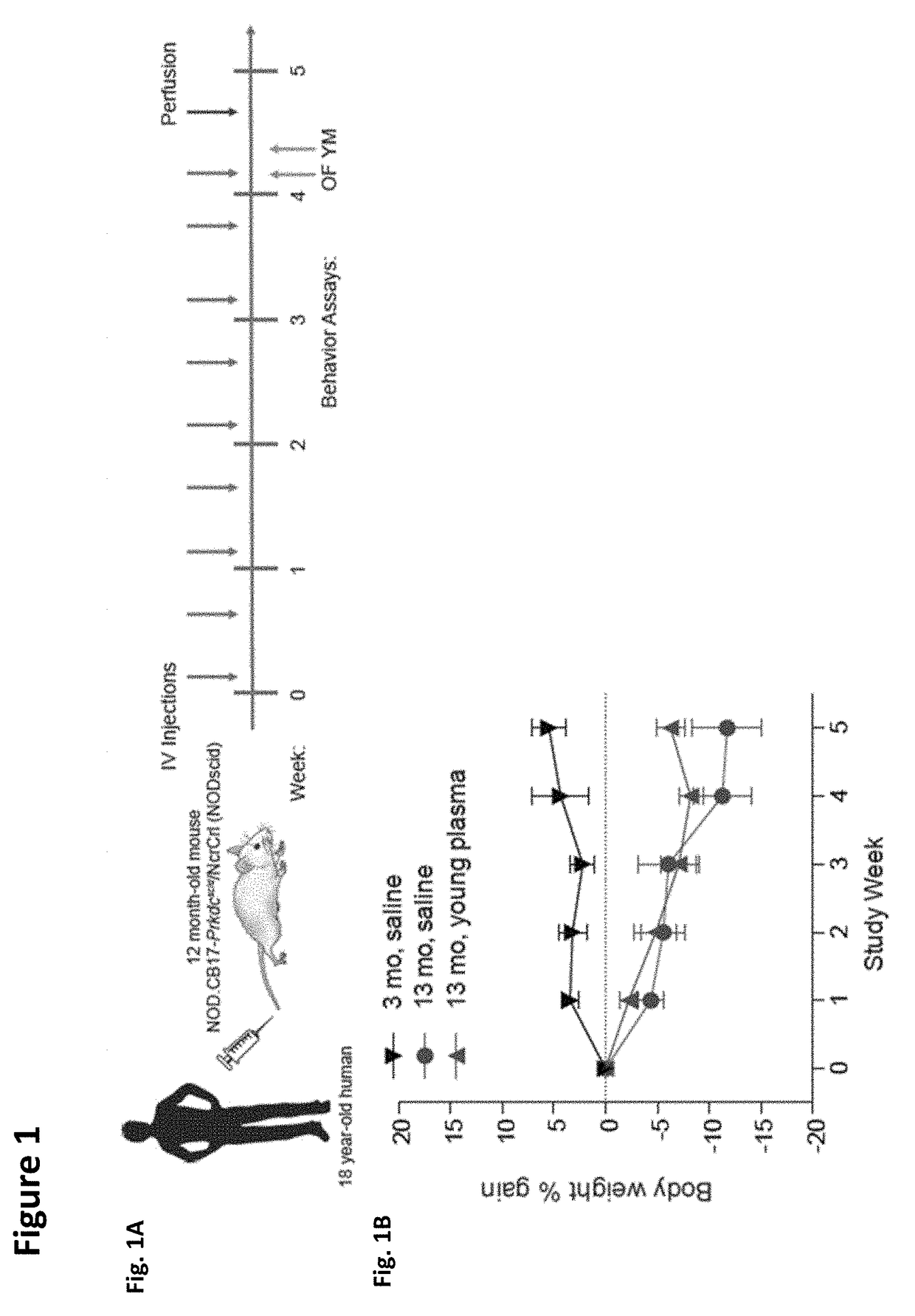
Methods and compositions are provided for screening candidate compositions for activity with respect to treatment of aging-associated conditions, e.g., cognitive impairment conditions or age-related dementia. Aspects of the methods include administering a human blood product comprising plasma, to an immunocompromised animal, e.g., a mouse or a rat, and measuring the effect of the product on an endpoint, e.g., neurogenesis, locomotor activity, anxiety, spatial memory, and hippocampus-dependent memory.
Owner:ALKAHEST INC +2
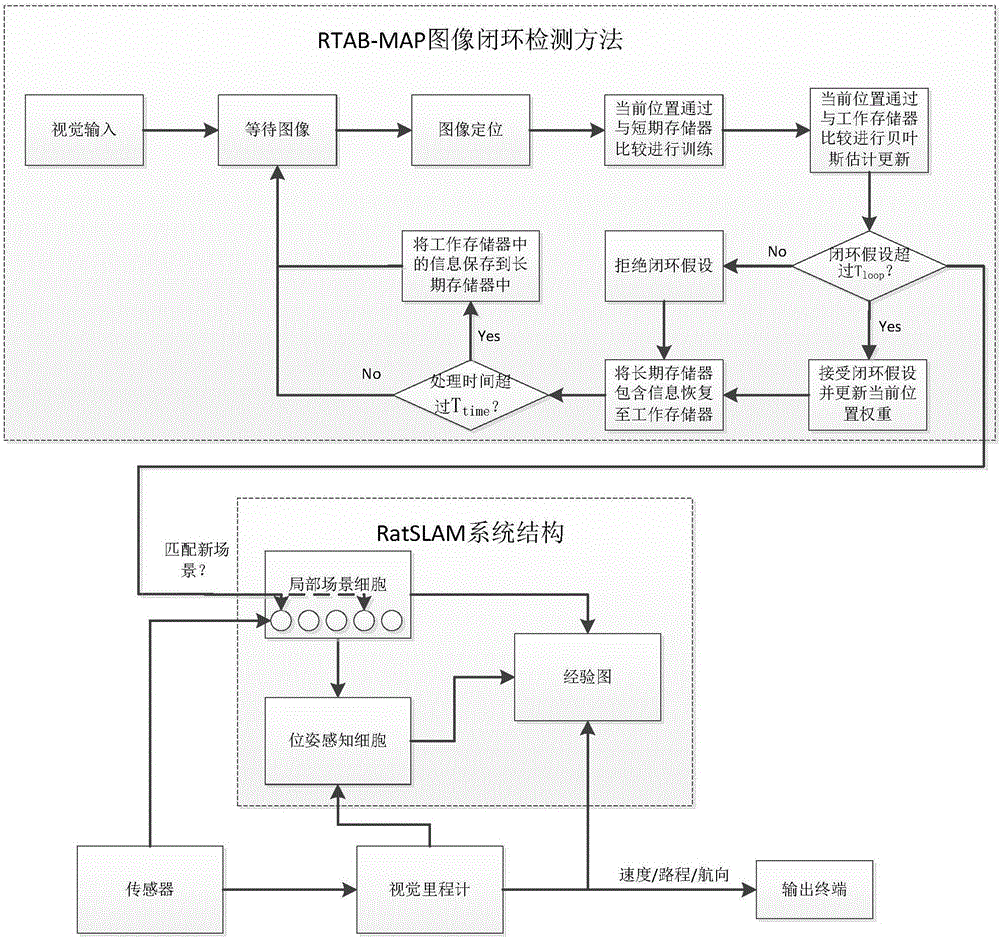
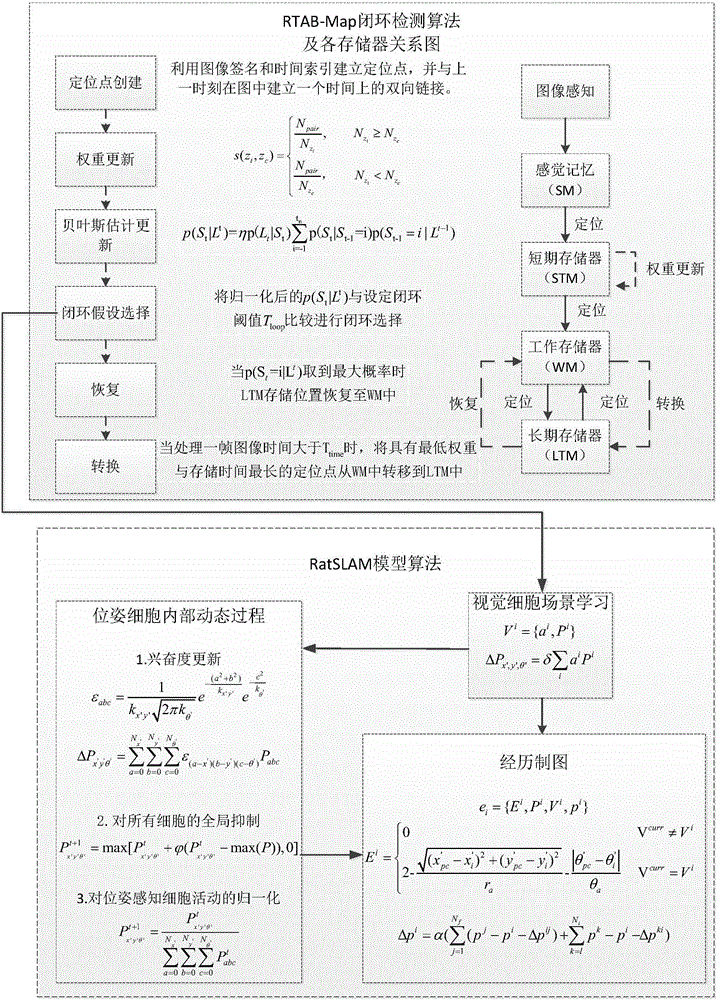
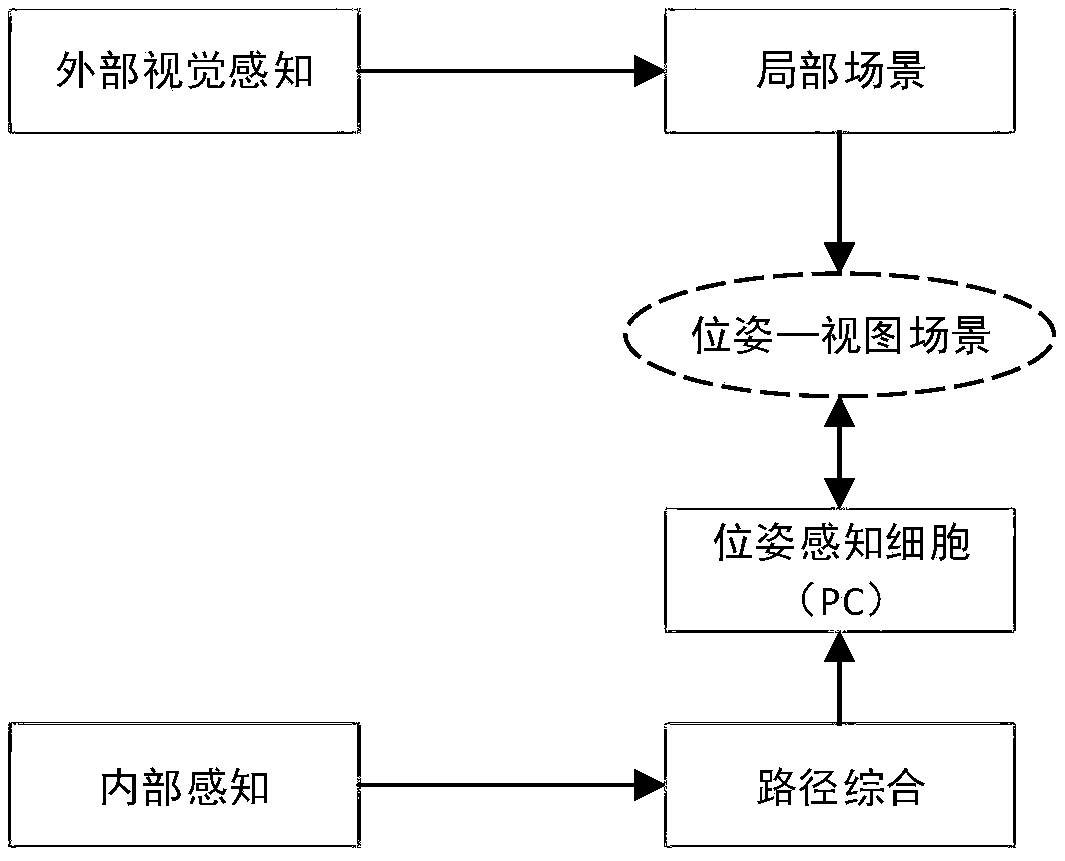
SLAM method based on rodent model and RTAB-Map closed-loop detection algorithm
ActiveCN106814737ACorrection of driftImprove real-time performanceImage enhancementImage analysisSpatial positioningRodent model
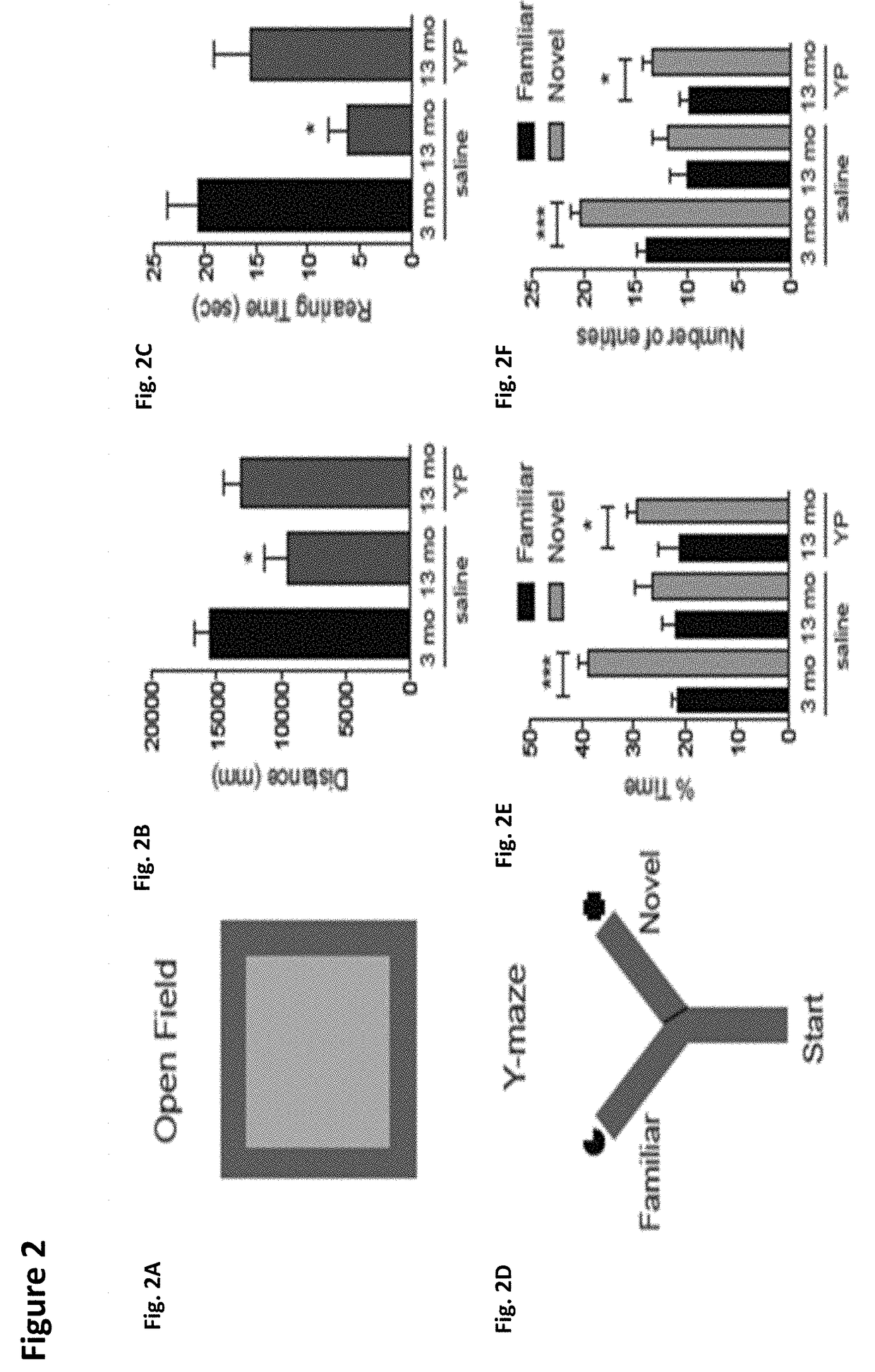
The invention discloses an SLAM method based on a rodent model and an RTAB-Map closed-loop detection algorithm. Spatial positioning can be performed by means of hippocampal neurons of the rodent model. A new bionic navigation model is constructed according to an existing closed-loop detection RTAB-MAP algorithm. On the condition that high real-time performance of the system is ensured, correction of a long-term accumulated error in a macroenvironment can be realized, and furthermore low navigation stability caused by indoor light change to a certain extent.
Owner:光武惠文生物科技(北京)有限公司
Non-Human Animal Models for B-cell Non-Hodgkin's Lymphoma and Uses Thereof
InactiveUS20080070256A1Compounds screening/testingAnimal cellsBiologyB-cell chronic lymphocytic leukemia
Disclosed are non-human animal models, and preferably, rodent models, and more preferably, mouse models, of B-cell Non-Hodgkin's Lymphoma (NHL). In particular, the present invention provides animal models of B cell NHL including, B cell chronic lymphocytic leukemia / lymphoma (B-CLL), Burkitt's lymphoma (BL), Follicular-like lymphoma (FLL) and Diffuse large B-cell lymphoma (DLBCL), as well as various methods for producing these non-human animal models. These animal models, as well as cell lines produced from or derived from these models, are useful tools for a variety of methods, including, but not limited to, preclinical testing of drug candidates, and particularly drug candidates that are specific for human proteins, and any research, development, pharmaceutical, or clinical purpose, including but not limited to, the identification, development, and / or testing of drugs (therapeutics, prophylactics, etc.), targets, markers, and / or research tools for use in the diagnosis of, study of, or treatment of any Non-Hodgkin's Lymphoma, such as those described herein, or for any related condition.
Owner:NAT JEWISH MEDICAL & RES CENT
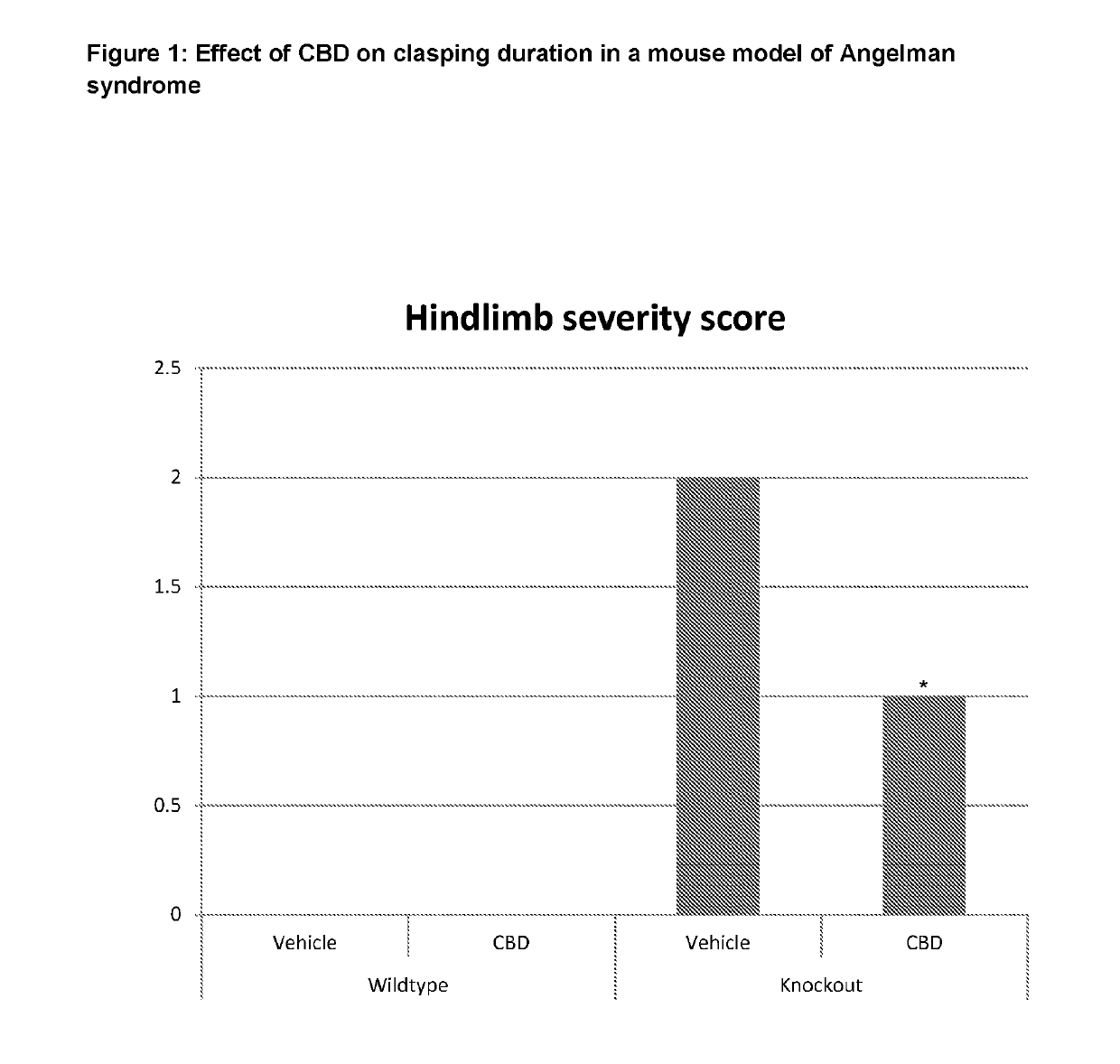
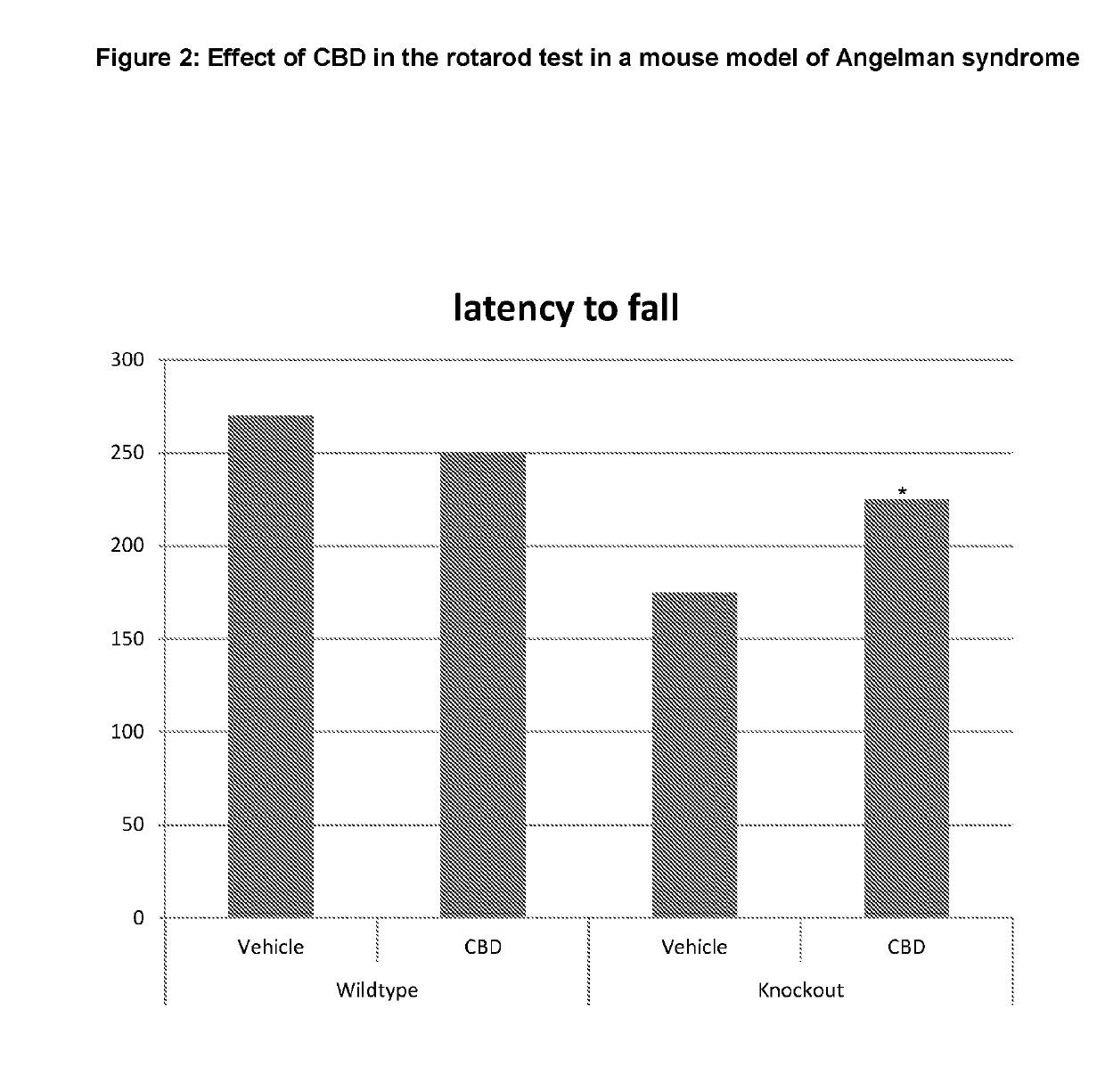
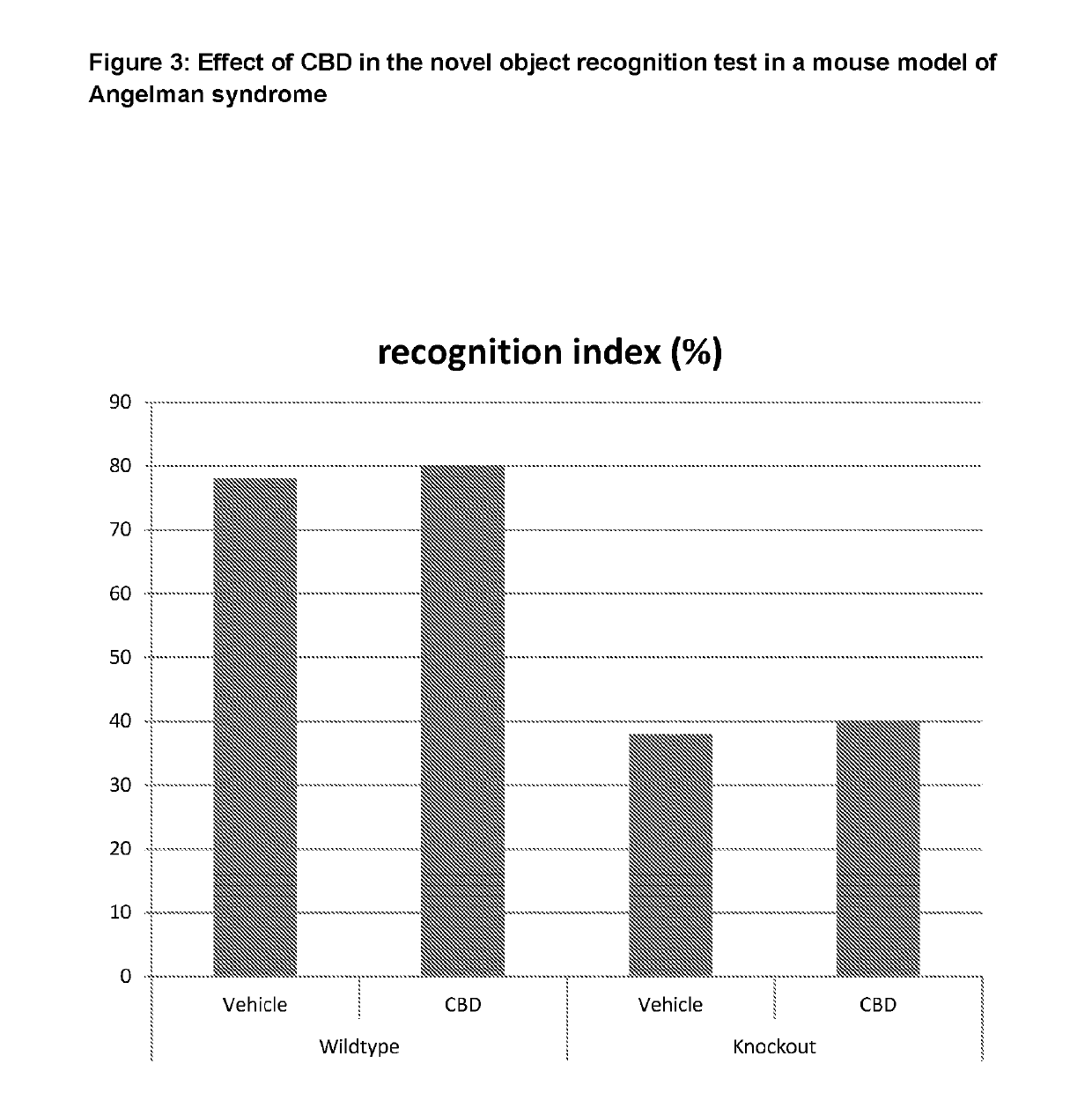
Use of cannabinoids in the treatment of angelman syndrome
ActiveUS20190321307A1Symptoms improvedNervous disorderHydroxy compound active ingredientsRodent modelAnxiety
The present invention relates to the use of cannabidiol (CBD) in the treatment of Angelman syndrome (AS). CBD has It been shown to be particularly effective in improving anxiety in rodent models of AS. The CBD is preferably substantially pure. It may take the form of a highly purified extract of Cannabis such that the CBD is present at greater than 98% of the total extract (w / w) and the other components of the extract are characterised. Alternatively, the CBD is synthetically produced. Alternatively, the CBD may be used as a botanical drug substance (BDS) from a Cannabis plant in which CBD is the predominant cannabinoid. The CBD may also be present in combination with other cannabinoids and non-cannabinoid components such as terpenes. In use the CBD may be used concomitantly with one or more other medicaments. Alternatively, the CBD may be formulated for administration separately, sequentially or simultaneously with one or more medicaments or the combination may be provided in a single dosage form. Where the CBD is formulated for administration separately, sequentially or simultaneously it may be provided as a kit or together with instructions to administer the one or more components in the manner indicated. It may also be used as the sole medication, i.e. as a monotherapy.
Owner:GW RES LTD
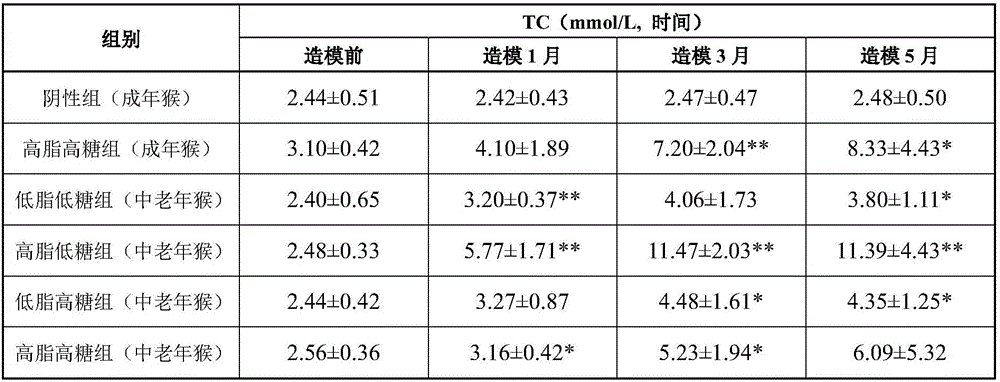
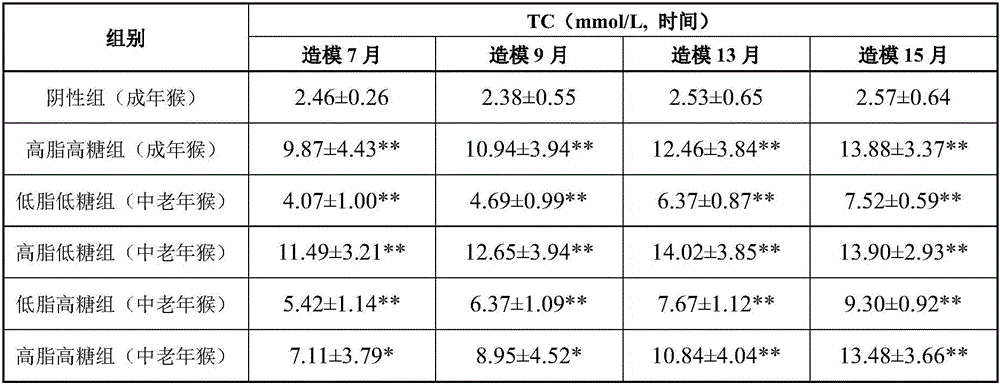
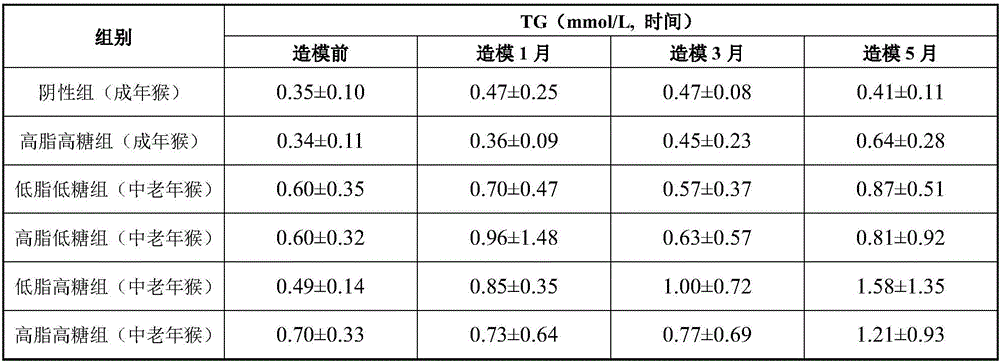
High-fat high-sugar semi-liquid diet for inducing hyperlipidemia primate model and an inducing method thereof
InactiveCN106577540ASpeed up the induction processAccessory food factorsRodent modelHigh fat high sugar
The invention discloses a high-fat high-sugar semi-liquid diet for inducing a hyperlipidemia primate model and an inducing method thereof. According to the high-fat high-sugar semi-liquid diet for inducing the hyperlipidemia primate model and the inducing method thereof, the amount of the intake high fat and high sugar of each animal every day can be quantized; moreover, the worry of animal intake amount insufficiency caused by taste change is avoided; high fat and high sugar can be taken timely and quantitatively, so that an inducing process of a hyperlipidemia model is greatly accelerated. The hyperlipidemia primate model manufactured by the invention has the advantages which cannot be unmatched by those of the hyperlipidemia model which is induced by a rodent, and can be applied to various types of lipid-reducing medicaments, particularly the research, development and evaluation of the biotechnological medicaments which are not suitable for a rodent model.
Owner:GUANGDONG INST OF APPLIED BIOLOGICAL RESOURCES
Establishment and application of rhesus monkey pulmonary fibrosis model
ActiveCN105126075AEasy to operateEasy to repeatSaccharide peptide ingredientsRespiratory disorderRodent modelMedicine
The invention discloses a method for preparing an animal pulmonary fibrosis model. According to the method, bleomycin is applied to a primate, the application dose of the bleomycin is 7 mg / Kg for each time, and application is performed once for each week and totally performed twice. The invention further provides a rhesus monkey pulmonary fibrosis model prepared with the method and an application of the rhesus monkey pulmonary fibrosis model. The rhesus monkey pulmonary fibrosis model is established successfully, an operation process is simple and easy to repeat, and the model can be used for new biotechnological drug evaluation which cannot be finished by a rodent model and has bright clinical application prospects.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV +1
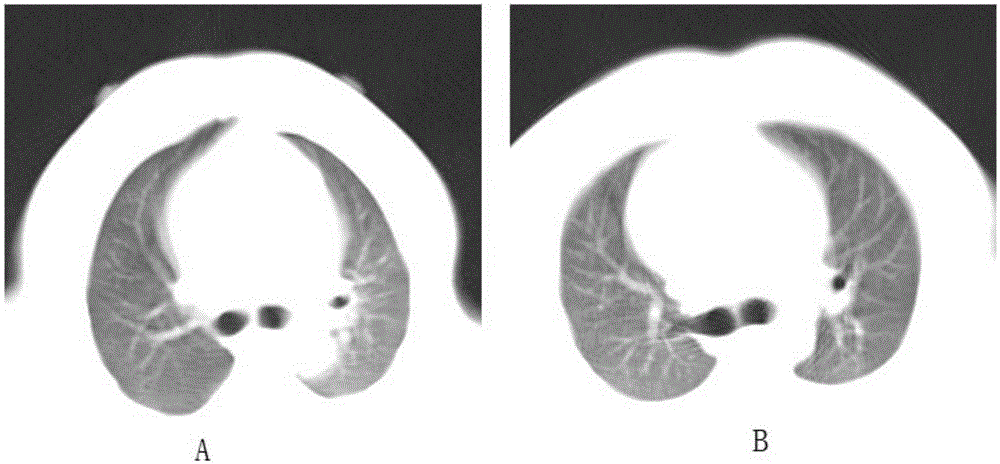
Inducing method of dry age-related macular degeneration primate model
ActiveCN107787912ARelieve concerns about insufficient intakePromote formationCompounds screening/testingAccessory food factorsPrimateRodent model
The invention discloses an inducing method of a dry age-related macular degeneration primate model. The inducing method comprises the steps that a high-glucose and high-fat semifluid diet is injectedinto the stomach of a primate so as to induce the primate to suffer from hypercholesteremia, and then the dry age-related macular degeneration primate model is obtained. By means of the inducing method, the high glucose and high fat which each primate uptakes every day can be quantified without worry about insufficient animal intake caused by taste change, and each primate can regularly and quantificationally uptake the high glucose and high fat, so that the formation of hypercholesteremia is greatly accelerated, and meanwhile, the inducing process of the dry AMD model is accelerated. Comparedwith AMD models induced by other methods, the dry age-related macular degeneration (AMD) primate model has the incomparable advantages and can be used for research, development and assessment of desiccation-resistant AMD drugs, especially for the research, development and assessment of the biotechnology drugs for which a rodent model is not suitable.
Owner:INST OF ZOOLOGY GUANGDONG ACAD OF SCI
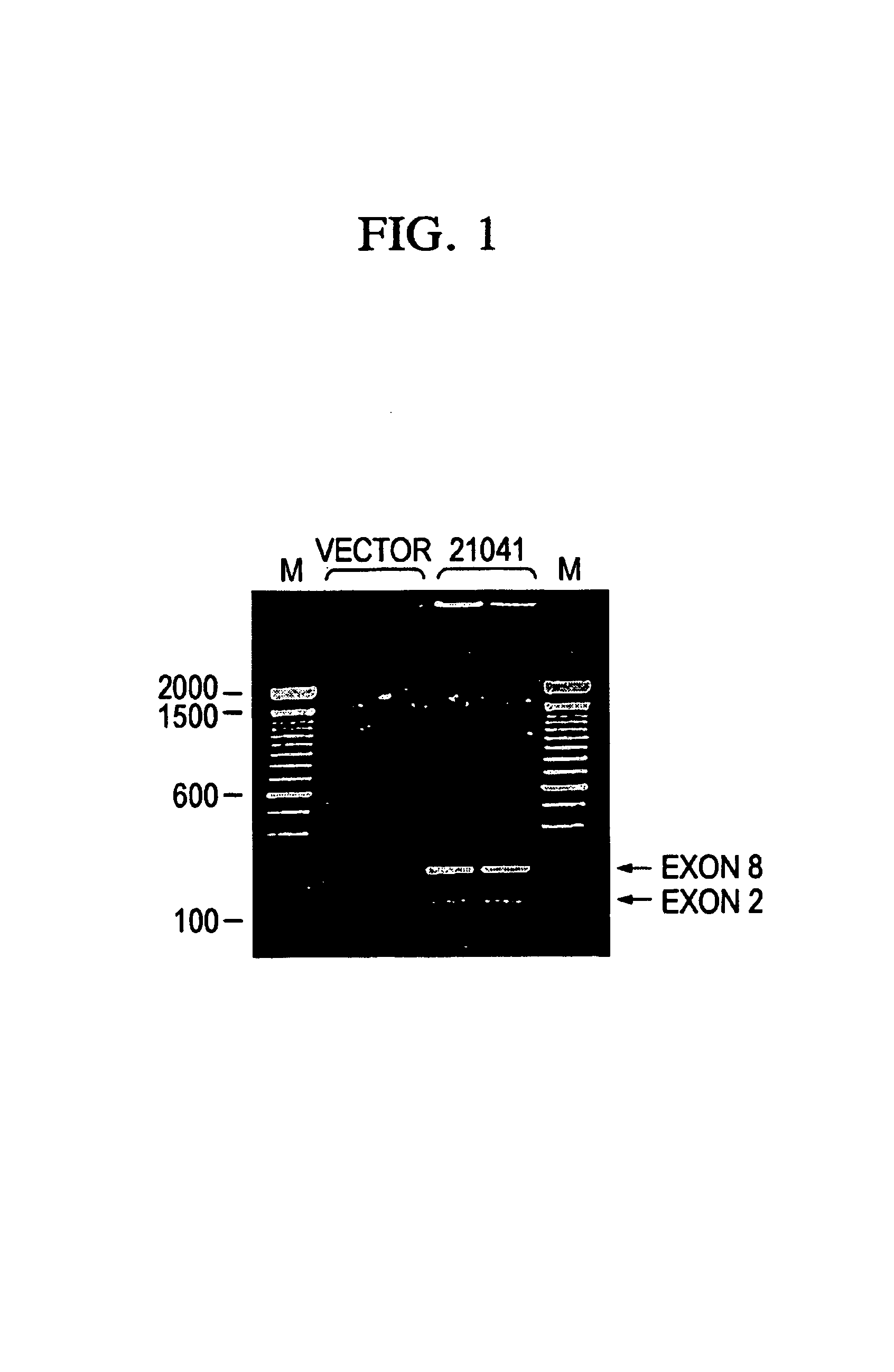
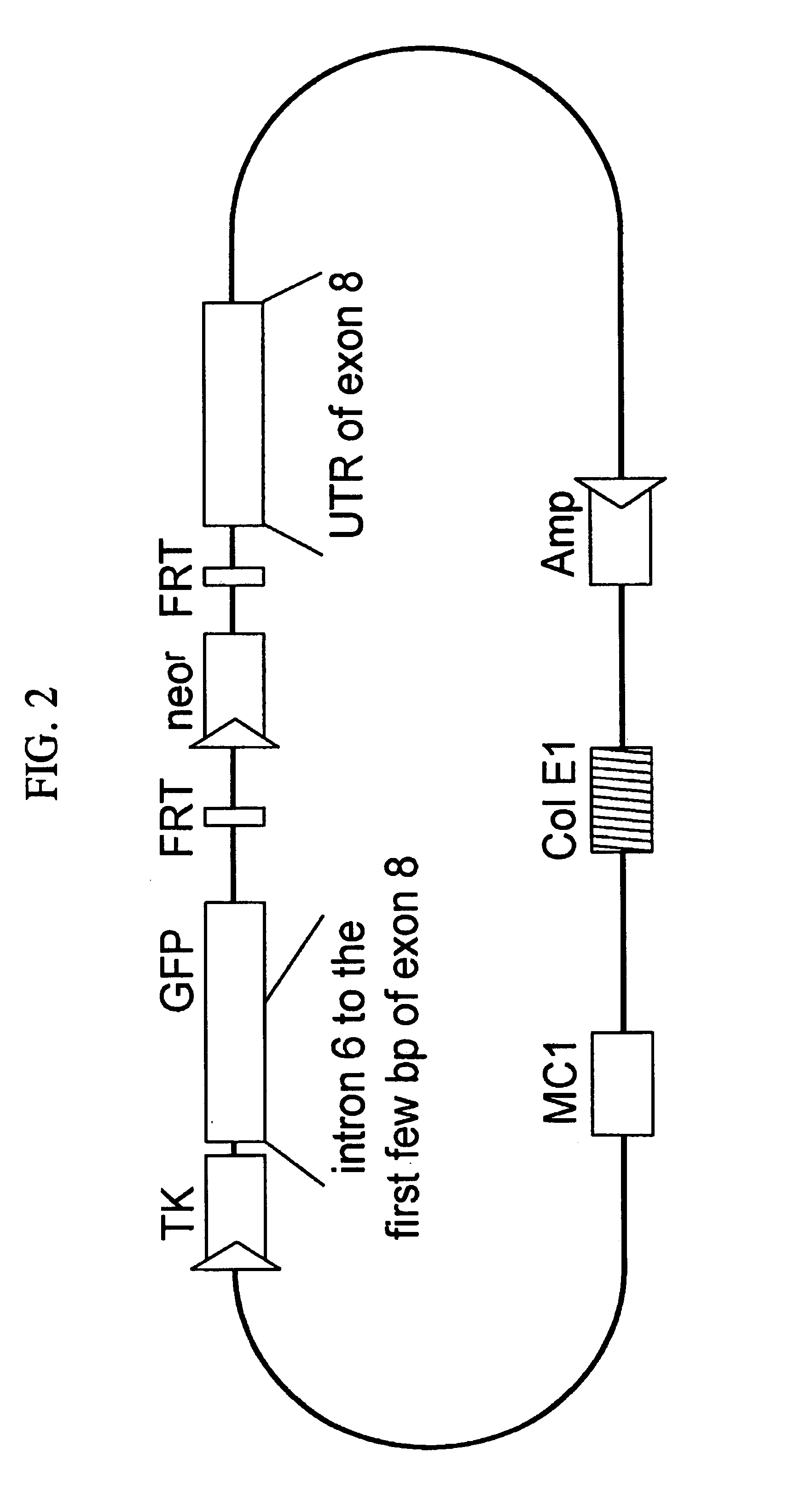
Mice heterozygous for WFS1 gene as mouse models for depression
InactiveUS6984771B2Disrupting functionImprove performanceCell receptors/surface-antigens/surface-determinantsNucleic acid vectorHeterologousRodent model
The present invention describes a recombinant rodent model for depression. More particularly, the rodent comprises cells expressing mutations in the WFS1 gene. The rodent is preferably a mouse heterologous for mutations in exon 8 of the WFS1 gene. Preferably, the mutations yield a non-functional wolframin protein that lacks all or some of it transmembrane regions. Methods and compositions for making and using the mouse and cells thereof are disclosed.
Owner:PHARMACIA & UPJOHN CO
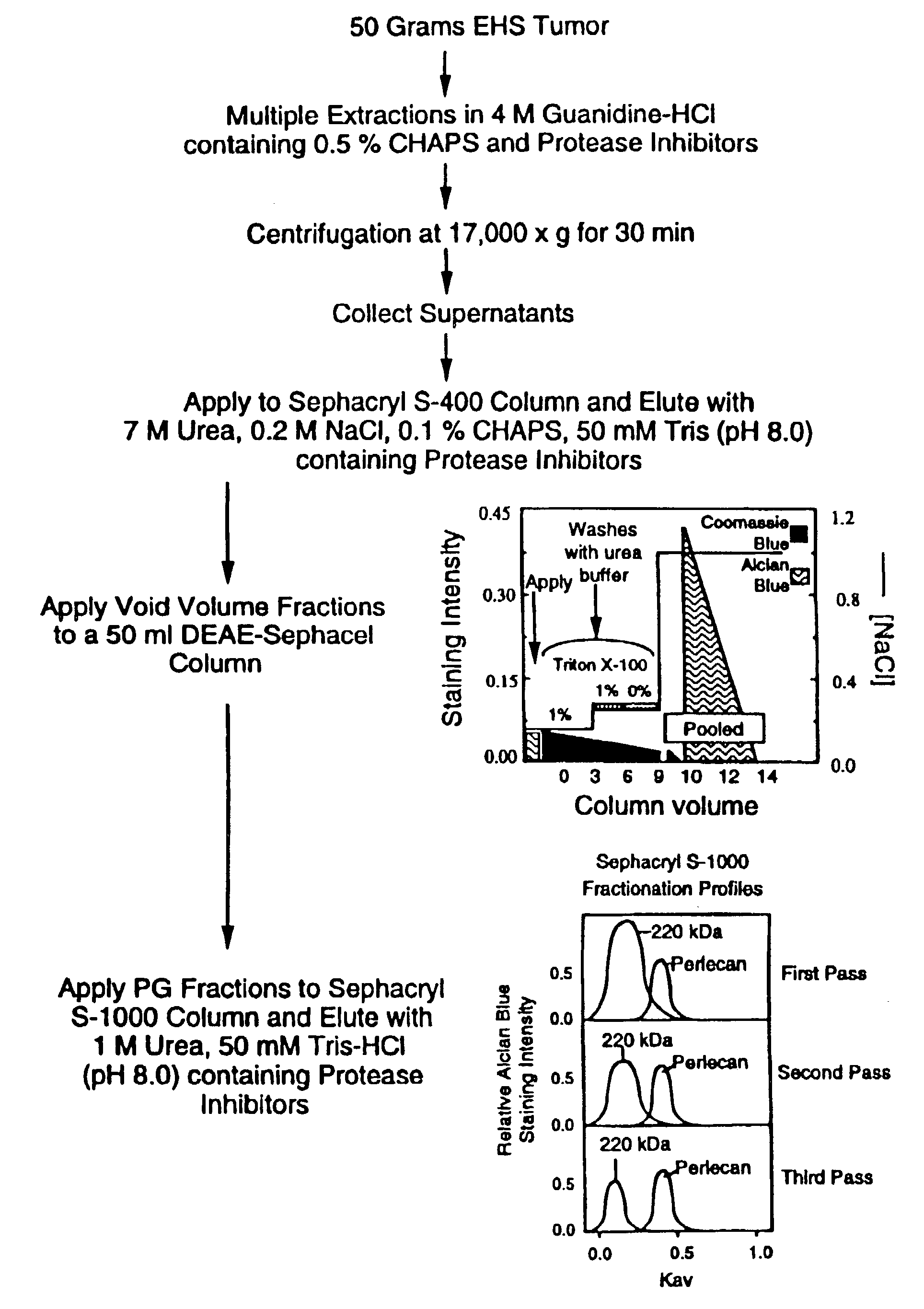
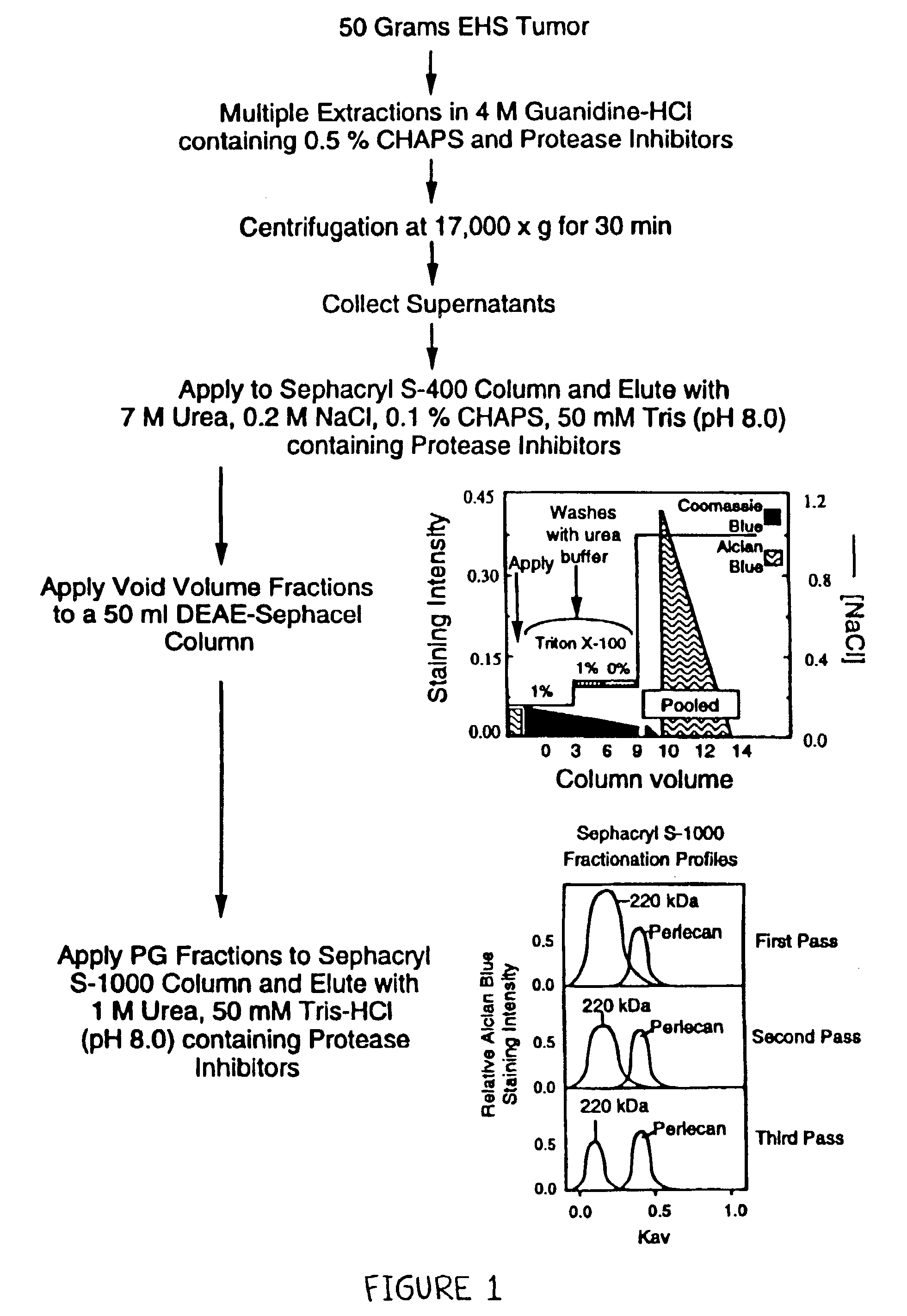
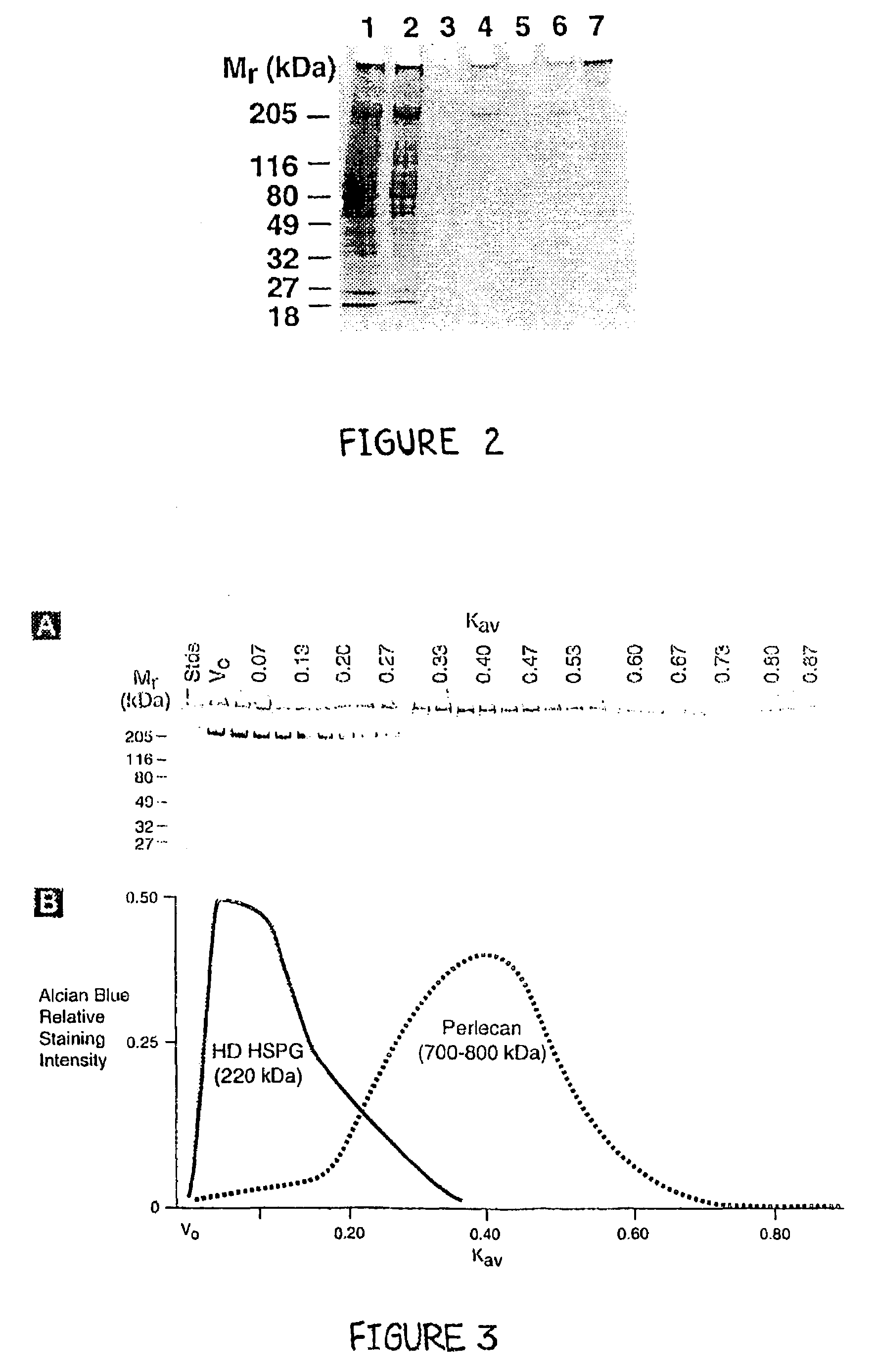
Methods for producing pure perlecan and other heparan sulfate proteoglycans
InactiveUS7094580B2Easy to useEffective isolationCompounds screening/testingLuminescence/biological staining preparationFiltrationIon exchange
A method of perlecan isolation (from the EHS tumor) which produces “clean” (i.e. substantially “pure”) perlecan is disclosed. Clean perlecan is thus produced in sufficient quantities for use in a number of different in vitro and in vivo assays. In addition, this isolation method exploits a newly discovered aggregating property of a ˜220 kDa heparan sulfate proteoglycan (HSPG) observed during gel filtration chromatography, which allows it to be effectively separated from non-aggregating perlecan. The method employs specific cation exchange, anion exchange, molecular sieve chromatography and immobilized GAG affinity chromatography. It is demonstrated that there are no other contaminating proteins in the perlecan and HSPG preparations, and that the perlecan core protein is intact. Improved, clean perlecan based, rodent models of fibrillar amyloid protein deposition, accumulation and / or persistence in tissues are disclosed.
Owner:UNIV OF WASHINGTON
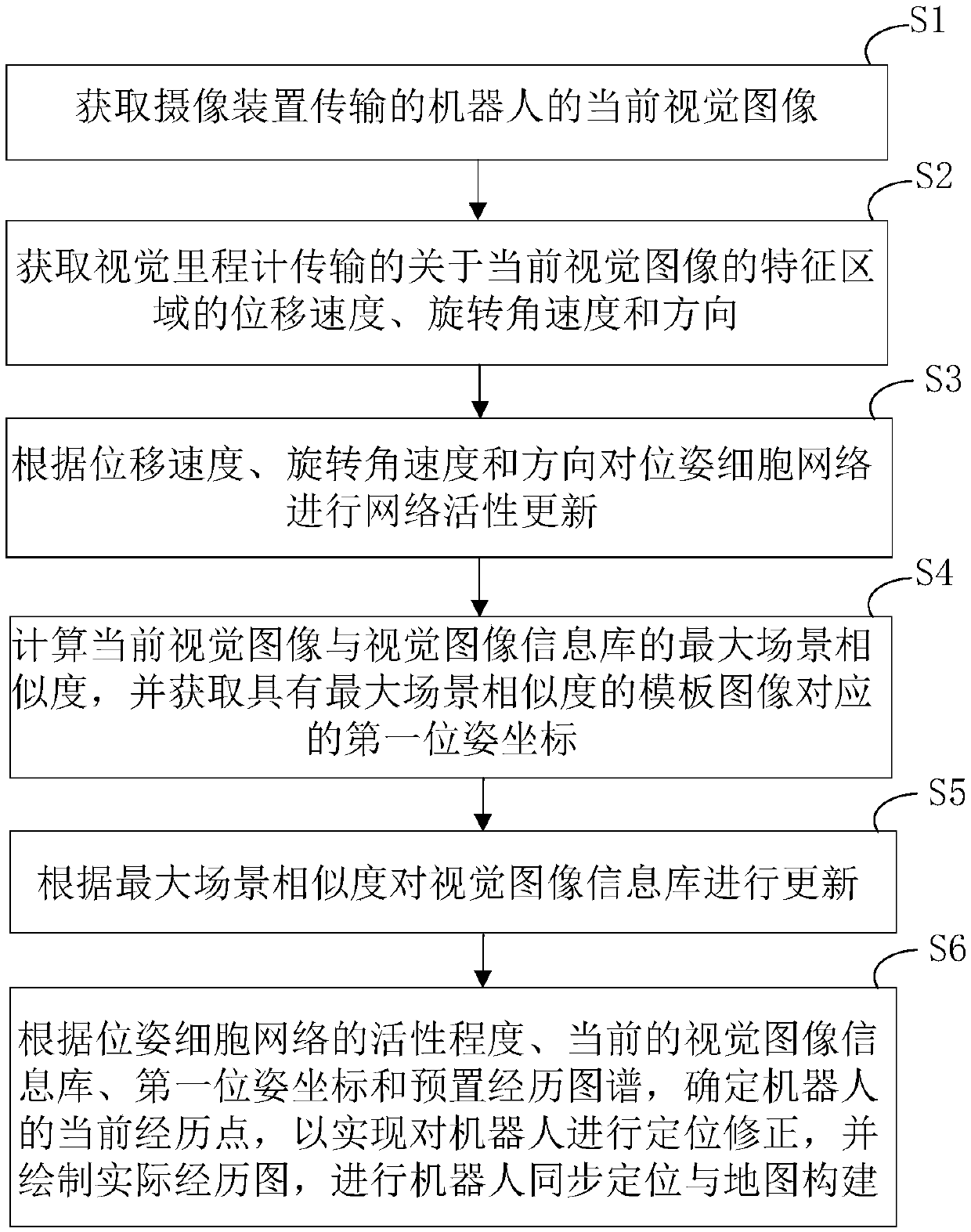
Simultaneous localization and mapping method and device based on rodent model
ActiveCN108680177AImplement positioning fixesAchieve precise positioningInstruments for road network navigationLocation information based serviceNetwork activitySimultaneous localization and mapping
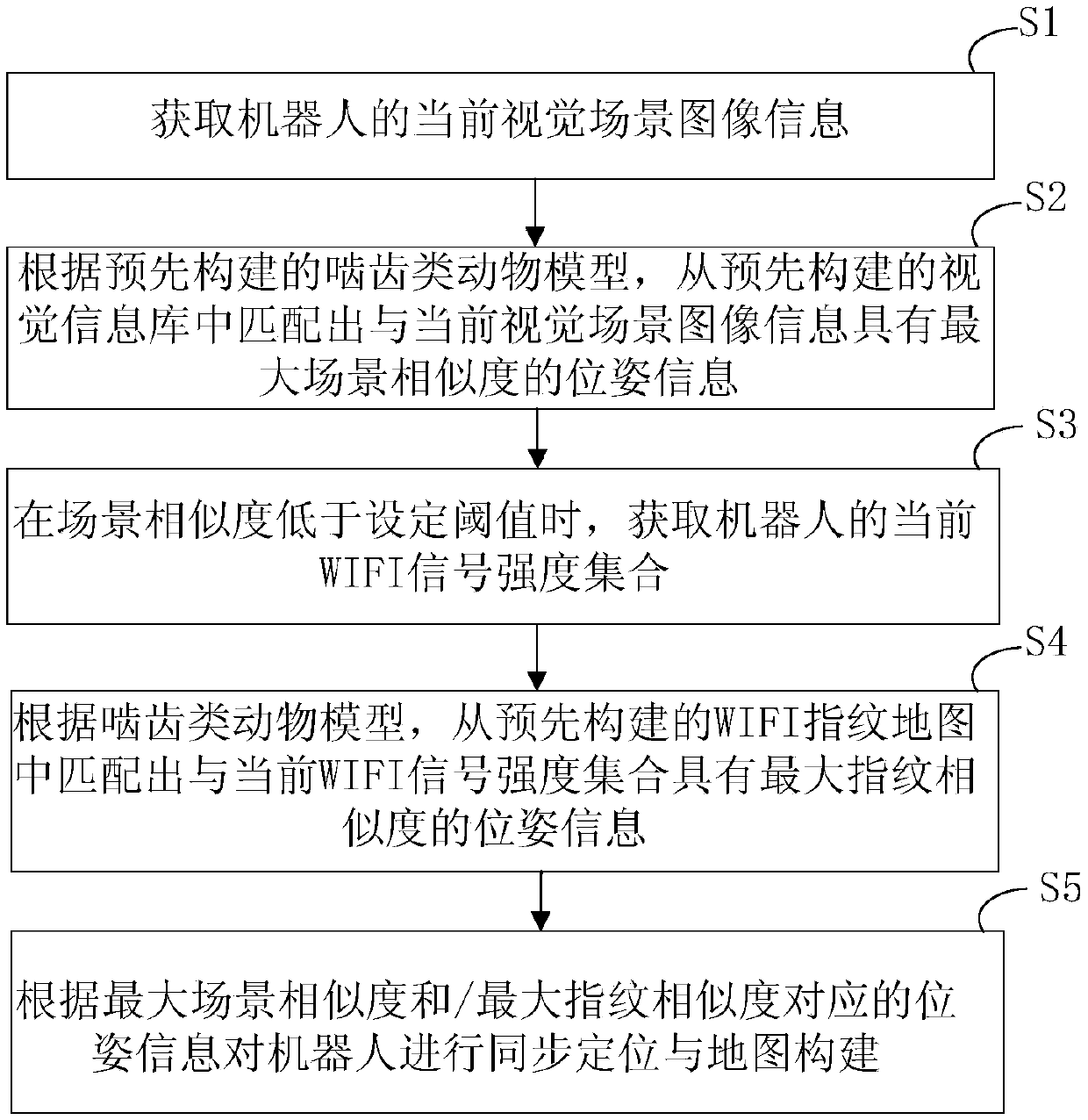
The invention provides a simultaneous localization and mapping method and device based on a rodent model. The method comprises the following steps: acquiring a current visual image of a robot transmitted by a pick-up device; acquiring the displacement speed, the angular velocity of rotation and the direction of a characteristic region of the current vision image, transmitted by a visual odometer;performing network activity updating on the pose cellular network according to the displacement speed, the rotating angular velocity and the direction; calculating the maximal scene similarity of thecurrent vision image and a vision image information base, and acquiring the corresponding first pose coordination of a template image with the maximal scene similarity; updating the vision image information base according to the maximal scene similarity; and determining the current experience point according to the activity degree of the pose cellular network, the current vision image informationbase, the first pose coordination and a preset experience map so as to realize localization and correction on the robot and draw an actual experience chart to perform the simultaneous localization andmapping on the robot.
Owner:ANHUI UNIVERSITY OF TECHNOLOGY AND SCIENCE
Macaca mulatta hepatocellular carcinoma model, macaca mulatta hepatocellular carcinoma cell line and application of macaca mulatta hepatocellular carcinoma model and macaca mulatta hepatocellular carcinoma cell line
ActiveCN105343897ATumorigenicOrganic active ingredientsIn-vivo testing preparationsSurgical treatmentImage diagnosis
The invention discloses a macaca mulatta hepatocellular carcinoma model preparation method. According to the method, diethylnitrosamine is applied to primates, and the application dosage of the diethylnitrosamine is 40mg / Kg / per time. The invention further provides a macaca mulatta hepatocellular carcinoma cell line and application thereof. A macaca mulatta hepatocellular carcinoma model can be used for evaluating hepatocellular carcinoma prevention factors, new anti-hepatocellular carcinoma medicine, hepatocellular carcinoma radiotherapy, hepatocellular carcinoma surgical treatment and novel technologies such as imaging diagnosis which cannot be completed by a rodent model.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV +1
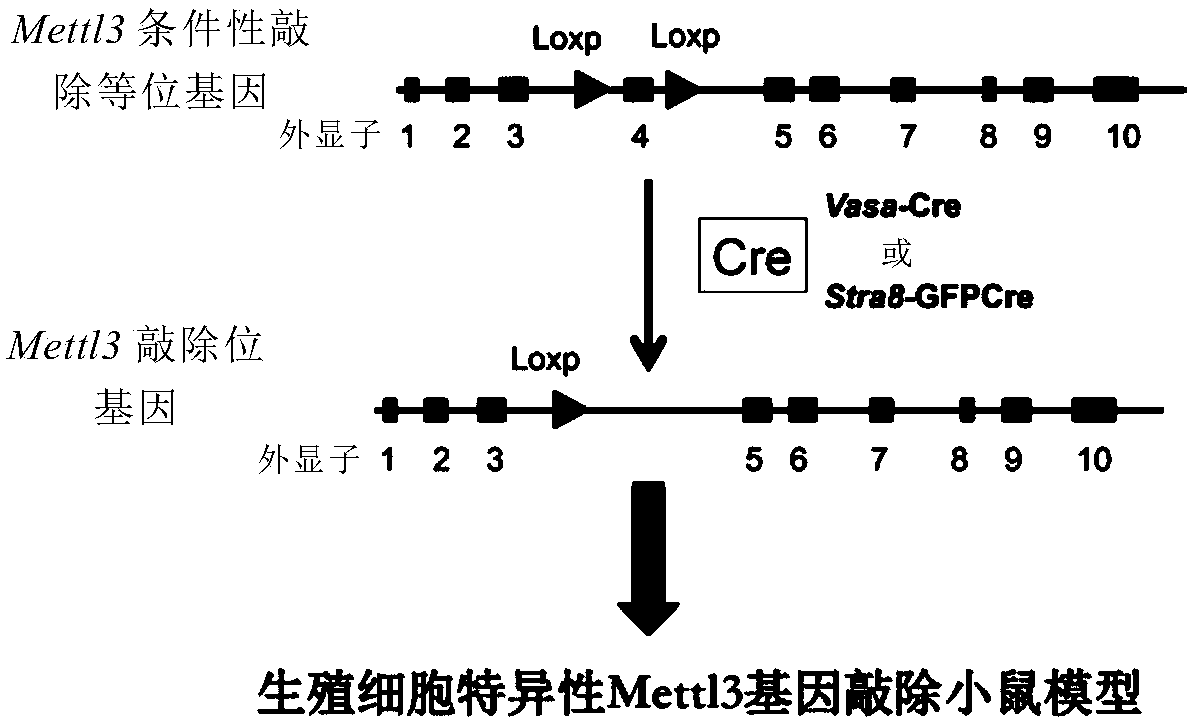
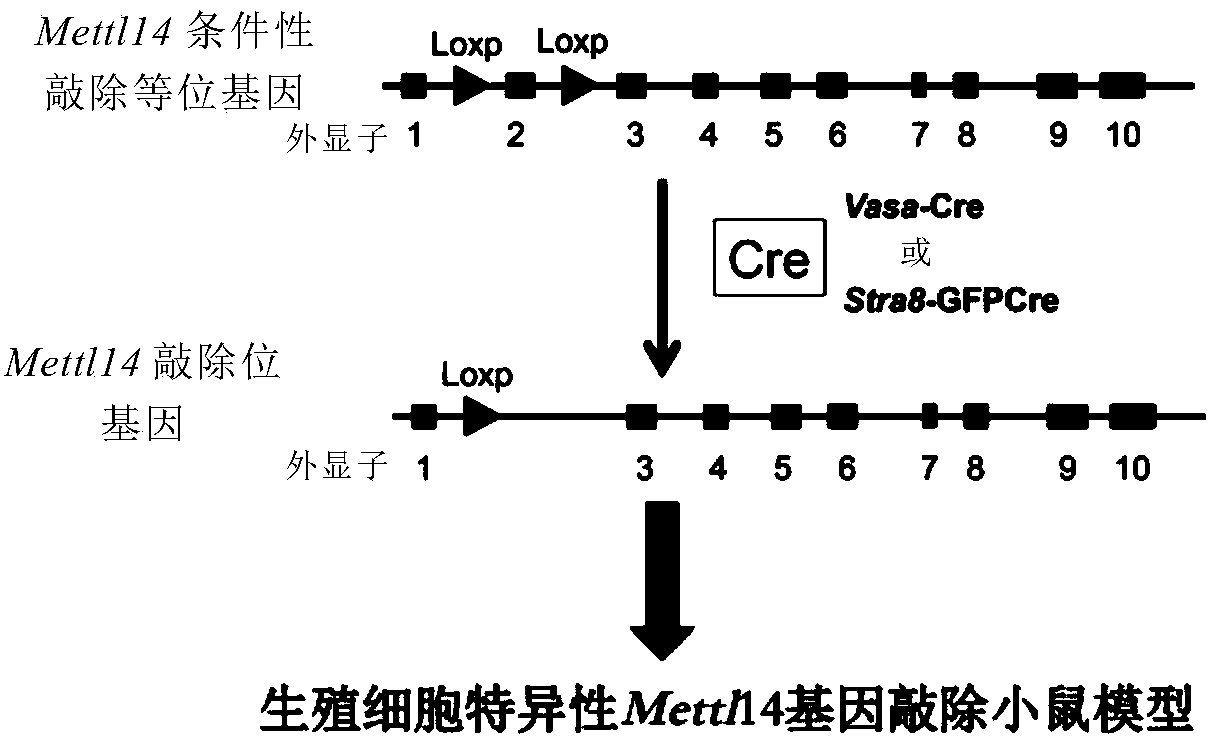
Mettl3-knockout spermatogenesis disorder animal model and building method thereof
A Mettl3-knockout spermatogenesis disorder animal model and a building method thereof are provided. The method includes building a Mettl3 conditionally gene-knockout rodent by applying a CRISPR / Cas9 technique; and mating the Mettl3 conditionally gene-knockout rodent and a rodent of the same kind with germ cells capable of specific expression of Cre so as to obtain a Mettl3-knockout spermatogenesisdisorder rodent model with germ cell specificity. The model built provides new ideas and new means for the cause diagnosis, prevention and treatment of human male infertility and prevention and reduction of paternal birth defects.
Owner:CENT FOR EXCELLENCE IN MOLECULAR CELL SCI CHINESE ACAD OF SCI
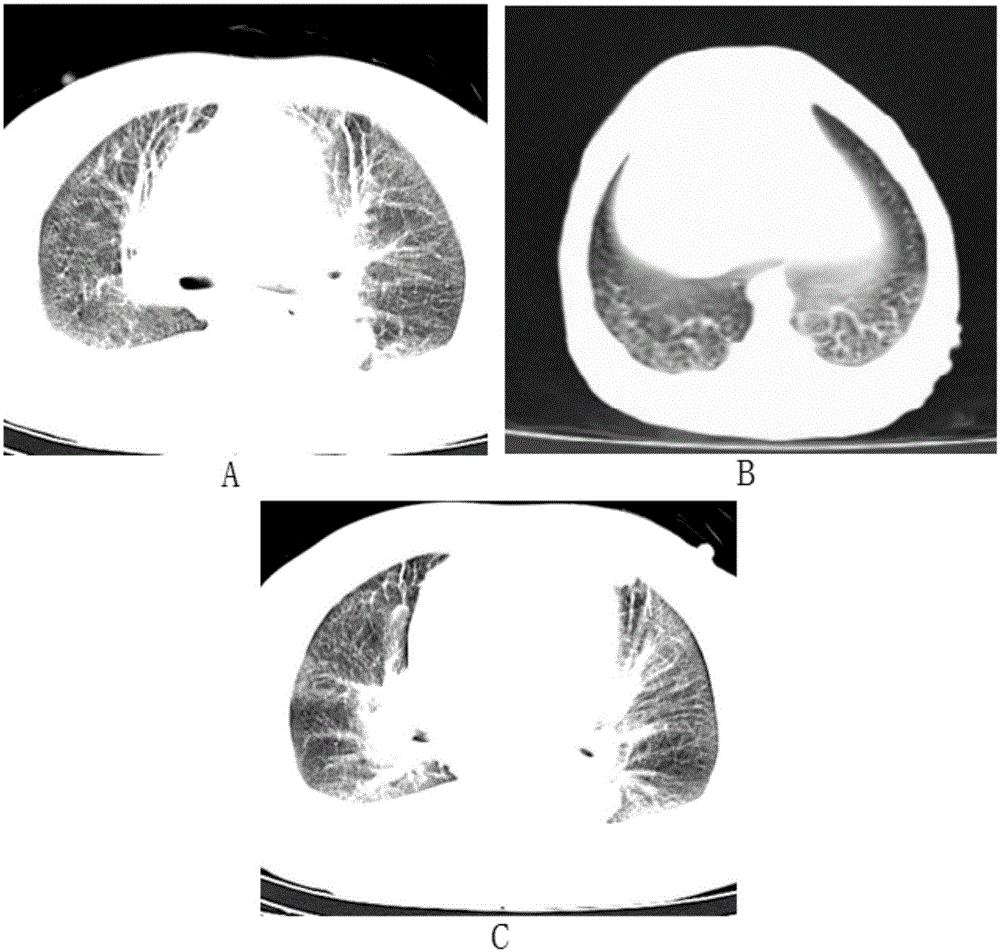
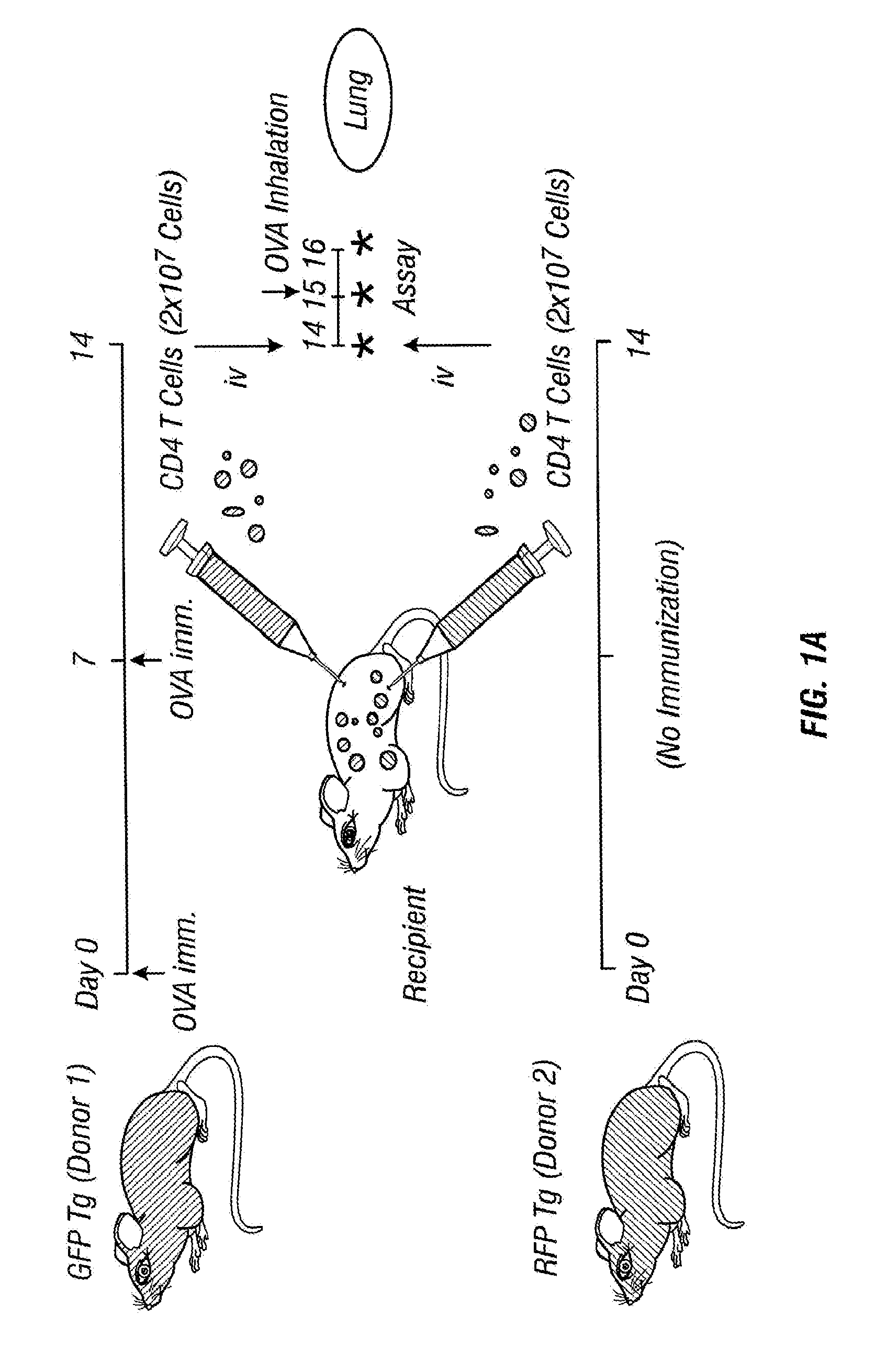
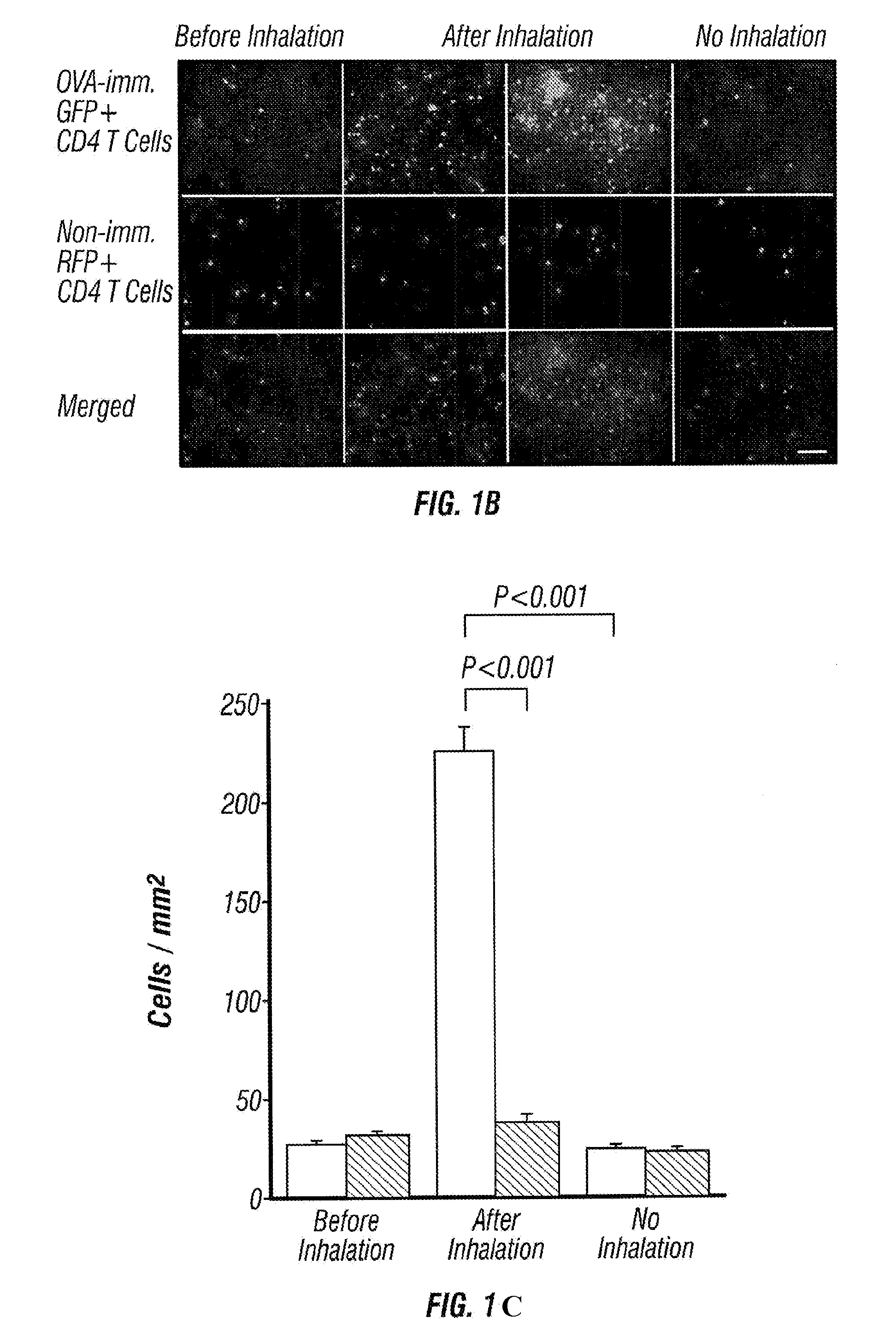
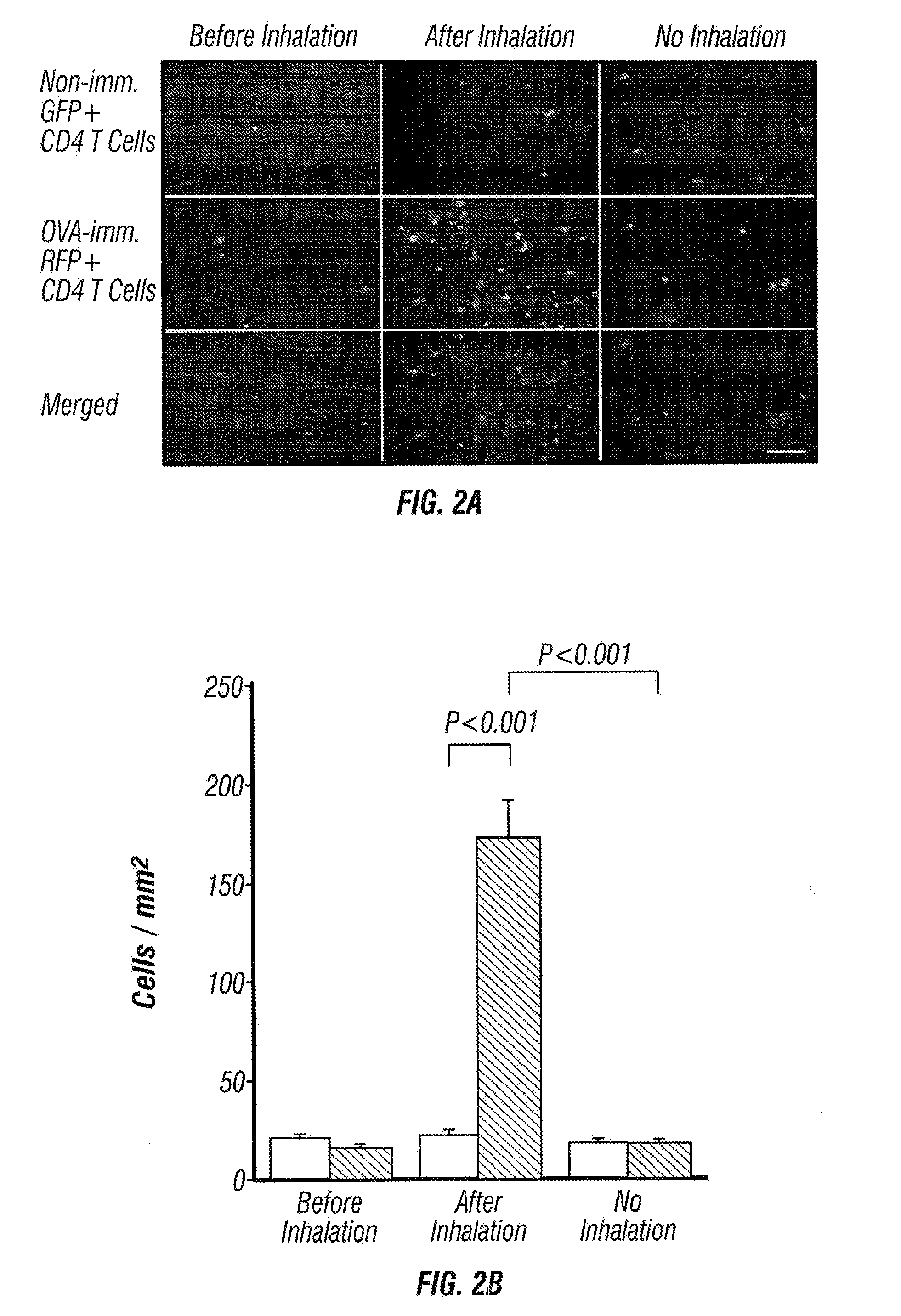
Imageable rodent model of asthma
An imageable rodent model for asthma is described. The invention provides a rodent model for asthma wherein a rodent is provided with fluorescently labeled lymphocytes sensitized to an allergen which can be monitored after inducing an asthmatic response by the allergen. Methods to monitor trafficking of the fluorescently labeled cells in the rodent model for asthma are provided. Methods to determine the effectiveness of candidate drugs that regulate asthmatic responses using the rodent asthma model are also provided.
Owner:ANTICANCER
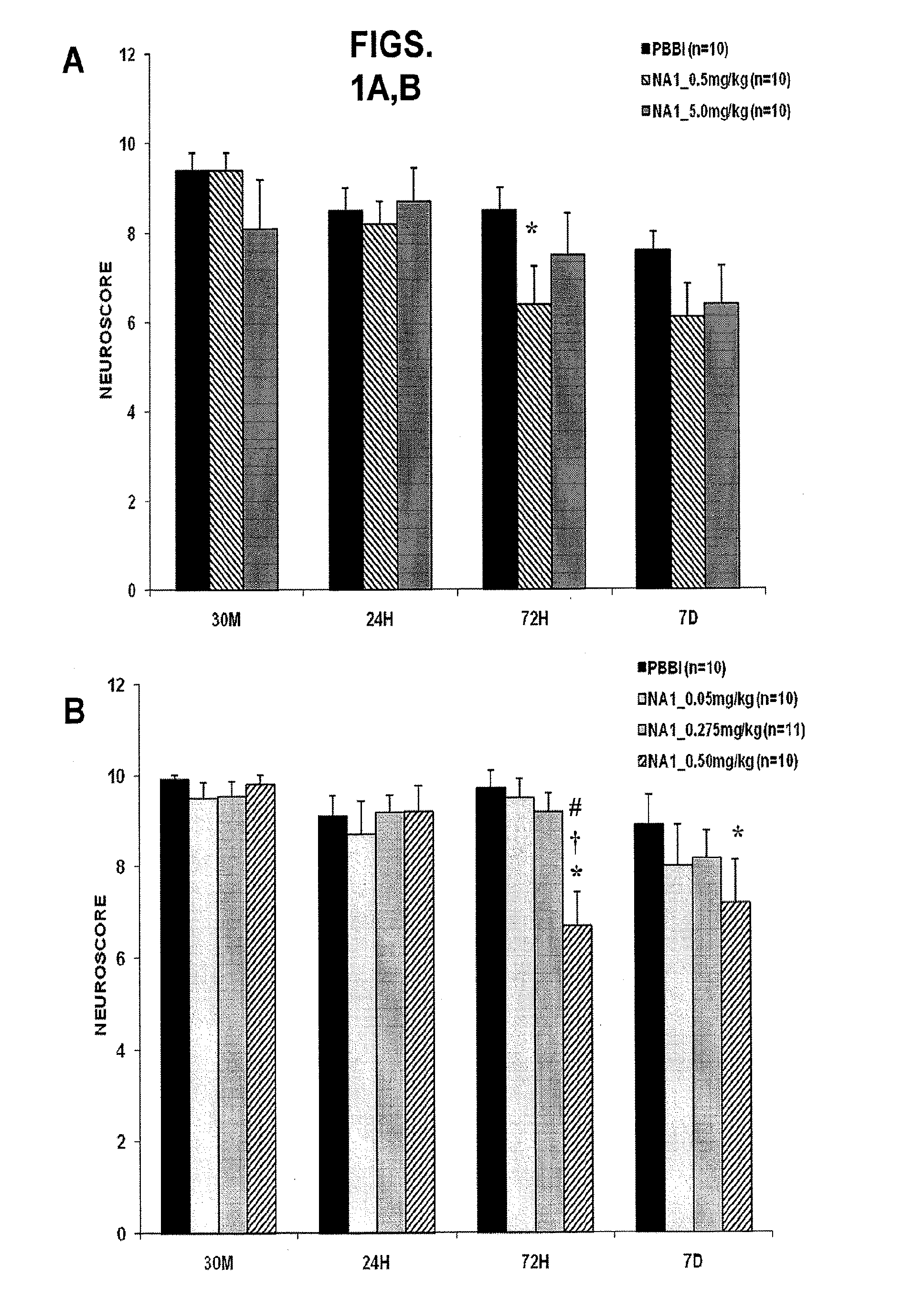
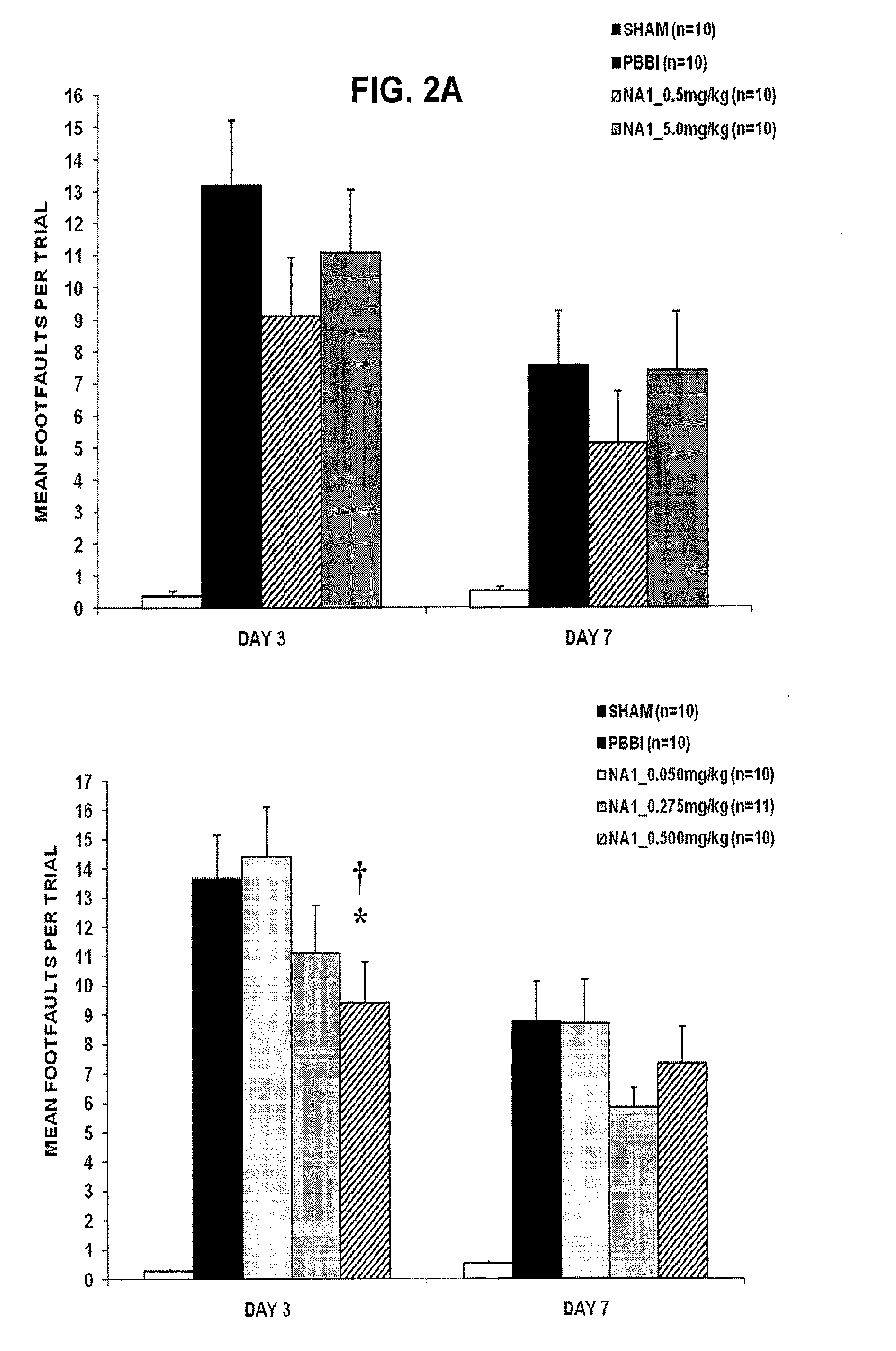
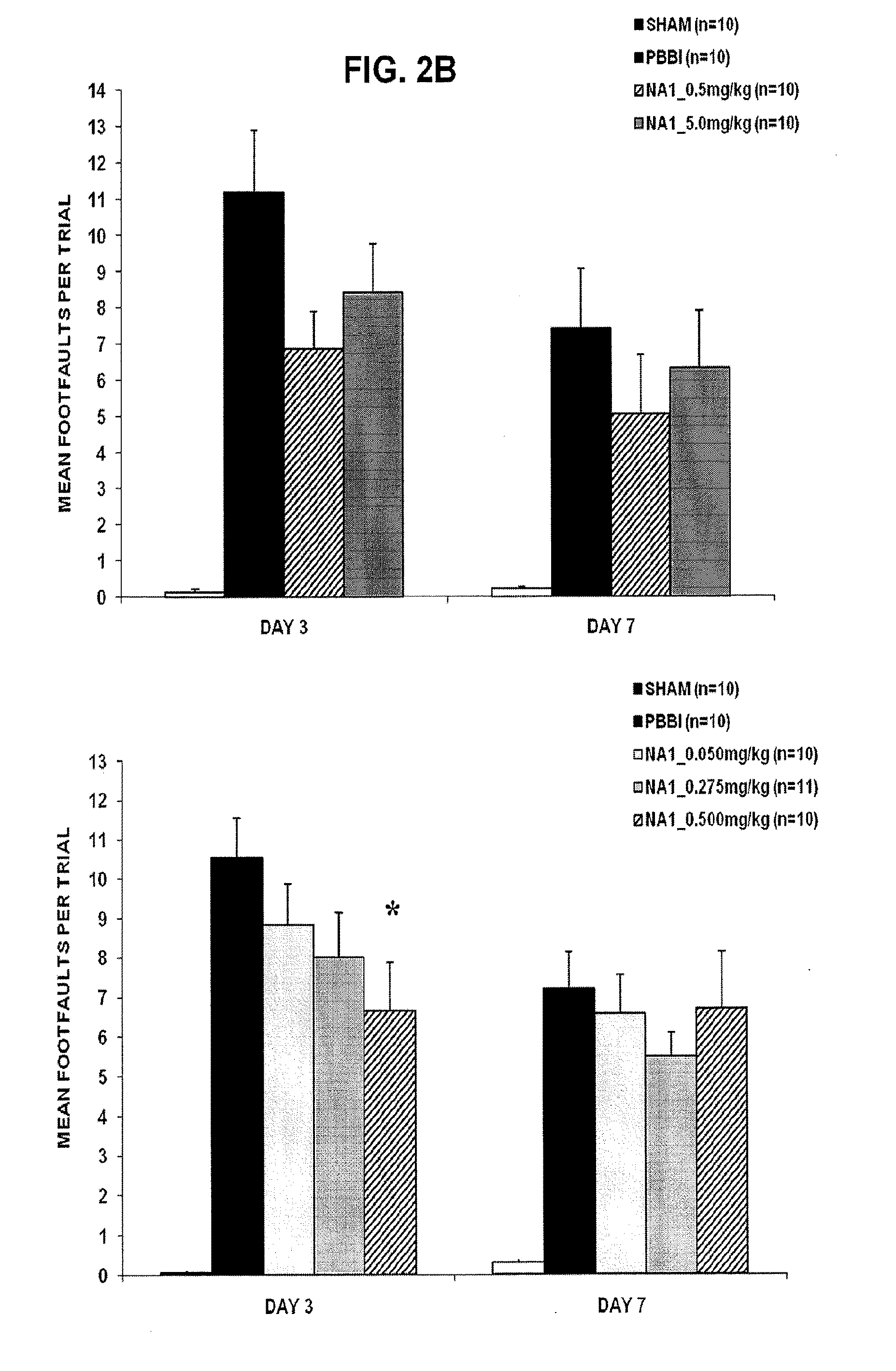
Treatment Of Penetrative Injury To The Brain
ActiveUS20130267470A1Reduce the temperaturePolypeptide with localisation/targeting motifCompound screeningPenetrating Brain InjuryInjury brain
The invention provides methods of treatment or prophylaxis of damaging effects of penetrative injury to the brain or other part of the central nervous system. The methods are based in part on results in a rodent model of penetrative ballistic injury showing that an inhibitor of PDF-95 NMDAR interaction is effective in inhibiting neurological deficits resulting from such injury. The methods are useful for treating subjects having or at risk of penetrative brain injury, including subjects who have been shot in the head or at risk of such injury (e.g., military or law enforcement personnel).
Owner:NONO INC
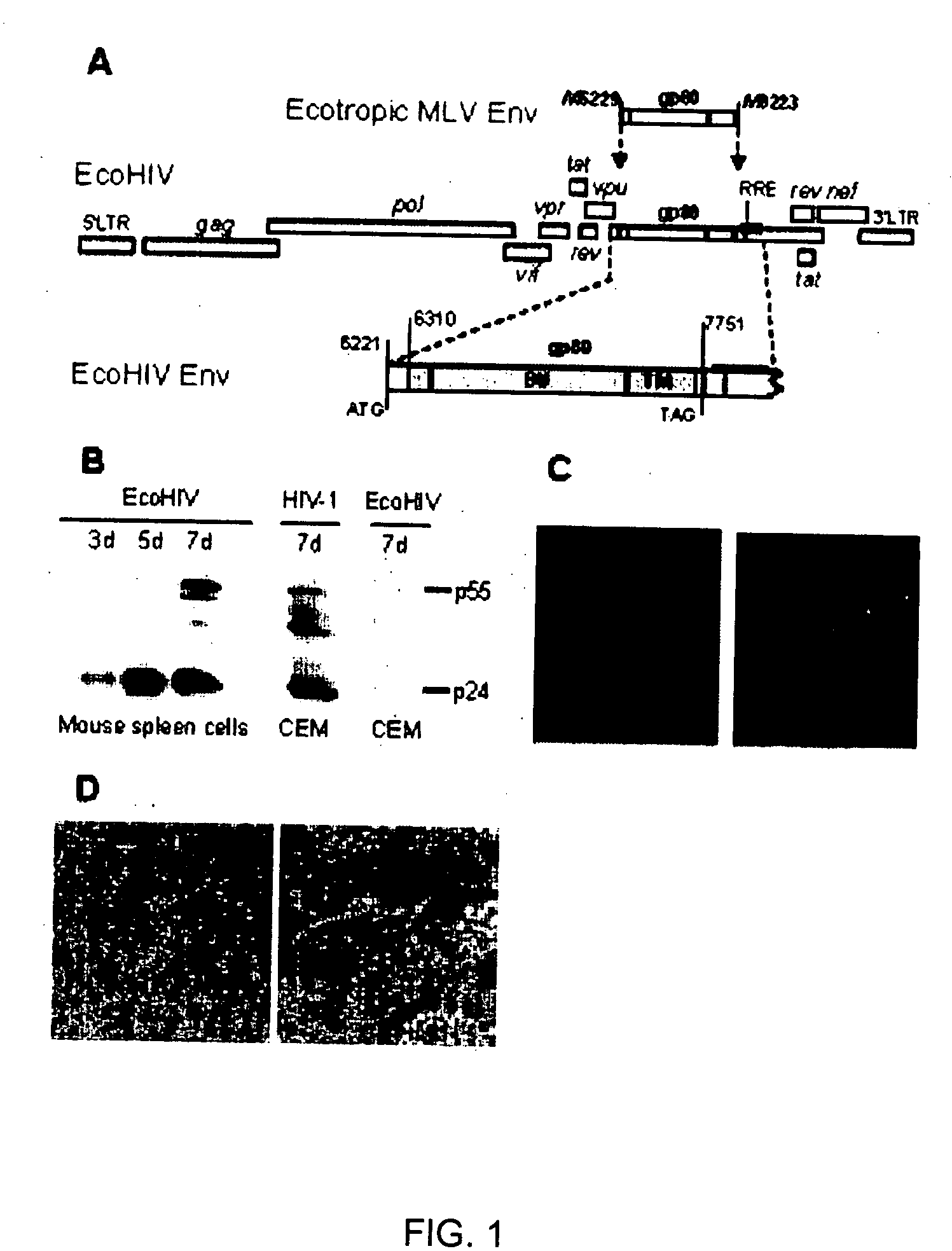
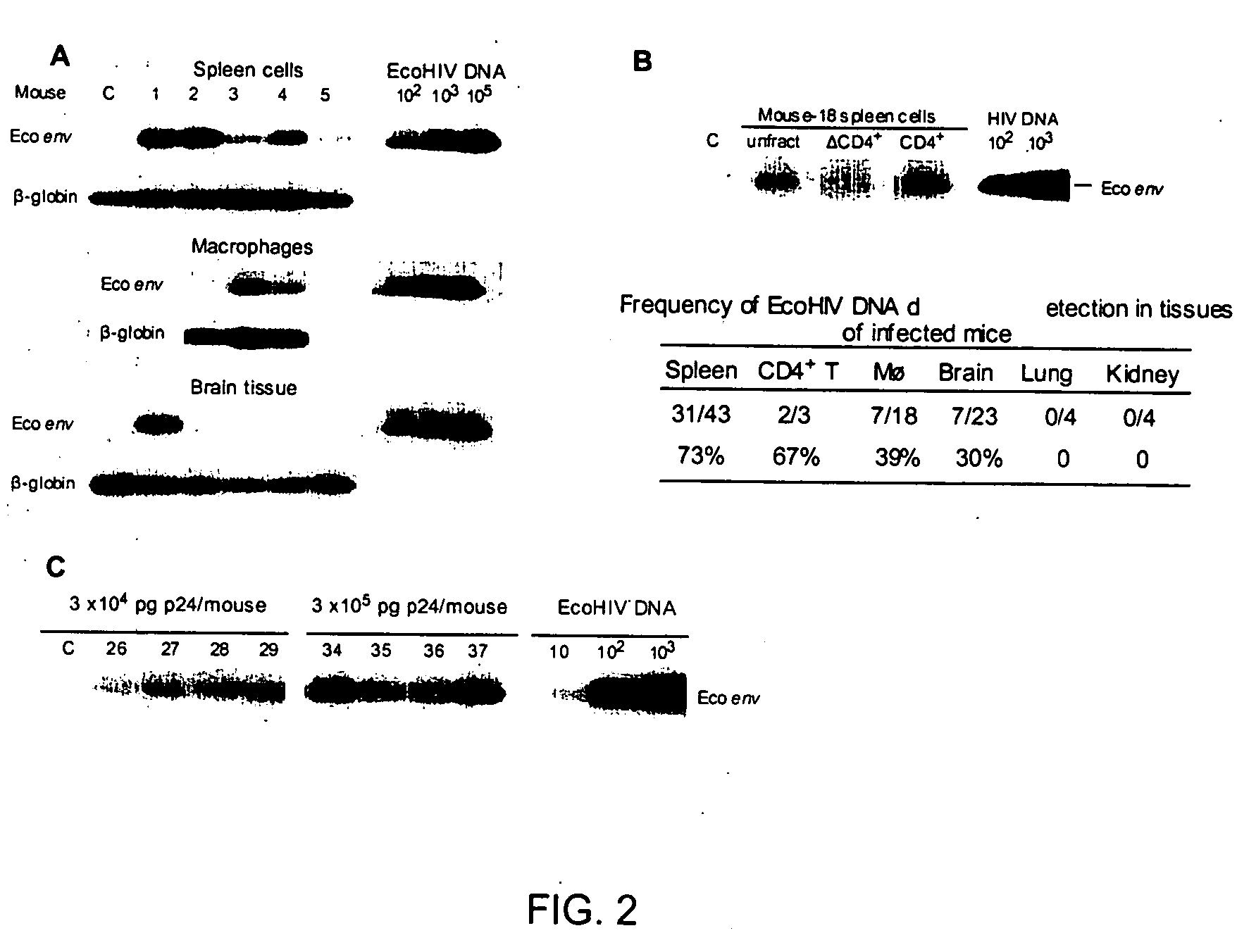
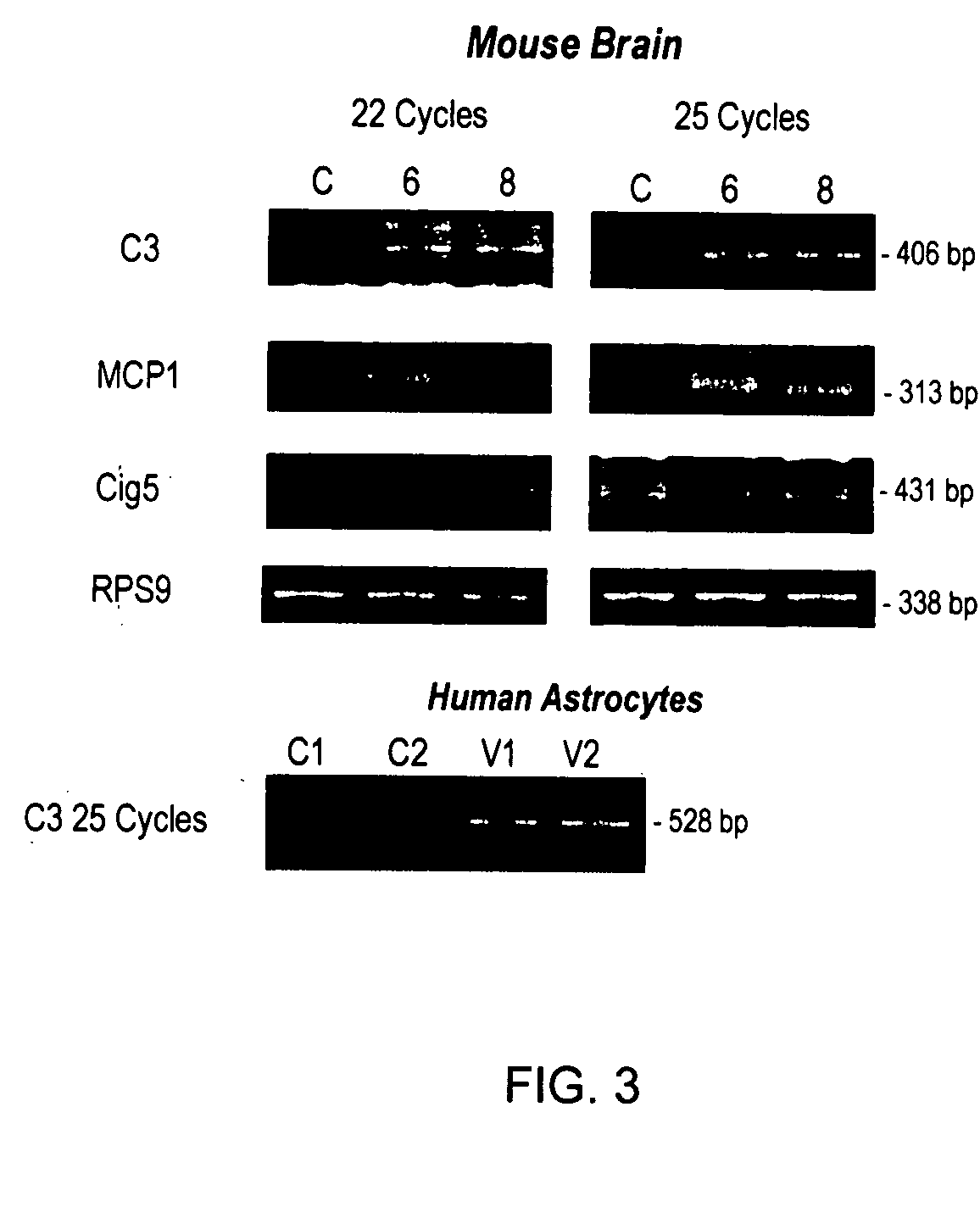
Development of a murine model of HIV-1 infection on the basis of construction of EcoHIV, a chimeric, molecular clone of human immunodeficiency virus type 1 and ecotropic moloney murine leukemia virus competent to infect murine cells and mice
The present invention provides a chimeric HIV-1 construct, EcoHIV, capable of replication in a rodent cell. The invention also provides a convenient and safe rodent model of HIV-1 infection and AIDS. Methods for producing a rodent model of HIV-1 infection are also provided. Additionally, the invention provides the means to test immunogenic compositions or pharmaceutical interventions effective in preventing infection, reducing viral load, or reducing disease symptoms in a subject.
Owner:POTASH MARY +1
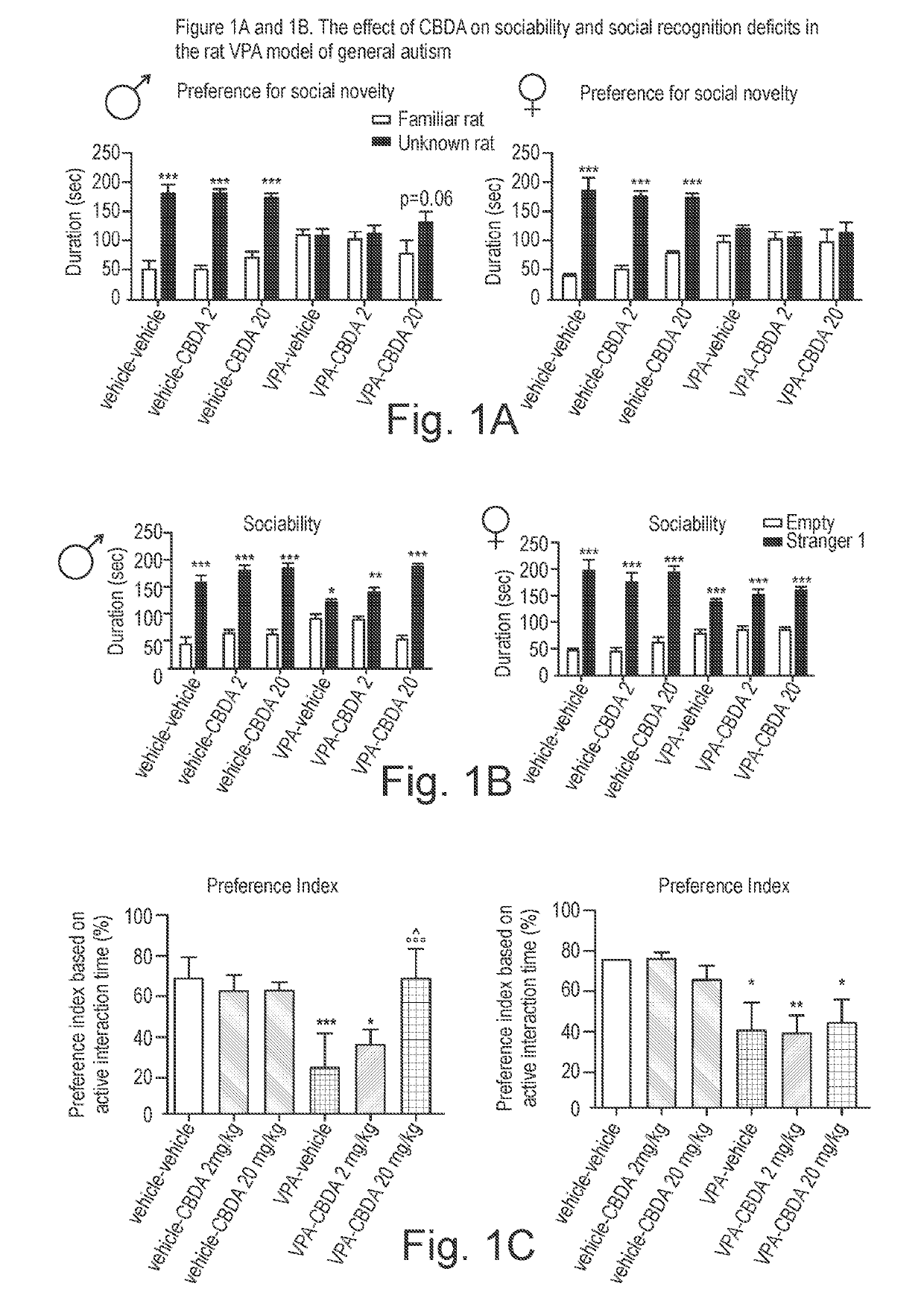
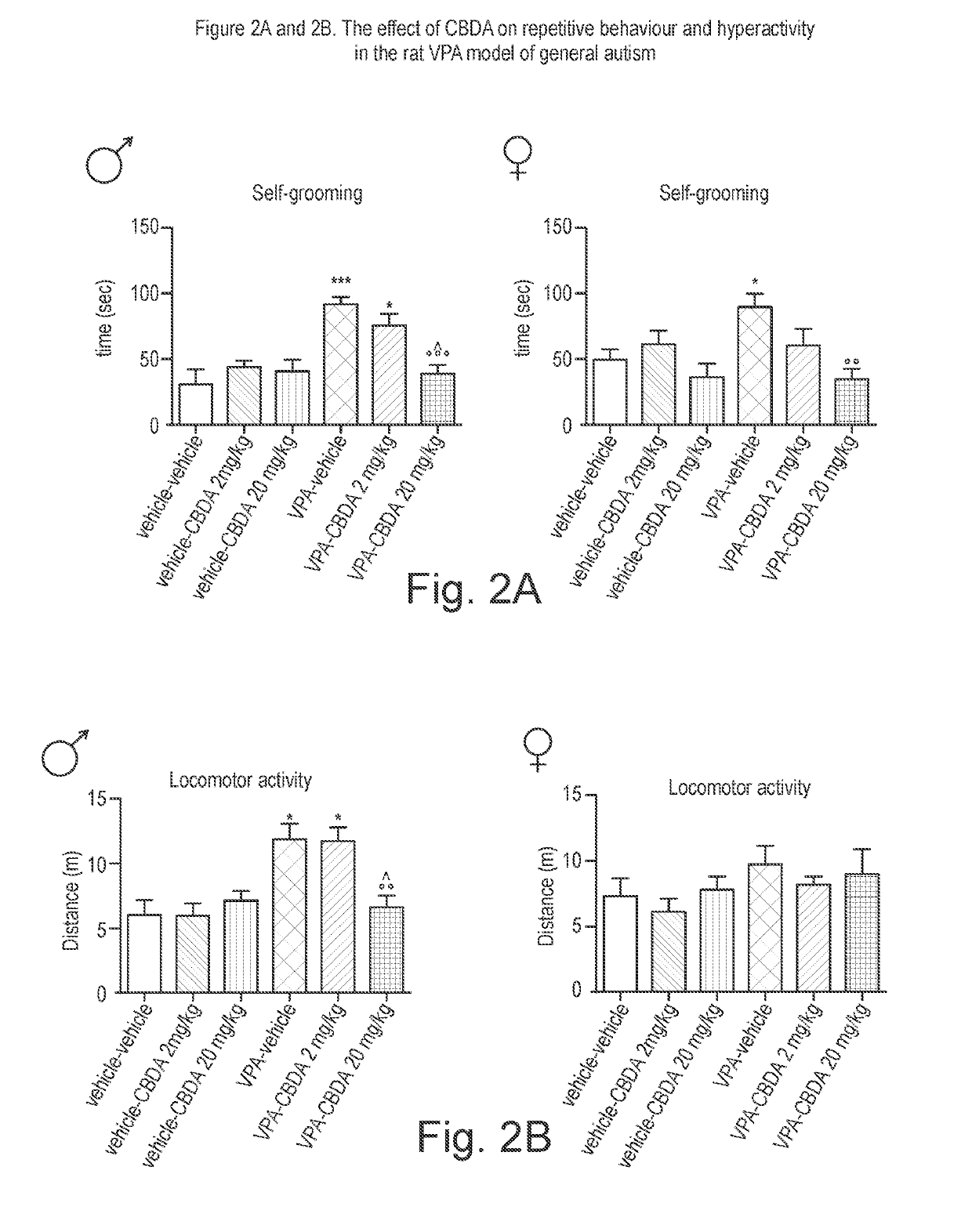
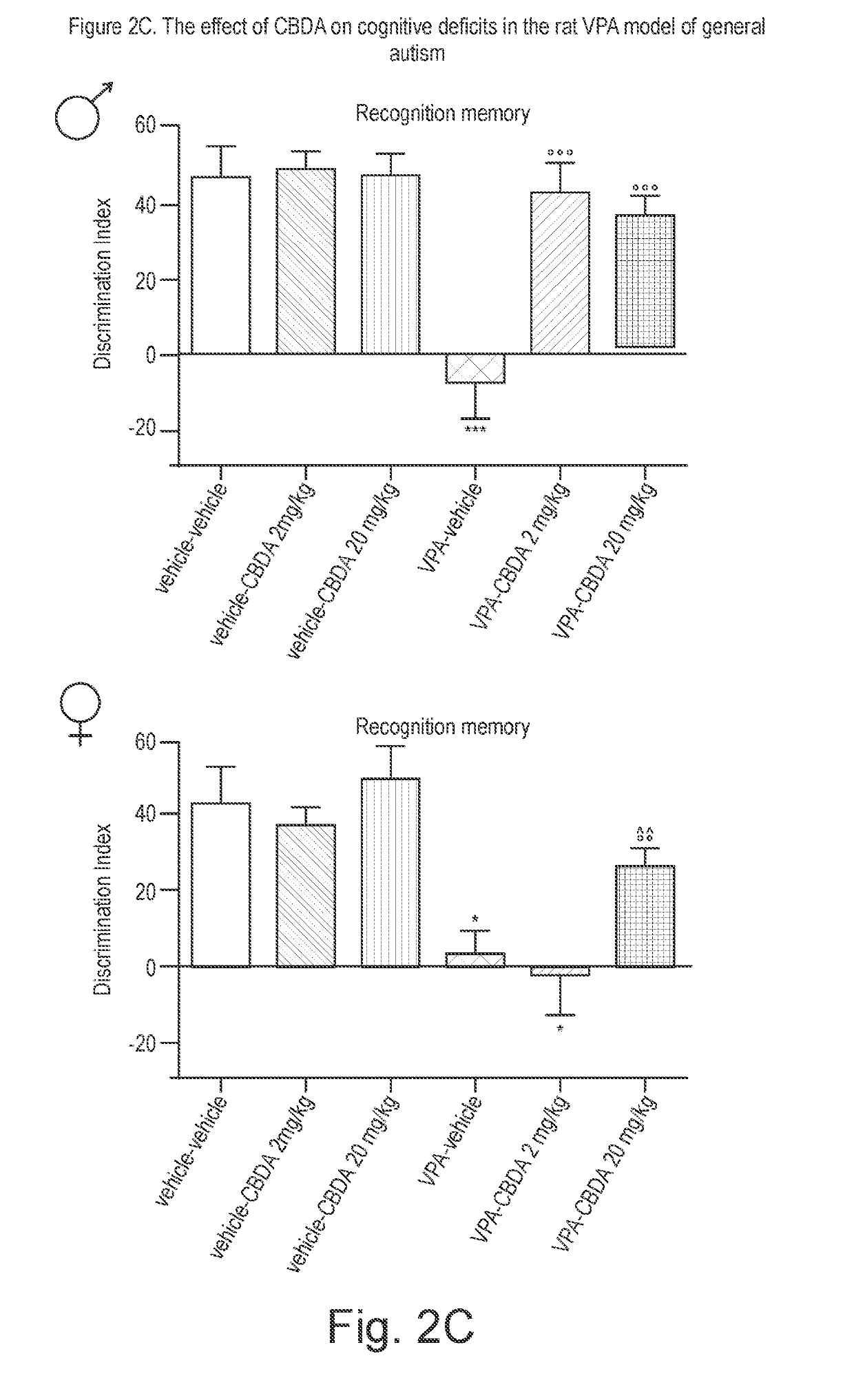
Use of cannabidiolic acid in the treatment of autism spectrum disorder and associated disorders
ActiveUS20190117619A1Symptoms improvedNervous disorderHydroxy compound active ingredientsDiseaseRodent model
The present invention relates to the use of cannabidiolic acid (CBDA) in the treatment of autism spectrum disorder (ASD) and ASD-associated disorders, such as Fragile X syndrome (FXS); Rett syndrome (RS); or Angelman syndrome (AS). CBDA has been shown to be particularly effective in improving cognitive dysfunction in rodent models of ASD, FXS, RS and AS. The CBDA is preferably substantially pure. It may take the form of a highly purified extract of cannabis such that the CBDA is present at greater than 95% of the total extract (w / w) and the other components of the extract are characterised. Alternatively, the CBDA is synthetically produced.
Owner:GW RES LTD
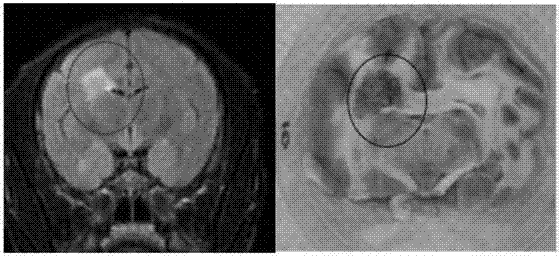
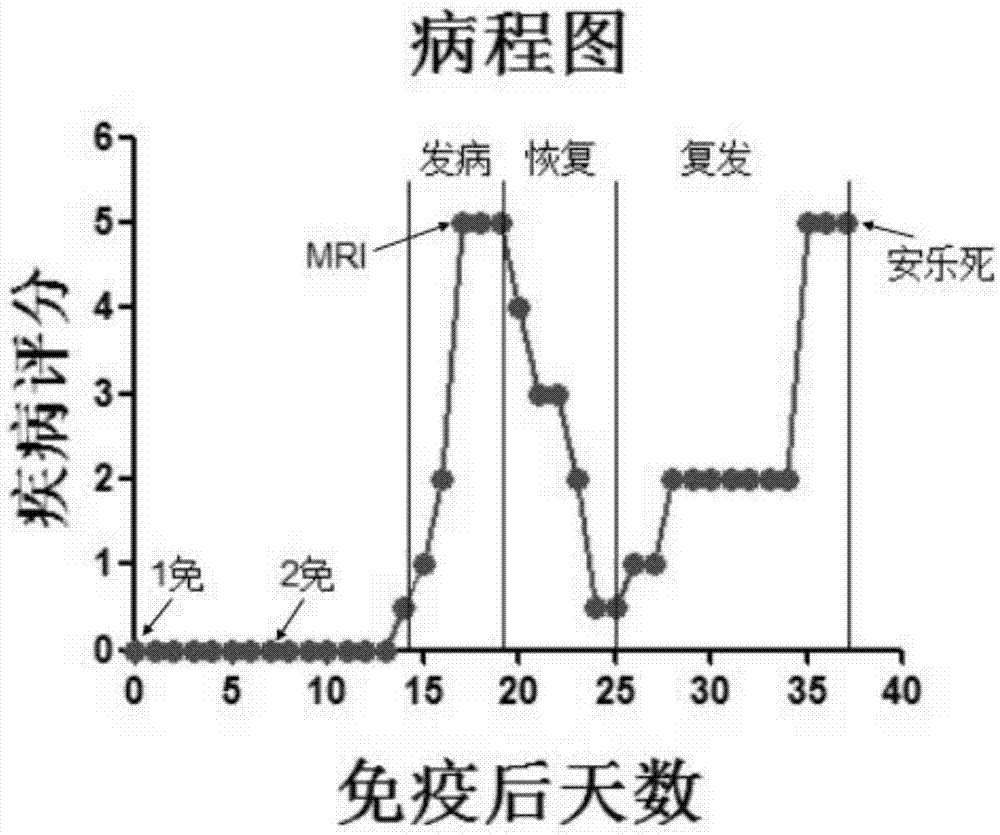
Method for establishing Macaca fascicularis experimental autoimmune encephalomyelitis model and application thereof
InactiveCN103933550AIndividual smallReduce usagePeptide/protein ingredientsEmulsion deliveryDiseaseNODAL
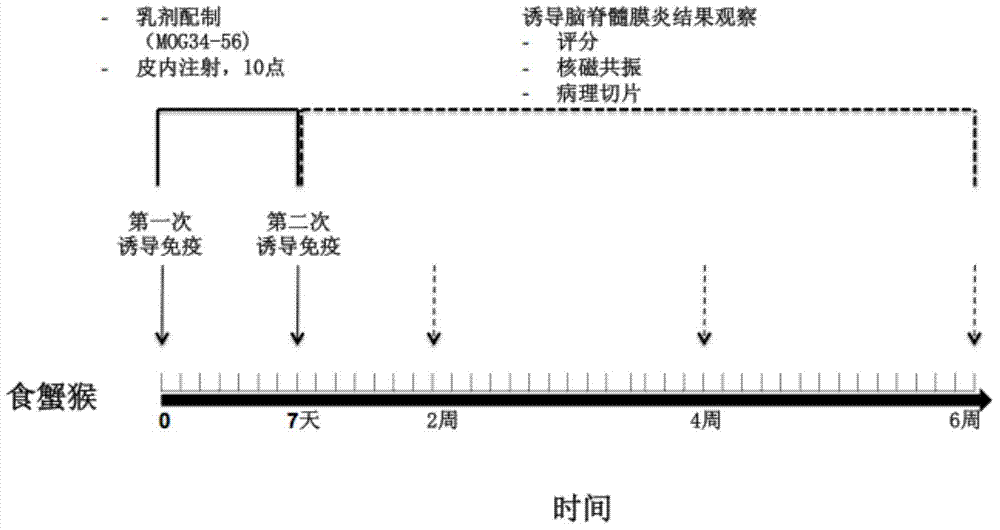
The invention discloses a method for establishing a Macaca fascicularis experimental autoimmune encephalomyelitis model and application thereof. According to a concrete technical scheme in the invention, the method comprises the following steps: preparing an emulsion (an MOG solution: CFA = 1: 1) from MOG 34-56 (100 mu g / ml); narcotizing Macaca fascicularis for experiments and injecting 1 ml of the prepared emulsion at 10 injection points; carrying out immunization injection of the emulsion (secondary immunization) according to the above-mentioned method and dose in 7 days after the primary immunization injection (primary immunization); carrying out clinical observation in one day after primary immunization and recording clinical scores; and determining pathogenic sites, degrees and the like by using an MRI iconographic method at pathogenic time nodes. The model established in the invention has an application value which cannot be achieved by other rodent models, has the characteristics of recurrence-alleviation type attacks, low cost, wide availability of Macaca fascicularis for experiments and the like compared with a Macaca rhesus model and has a wide application scope in fields related to multiple sclerosis diseases.
Owner:上海浦灵生物科技有限公司
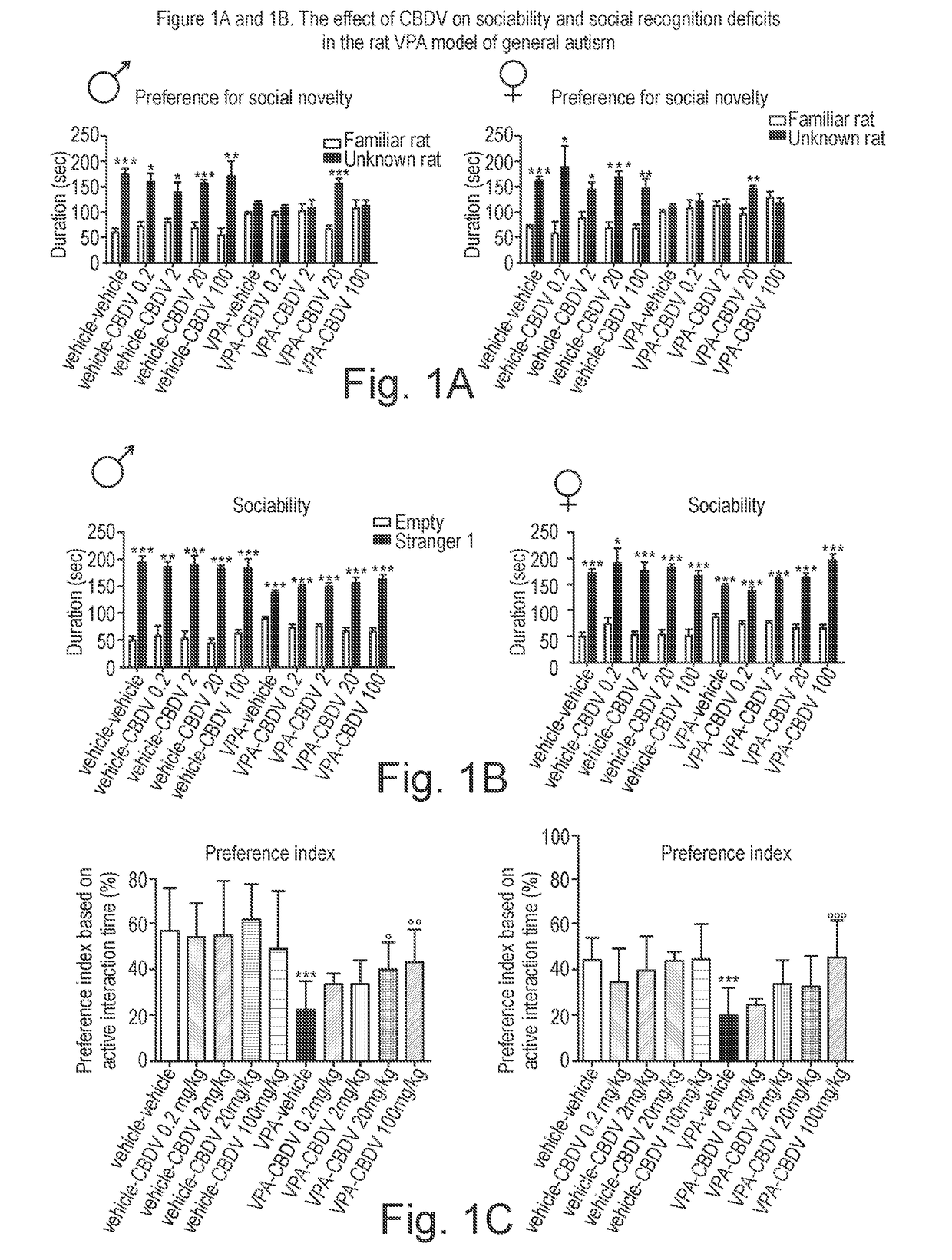
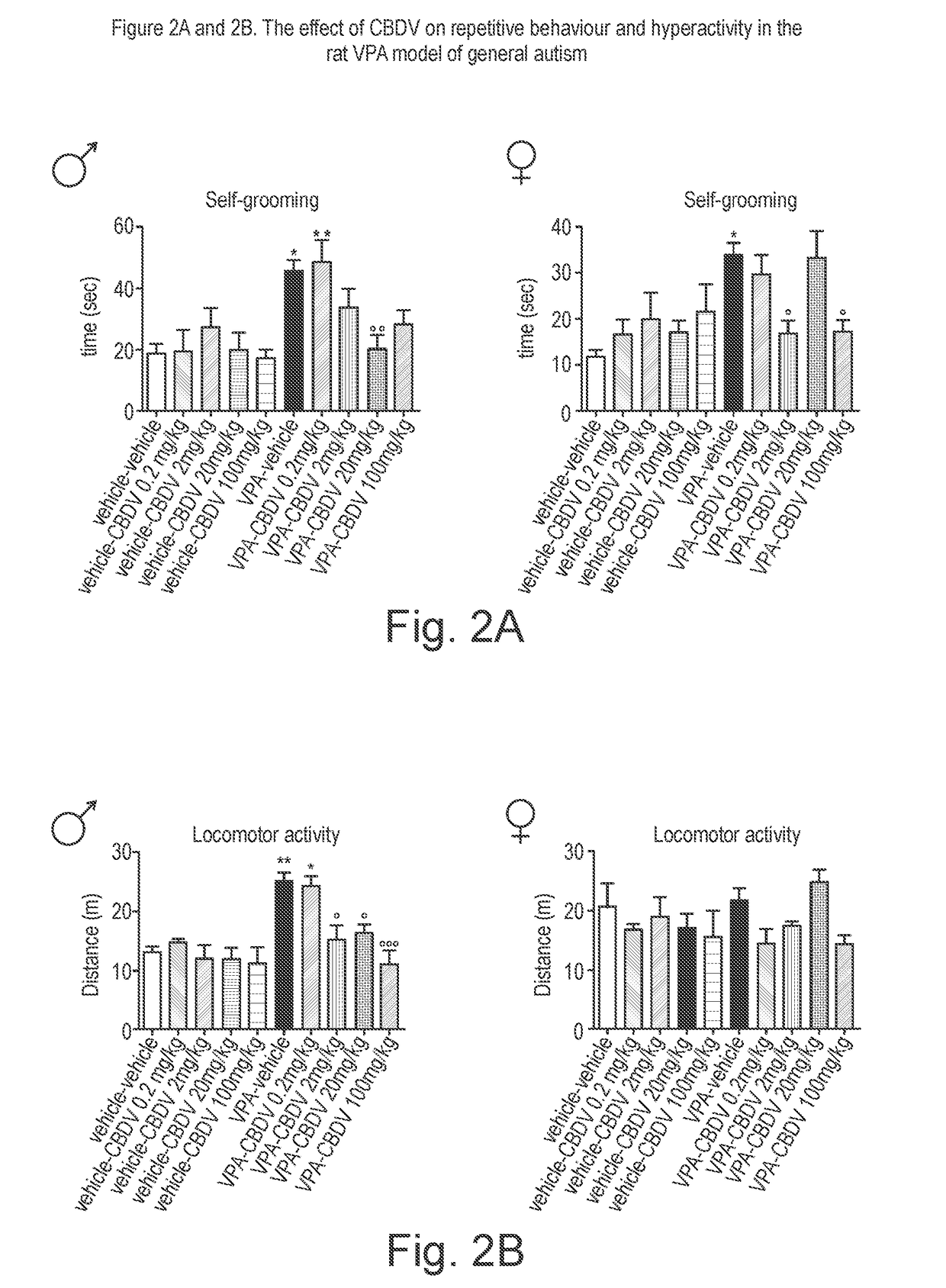
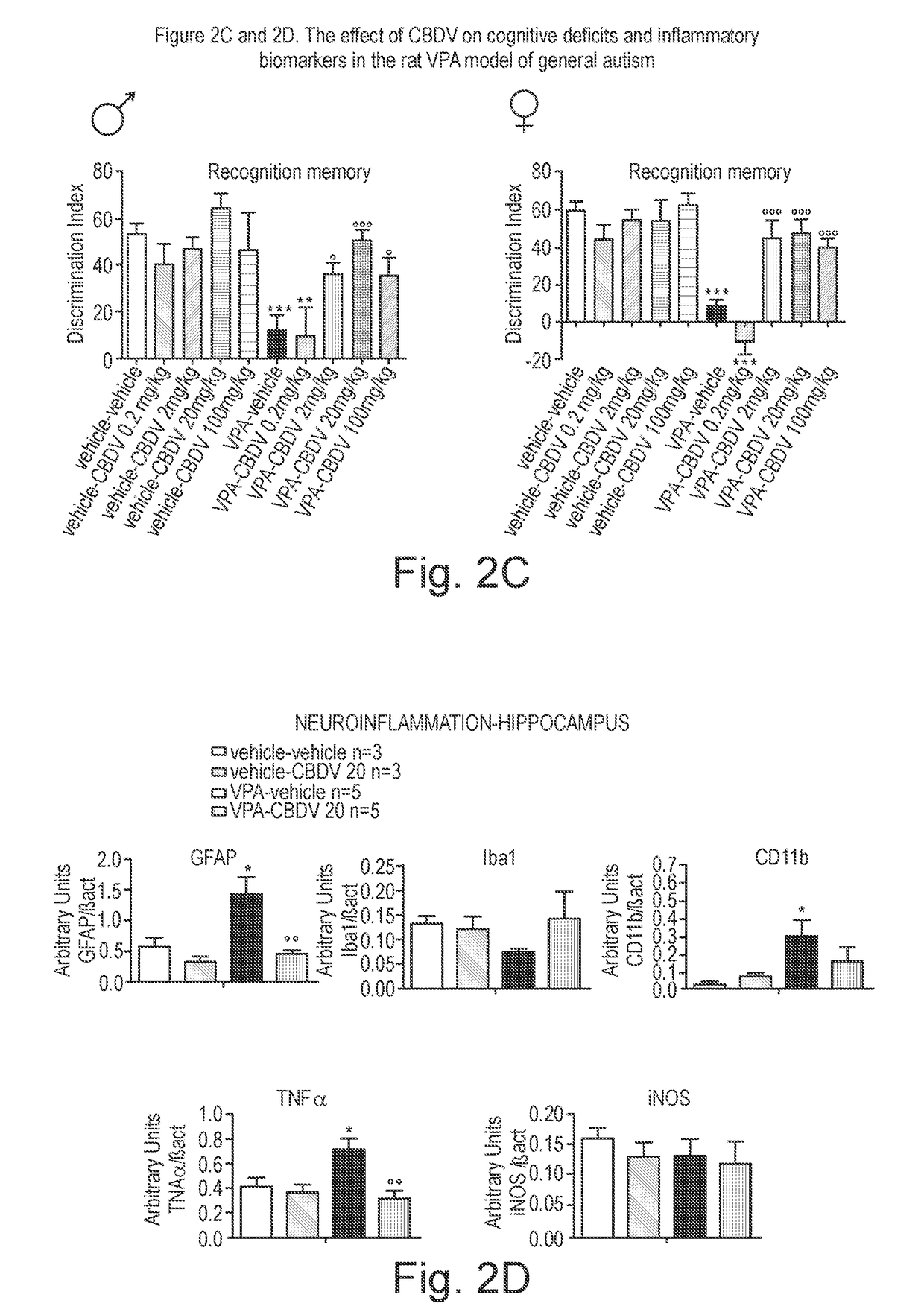
Use of cannabidivarin in the treatment of autism spectrum disorder, associated disorders and schizophrenia
InactiveUS20190070128A1Symptoms improvedNervous disorderHydroxy compound active ingredientsCannabisDisease
The present invention relates to the use of cannabidivarin (CBDV) in the treatment of autism spectrum disorder (ASD) and ASD-associated disorders such as Fragile X syndrome (FXS); Rett syndrome (RS); or Angelman syndrome (AS). In a further embodiment the invention relates to the use of CBDV in the treatment of schizophrenia. CBDV has been shown to be particularly effective in improving cognitive dysfunction in rodent models of ASD, FXS, RS, AS and schizophrenia. The CBDV is preferably substantially pure. It may take the form of a highly purified extract of cannabis such that the CBDV is present at greater than 95% of the total extract (w / w) and the other components of the extract are characterised. Alternatively, the CBDV is synthetically produced.
Owner:GW RES LTD
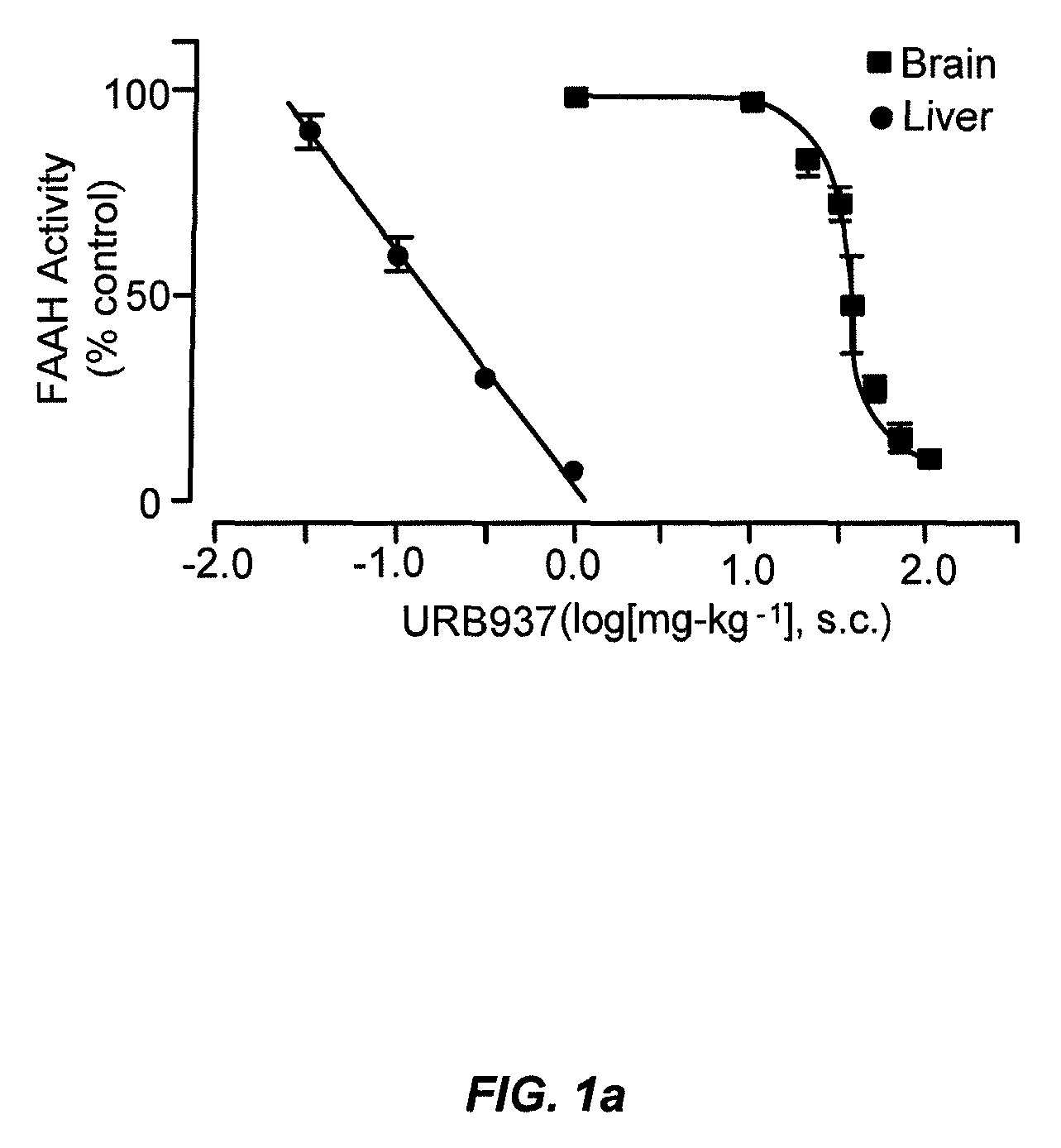
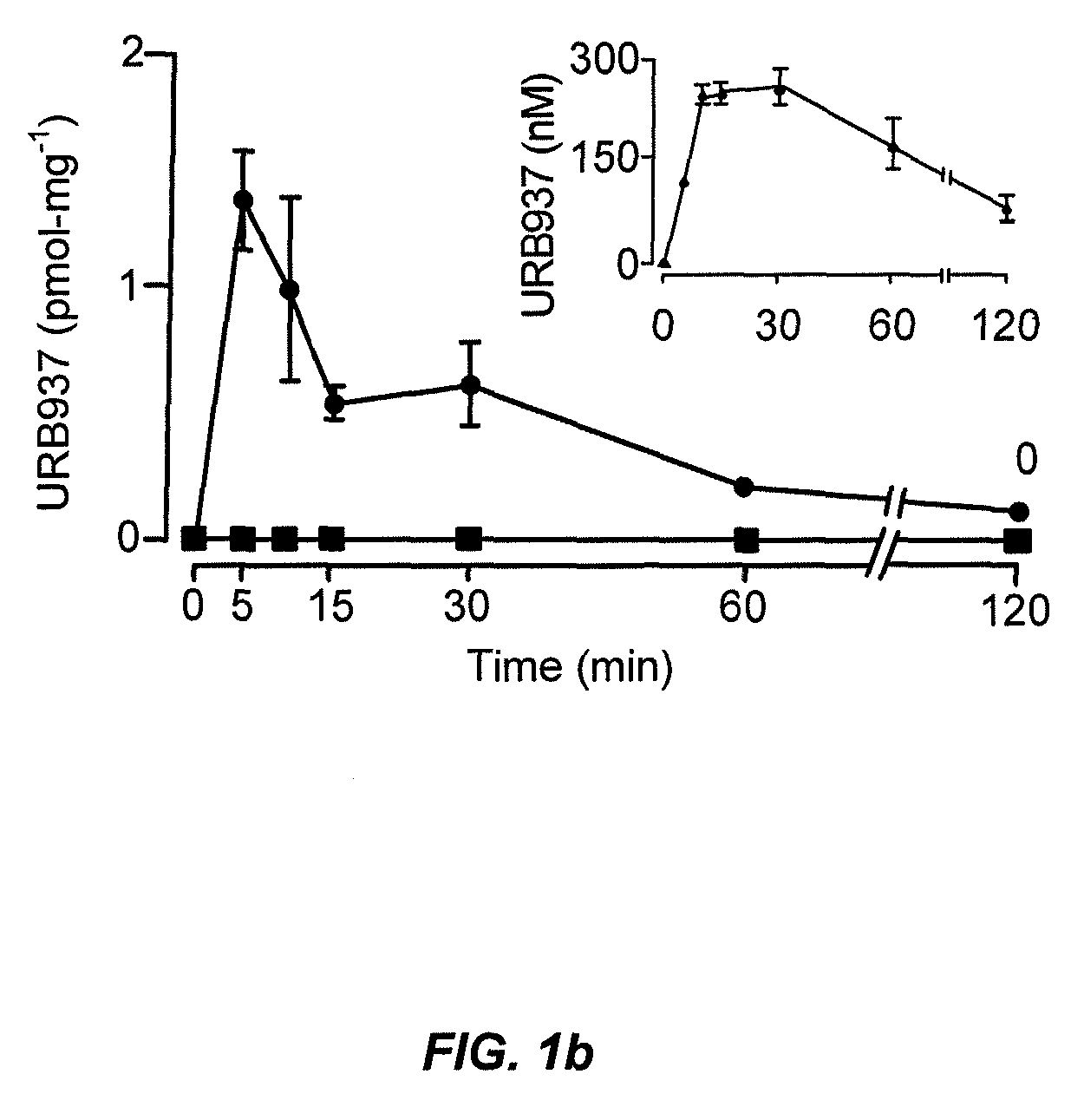
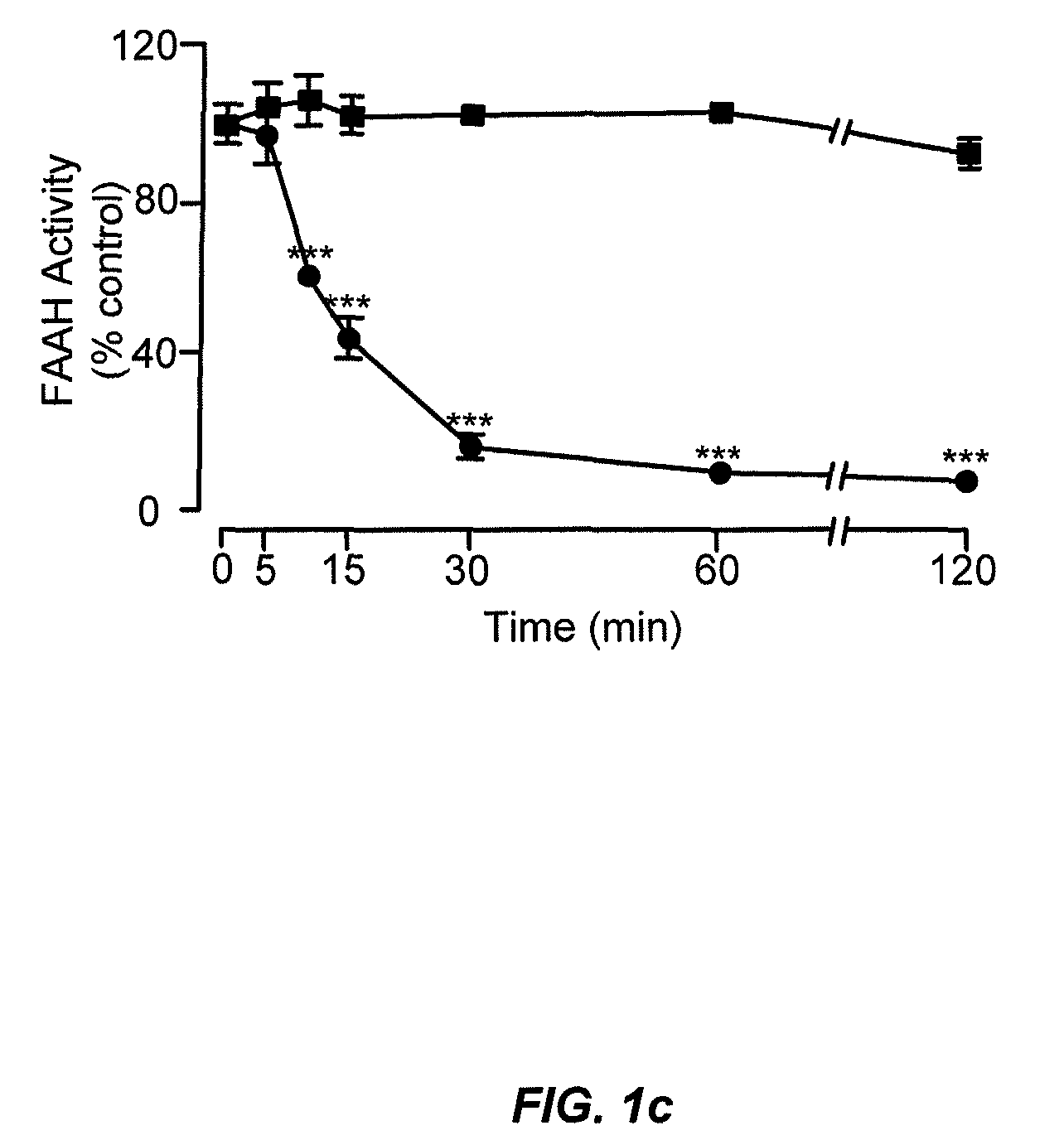
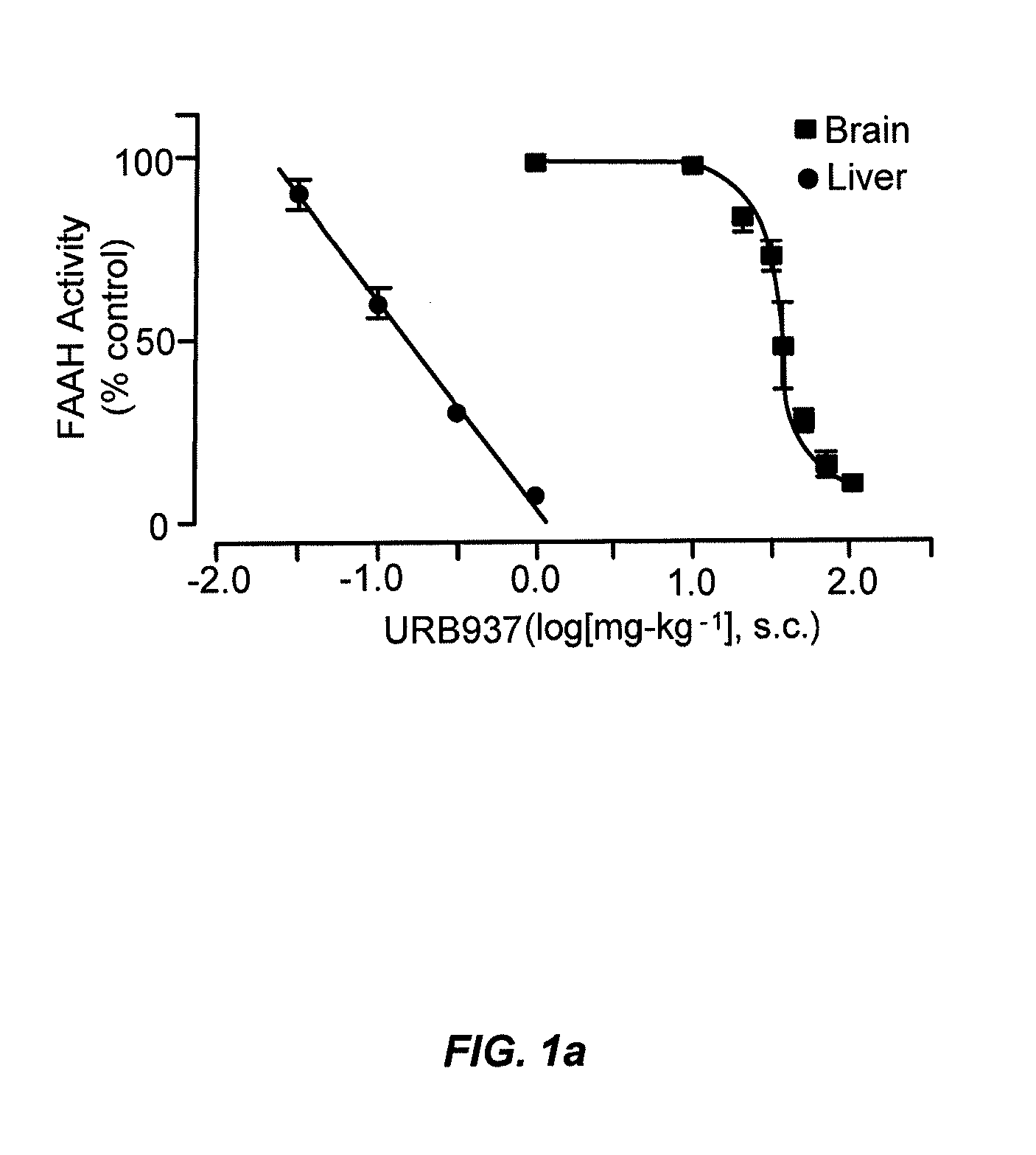
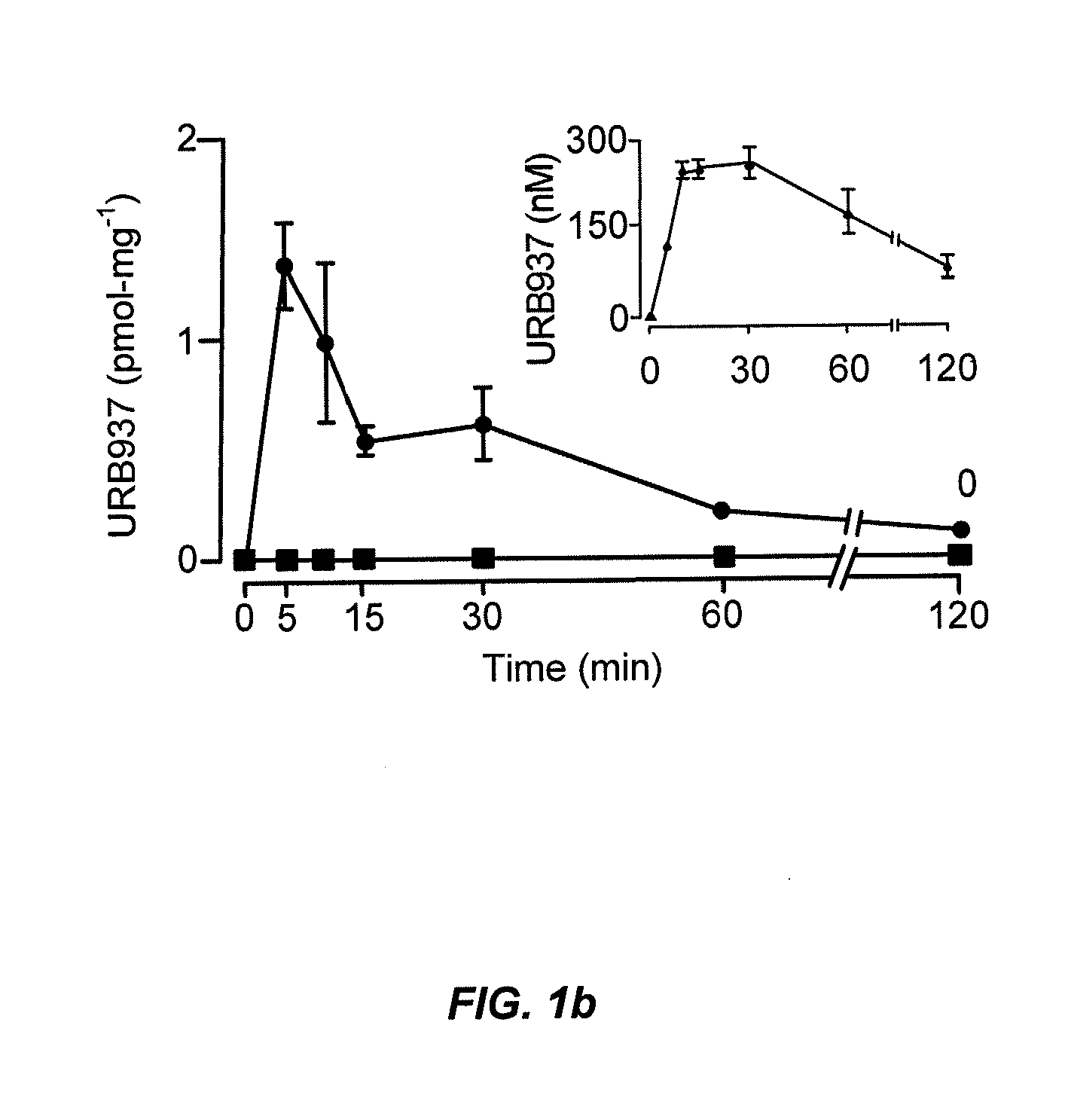
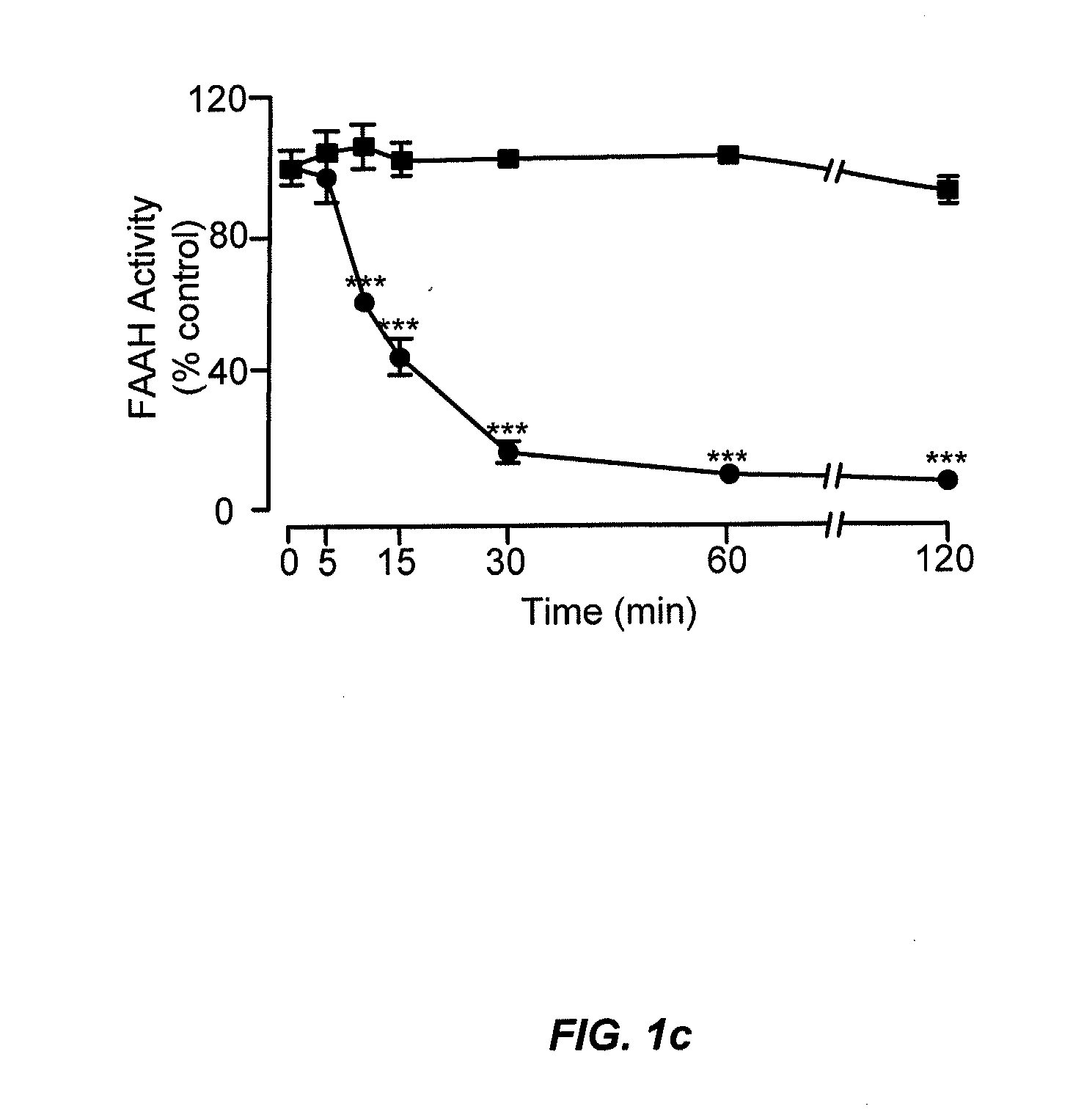
Peripherally restricted FAAH inhibitors
ActiveUS9187413B2Enhancing peripheral activityBiocideNervous disorderDepressantPharmaceutical medicine
Peripherally restricted inhibitors of fatty acid amide hydrolase (FAAH) are provided. The compounds can suppress FAAH activity and increases anandamide levels outside the central nervous system (CNS). Despite their relative inability to access brain and spinal cord, the compounds attenuate behavioral responses indicative of persistent pain in rodent models of inflammation and peripheral nerve injury, and suppresses noxious stimulus-evoked neuronal activation in spinal cord regions implicated in nociceptive processing. CBi receptor blockade prevents these effects. Accordingly, the invention also provides methods, and pharmaceutical compositions for treating conditions in which the inhibition of peripheral FAAH would be of benefit. The compounds of the invention are according to the formula (I): in which R1 is a polar group. In some embodiments, R1 is selected from the group consisting of hydroxy and the physiologically hydro lysable esters thereof. R2 and R3 are independently selected from the group consisting of hydrogen and substituted or unsubstituted hydrocarbyl; each R4 is independently selected from the group consisting of halogen and substituted or unsubstituted hydrocarbyl and n is an integer from 0 to 4; each R5 is independently selected from the group consisting of halo and substituted or unsubstituted hydrocarbyl and m is an integer from 0 to 3; and R6 is substituted or unsubstituted cyclohexyl; and the pharmaceutically acceptable salts thereof.
Owner:UNIV DEGLI STUDI DI URBINO +1
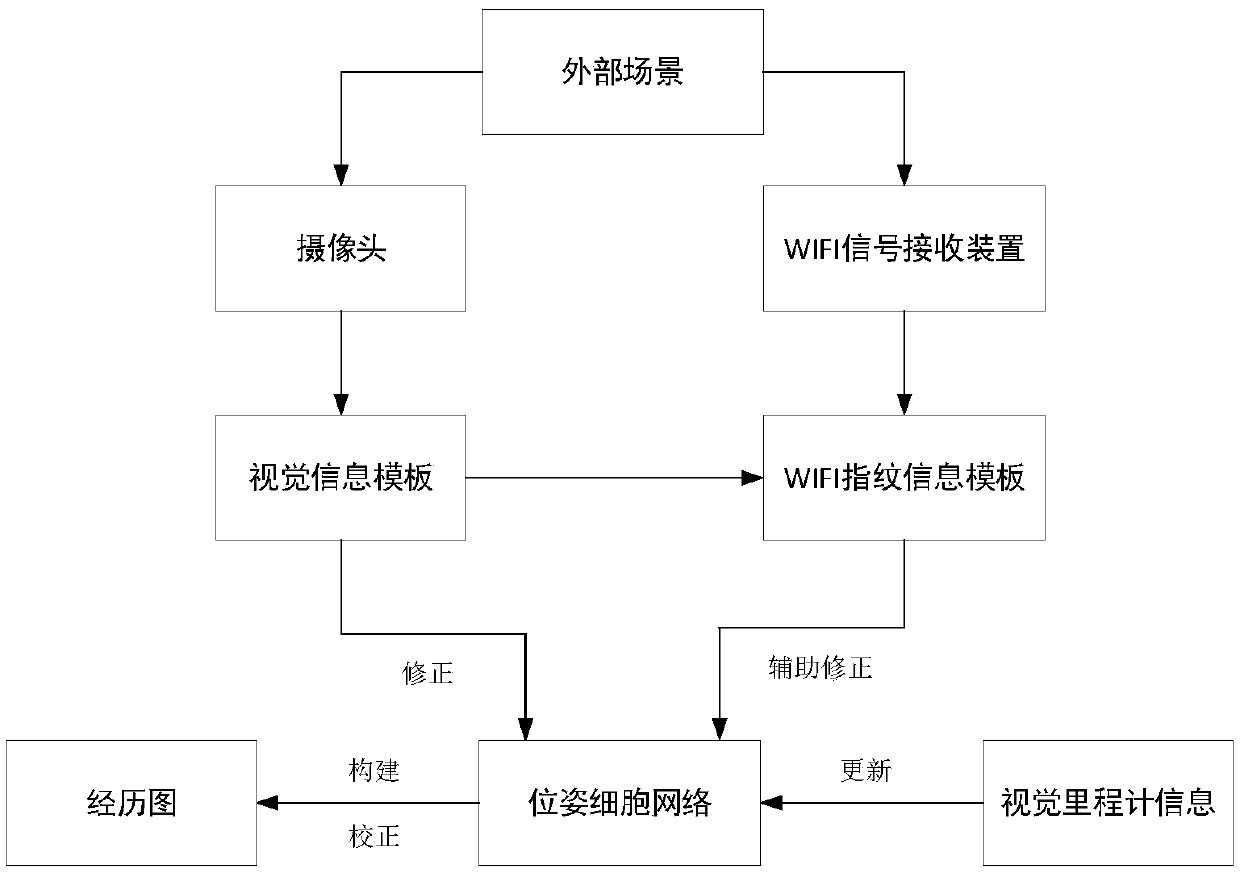
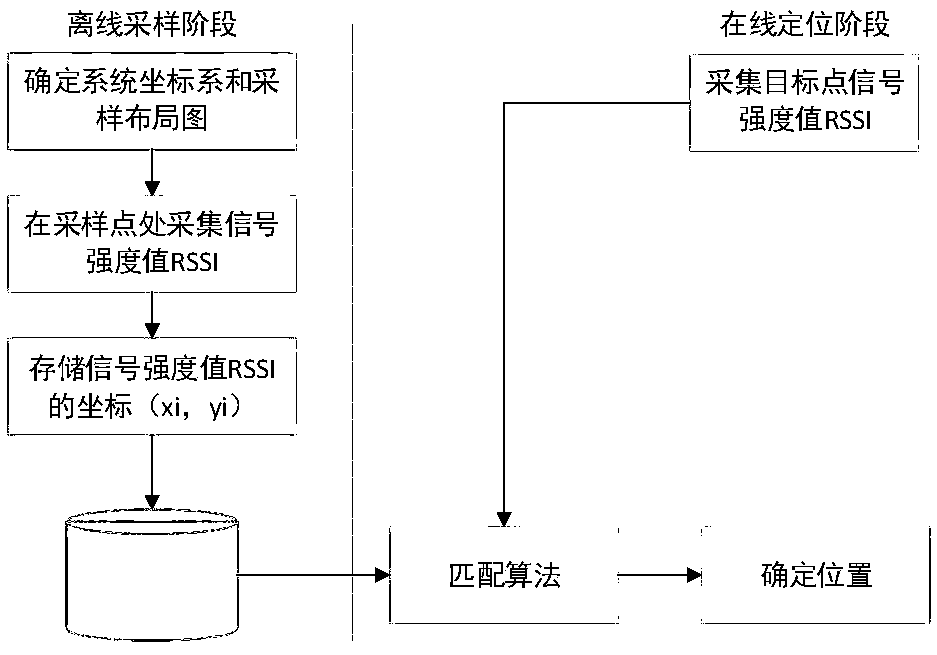
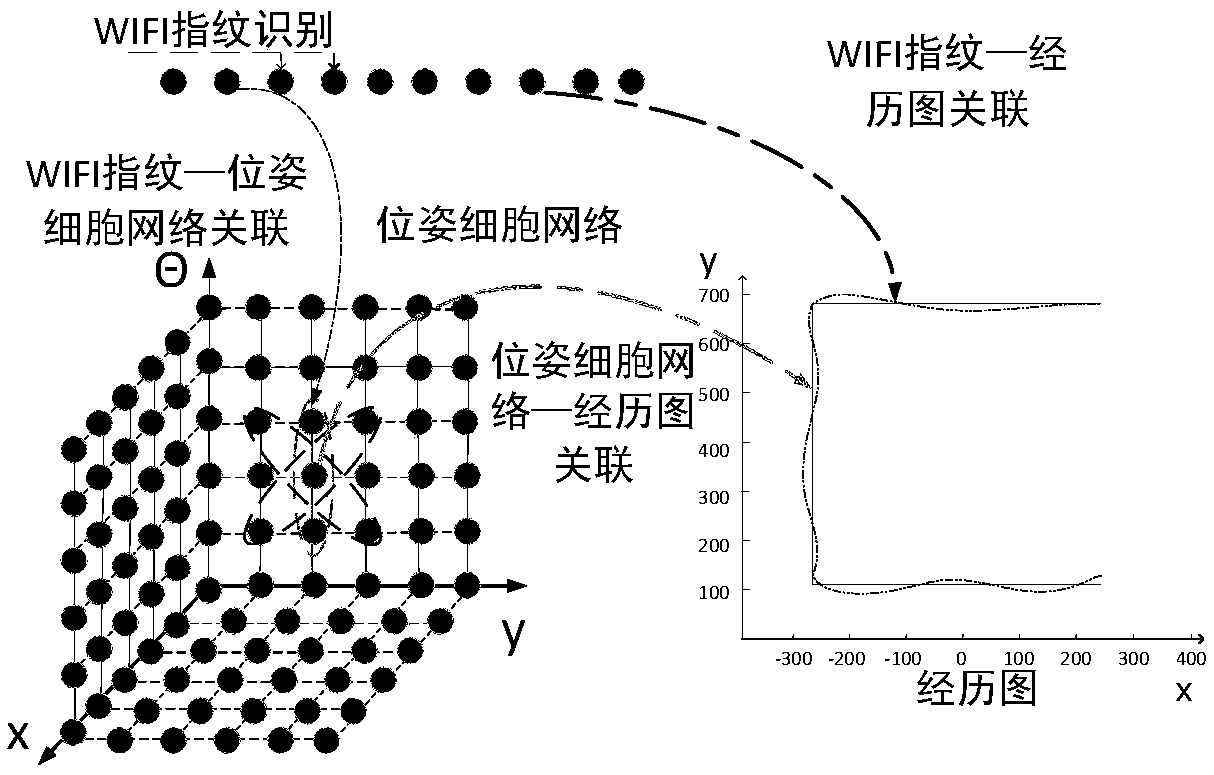
SLAM method based on rodent model and WIFI fingerprint
ActiveCN108712725AHigh positioning accuracyCorrective activityImage enhancementImage analysisRodent modelMobile robot
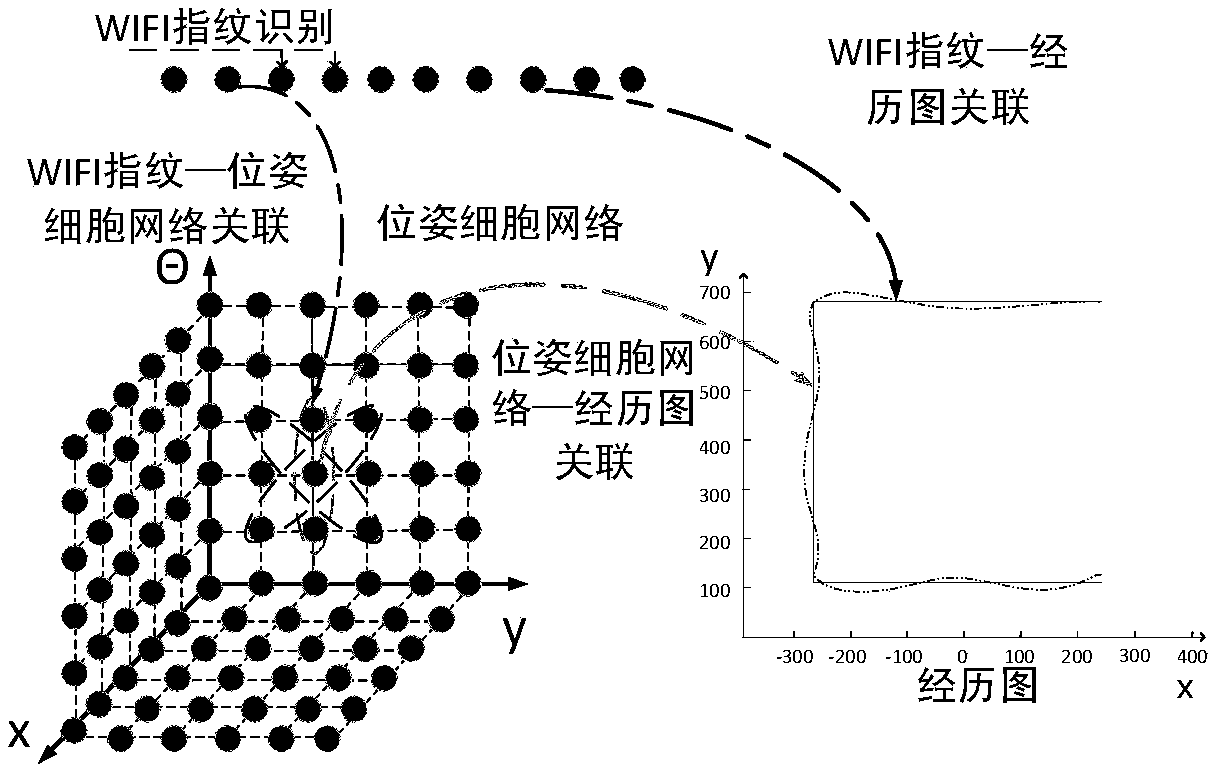
The invention discloses a SLAM method based on a rodent model and a WIFI fingerprint and relates to the field of bionics and wireless signal networks; a fingerprint recognition method based on WIFI signal strength is used; the WIFI fingerprint information is used for replacing local scene cellular network in the original rodent model; a location fingerprint database is established in an offline stage; a Bayesian algorithm is used for completing matching of WIFI signal strength fingerprint information in the online locating stage; the activity of the pose cellular network is corrected; finally,a more accurate experience map is obtained; and the SLAM method based on the rodent model and WIFI fingerprint in the present invention is adopted so that not only the locating accuracy of the mobilerobot is higher but also the stability of the system is improved and has good locating performance.
Owner:ANHUI UNIVERSITY OF TECHNOLOGY AND SCIENCE
Methods for screening human blood products comprising plasma using immunocompromised rodent models
ActiveUS10905779B2Compounds screening/testingMammal material medical ingredientsImmune compromisedNeurogenesis
Owner:ALKAHEST INC +2
Simultaneous localization and mapping method and simultaneous localization and mapping apparatus based on rodent model
InactiveCN108680175AImplement fixesAchieve precise positioningInstruments for road network navigationLocation information based serviceInformation repositorySimultaneous localization and mapping
The invention provides a simultaneous localization and mapping method and a simultaneous localization and mapping apparatus based on a rodent model. The simultaneous localization and mapping method comprises: acquiring the current visual field scene image information of a robot; according to a pre-constructed rodent model, matching to a pre-constructed visual field information database to obtain pose information, wherein the pose information and the current visual field scene image information have the maximum scene similarity; acquiring the current WIFI signal intensity set of the robot whenthe scene similarity is lower than a set threshold; according to the rodent model, matching to a pre-constructed WIFI fingerprint map to obtain pose information, wherein the pose information and the current WIFI signal intensity set have the maximum fingerprint similarity; and according to the pose information corresponding to the maximum scene similarity and / or the maximum fingerprint similarity,carrying out simultaneous localization and mapping on the robot.
Owner:ANHUI UNIVERSITY OF TECHNOLOGY AND SCIENCE
HIV-Env gene DNA allosteric recombinant envelope protein antigen immune response anti HIV experiment and method
InactiveCN101021539AEfficient removalIncrease variabilityMicrobiological testing/measurementBiological testingHIV ProteinsClinical trial
This invention is called: the experiment and method of the protein envelope which is allosteric restructuring of the HIV-Env gene DNA and its antigenic immunity responsive HIV. It relates to the domain of life sciences and medicine sciences. It is confirmed that gp120 of the HIV protein envelope or the gp160 of the antigenic vaccine combines with cell CD4+ that can induce the infirm immunogen of the alloantigen of the immune tolerant receptor CD4 and can not induce the HIV to infect the immune response of the parasitifer. It can also eliminate the multi-clone group of hill of the wild virus which is of fast copy the initial infection virus, high variability and sequence diversity. This invention can develop the non-spark human tolerant immunity HIV vaccine of the protein envelope which is allosteric restructuring of the HIV-Env gene DNA, the HIV preventive vaccine that can provide the scientific basis of the direct animal experiment and direct clinical trial of human being. It also provides the enforceable anti-HIV cell model, rodent model, non-human Primates model and the research program of human clinical trial.
Owner:叶新新
Method for establishing non-human primate autoimmune cerebrospinal meningitis model and application of model
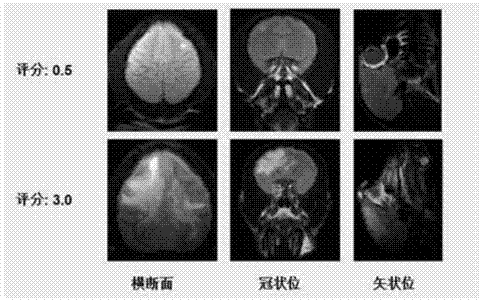
InactiveCN104758080AShorten the induction periodIndividual smallVeterinary instrumentsDiseaseMS multiple sclerosis
The invention discloses a method for establishing a non-human primate autoimmune cerebrospinal meningitis model and the application of the model. The specific technical scheme comprises the following steps; a machin is selected as the non-human primate experimental animals; MOG34-56(100Mug / ml) is prepared into an emulsion (MOG CFA=1; 1); after the experimental machin is anesthetized, the machin is intradermally injected with 1ml of prepared emulsion at 10 injection points; on the seventh day after the first injection, an MOG-CFA emulsion is prepared by use of the same method, and the second immune injection is performed at the same dosage and by use of the same method; the experimental autoimmune cerebrospinal meningitis model established by use of the method has the clinical characters of remission-relapse, and can be widely used to the related field of disseminated sclerosis diseases. Compared with the existing rhesus method and model, the method and the model also have the characteristics of short induction period, low cost, rich monkey resources and the like, and also have the application value which cannot be realized by rodent models.
Owner:上海浦灵生物科技有限公司
Establishment of rhesus monkey immunosuppression model
InactiveCN105030794AImprove the evaluation systemOrganic active ingredientsImmunological disordersDiseaseRodent model
The invention relates to establishment of a rhesus monkey immunosuppression model, and provides an application of cyclophosphamide in preparing the rhesus monkey immunosuppression model. Specifically, cyclophosphamide with different dosages are selected to carry out intravenous injection on the rhesus monkey, then the suitable immunosuppression molding dosage is determined, and a disease model consistent with the variation tend of various immune indices under human immunosuppression state is established, wherein the optimal use method and the optimal use amount of cyclophosphamide are as follows: intravenous injection is carried out at 15mg / kg each day. The invention further provides a method for preparing the rhesus monkey immunosuppression model, and an immunosuppression animal module prepared by the method. The animal mold can be used for the fields of biotechnology new drug research and development, immunosuppression mechanism study, virus infection modeling and the like, which can not be completed by rodent model, and provides a more ideal and more reliable experimental animal models and a perfect evaluation system for the above application compared with the rodent model.
Owner:SICHUAN AGRI UNIV
Peripherally restricted faah inhibitors
ActiveUS20130217764A1Enhancing peripheral activityBiocideNervous disorderBehavioral responseReceptor blockade
Peripherally restricted inhibitors of fatty acid amide hydrolase (FAAH) are provided. The compounds can suppress FAAH activity and increases anandamide levels outside the central nervous system (CNS). Despite their relative inability to access brain and spinal cord, the compounds attenuate behavioral responses indicative of persistent pain in rodent models of inflammation and peripheral nerve injury, and suppresses noxious stimulus-evoked neuronal activation in spinal cord regions implicated in nociceptive processing. CBi receptor blockade prevents these effects. Accordingly, the invention also provides methods, and pharmaceutical compositions for treating conditions in which the inhibition of peripheral FAAH would be of benefit. The compounds of the invention are according to the formula (I): in which R1 is a polar group. In some embodiments, R1 is selected from the group consisting of hydroxy and the physiologically hydro lysable esters thereof. R2 and R3 are independently selected from the group consisting of hydrogen and substituted or unsubstituted hydrocarbyl; each R4 is independently selected from the group consisting of halogen and substituted or unsubstituted hydrocarbyl and n is an integer from 0 to 4; each R5 is independently selected from the group consisting of halo and substituted or unsubstituted hydrocarbyl and m is an integer from 0 to 3; and R6 is substituted or unsubstituted cyclohexyl; and the pharmaceutically acceptable salts thereof.
Owner:UNIV DEGLI STUDI DI URBINO +1
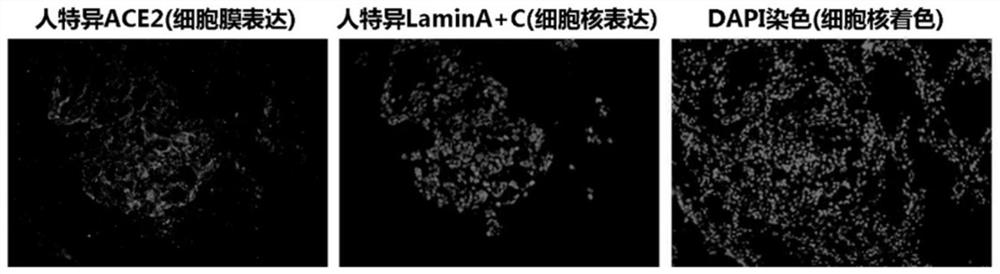
Novel coronavirus SARS-CoV-2 infected rodent model and construction method and application thereof
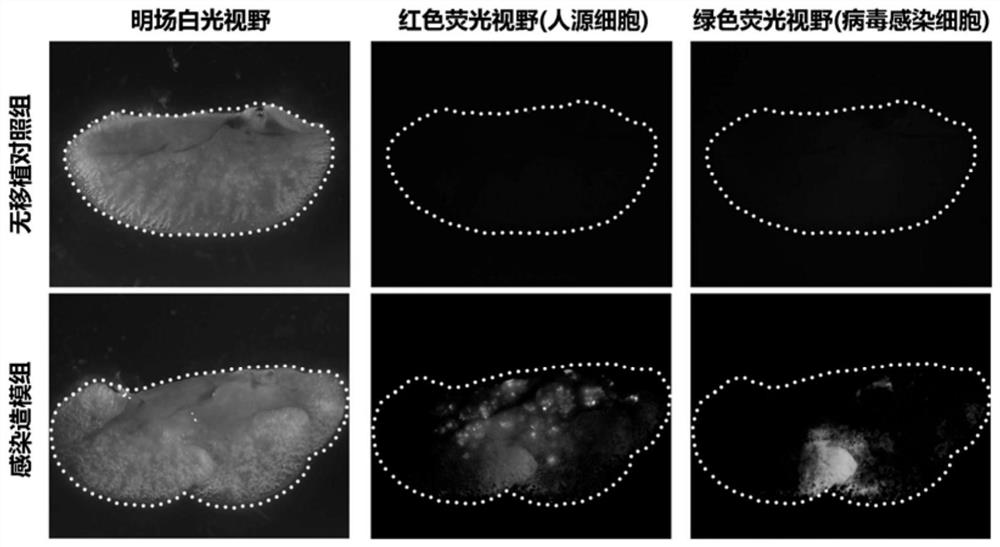
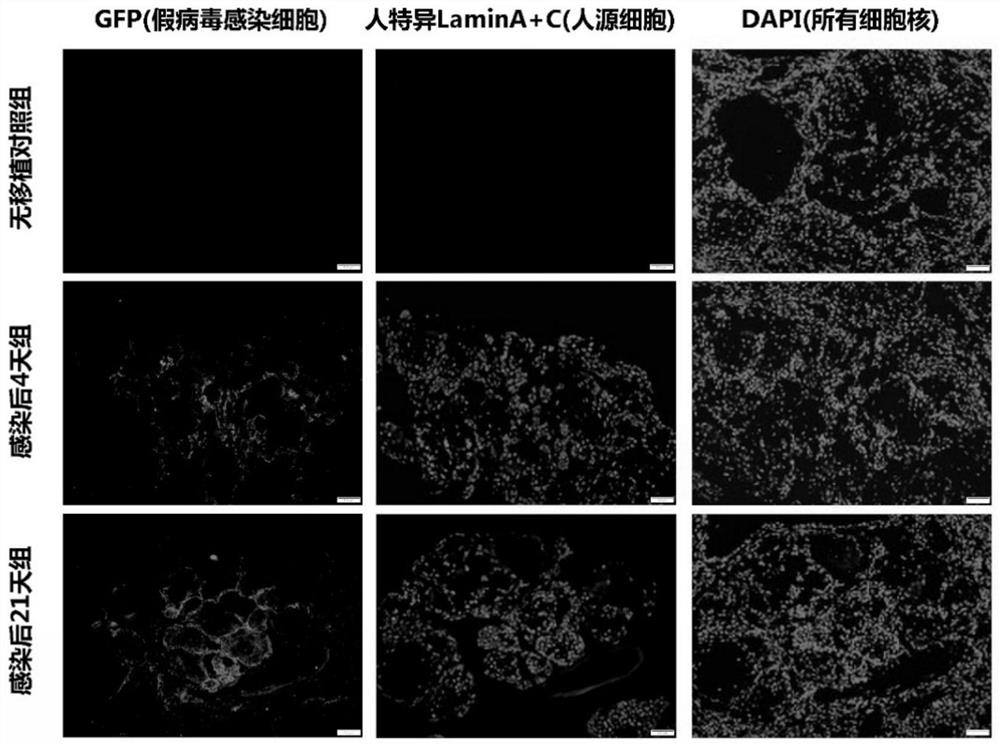
The invention relates to the technical field of animal model construction, and particularly provides a novel coronavirus SARS-CoV-2 infected rodent model and a construction method and application thereof. The construction method comprises the following steps of constructing a lung injury animal model, specifically, selecting an immunodeficient animal as an object to construct the lung injury animal model; conducting cell transplantation, specifically, transplanting human lung epithelial cells into the lung of the lung injury animal model; and conducting SARS-CoV-2 virus infection, specifically, transplanting SARS-CoV-2 viruses into the animal lung treated in the step of cell transplantation for infection, and establishing the novel coronavirus SARS-CoV-2 infected rodent model. By means of the method, the construction process is simple and convenient, operation is easy, the construction cost is low, and a normal human lung tissue structure and a human ACE2 receptor protein expression situation can be simulated to the greatest extent.
Owner:TONGJI UNIV +1
Rodent model of prostate cancer
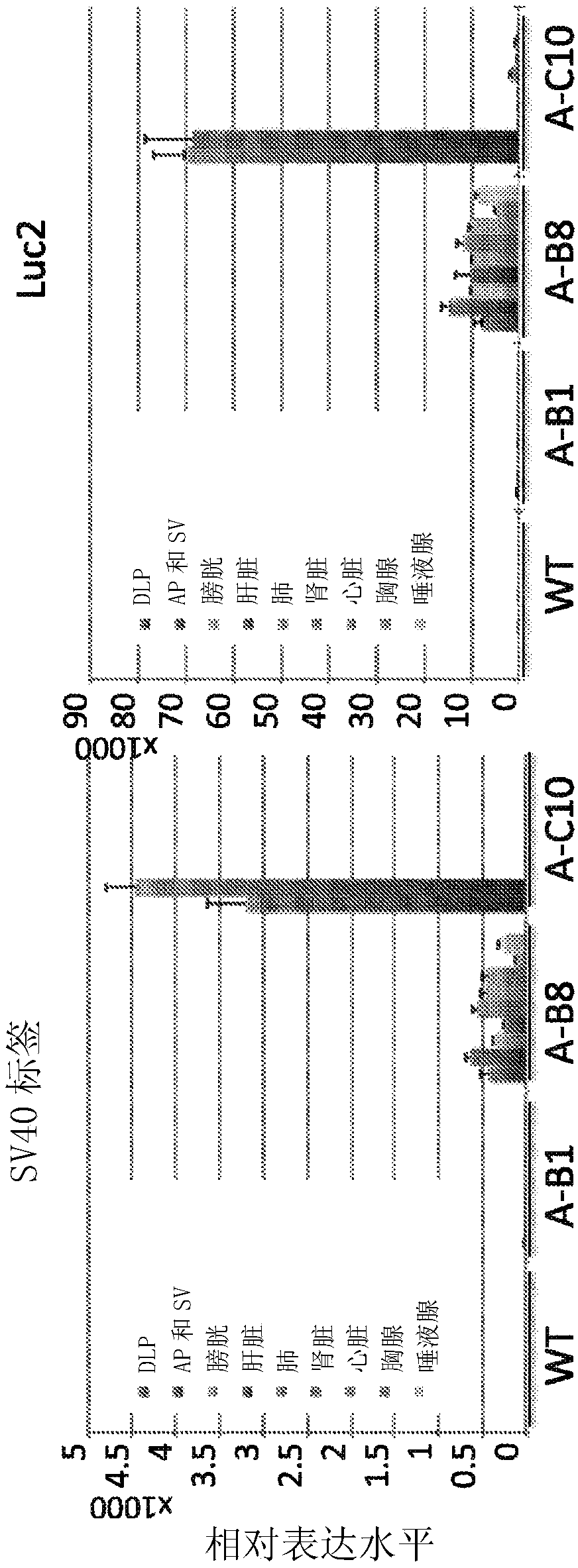
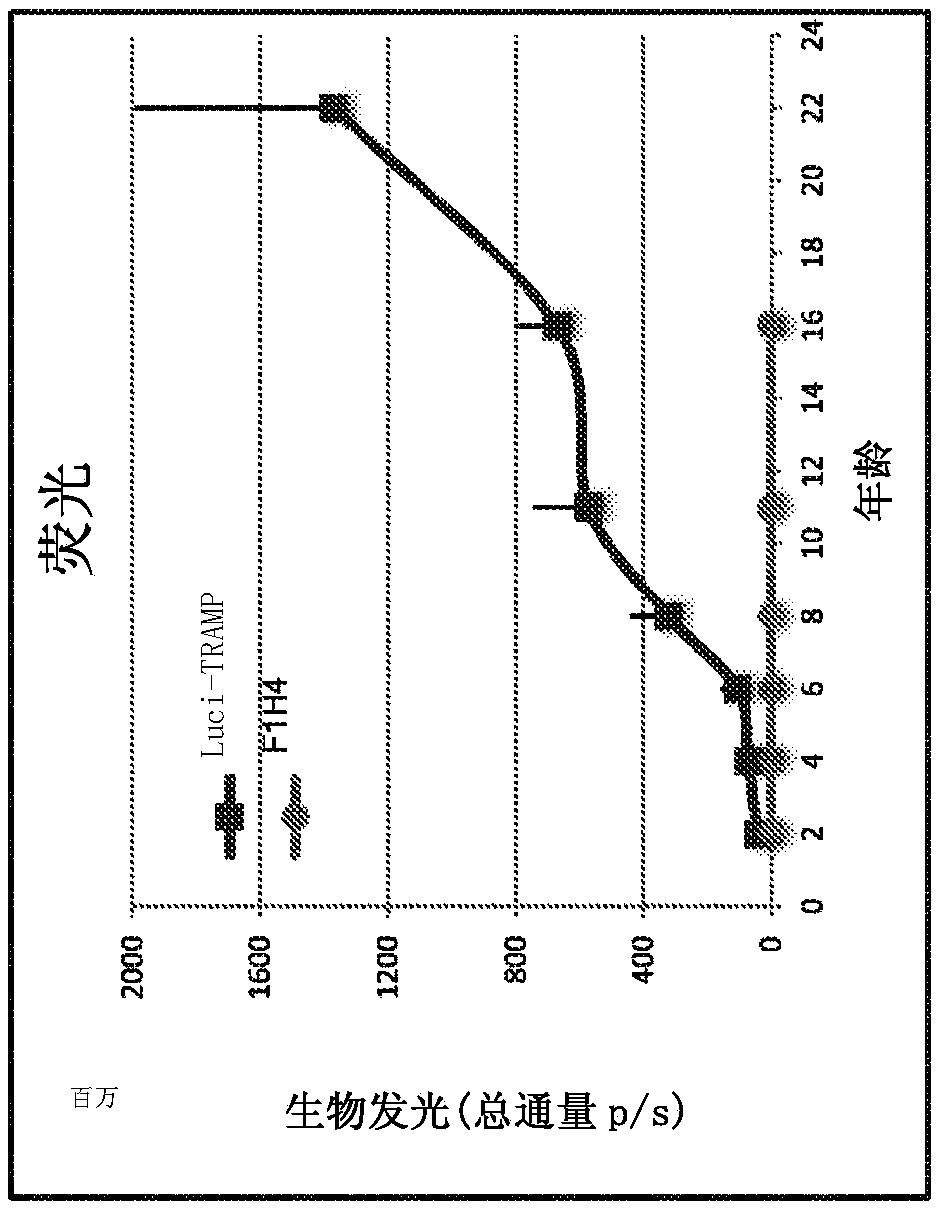
This disclosure provides a rodent model of prostate cancer. The rodents disclosed herein comprise a transgene that provides prostate-specific expression of an oncogenic protein (e.g, an SV40 tumor antigen) under the control of 5' and 3' regulatory regions of a mouse probasin gene. The rodents develop progressive forms of prostate tumor that resemble the development of human prostate cancer.
Owner:REGENERON PHARM INC
Rodent models with autistic features
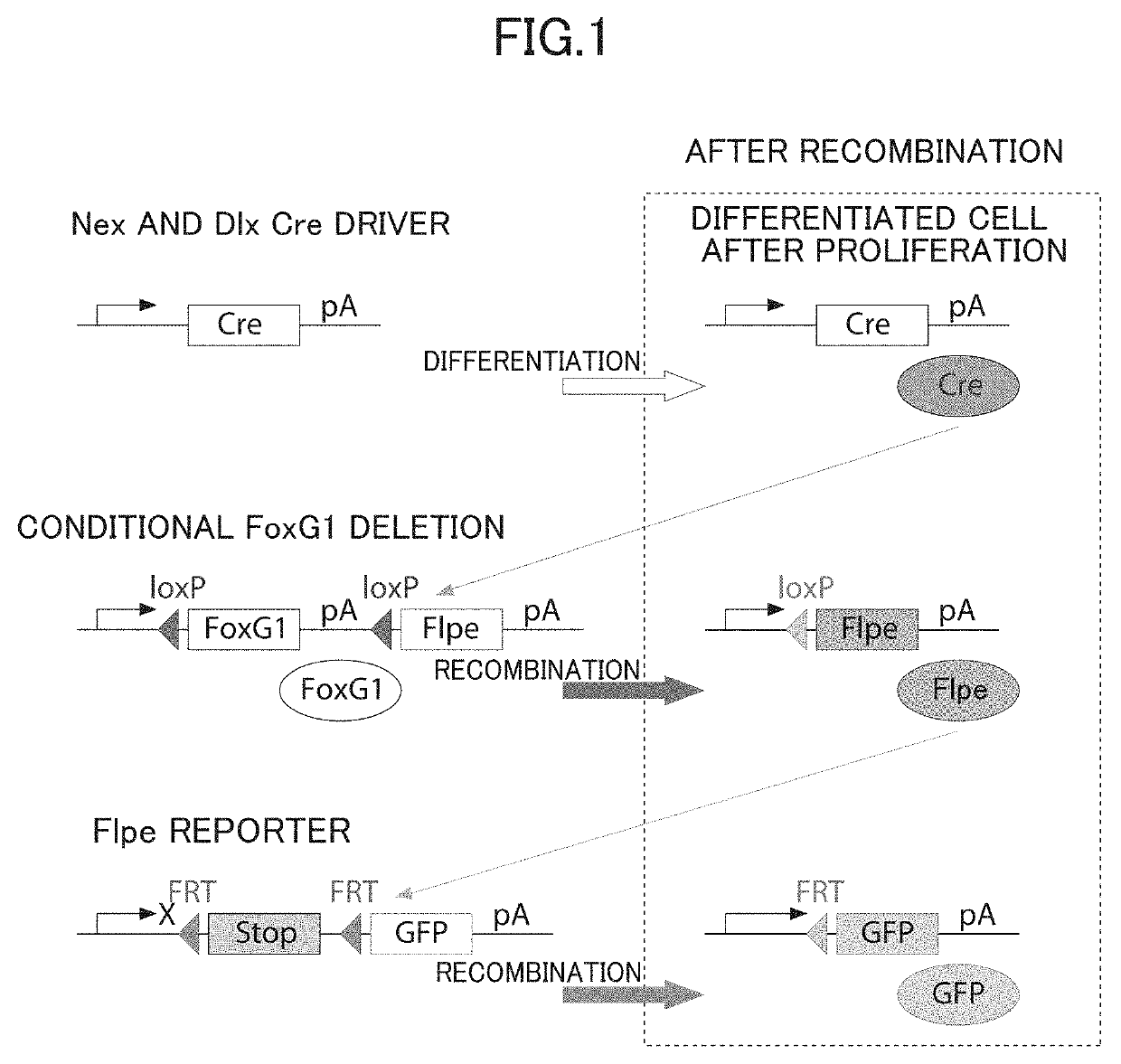
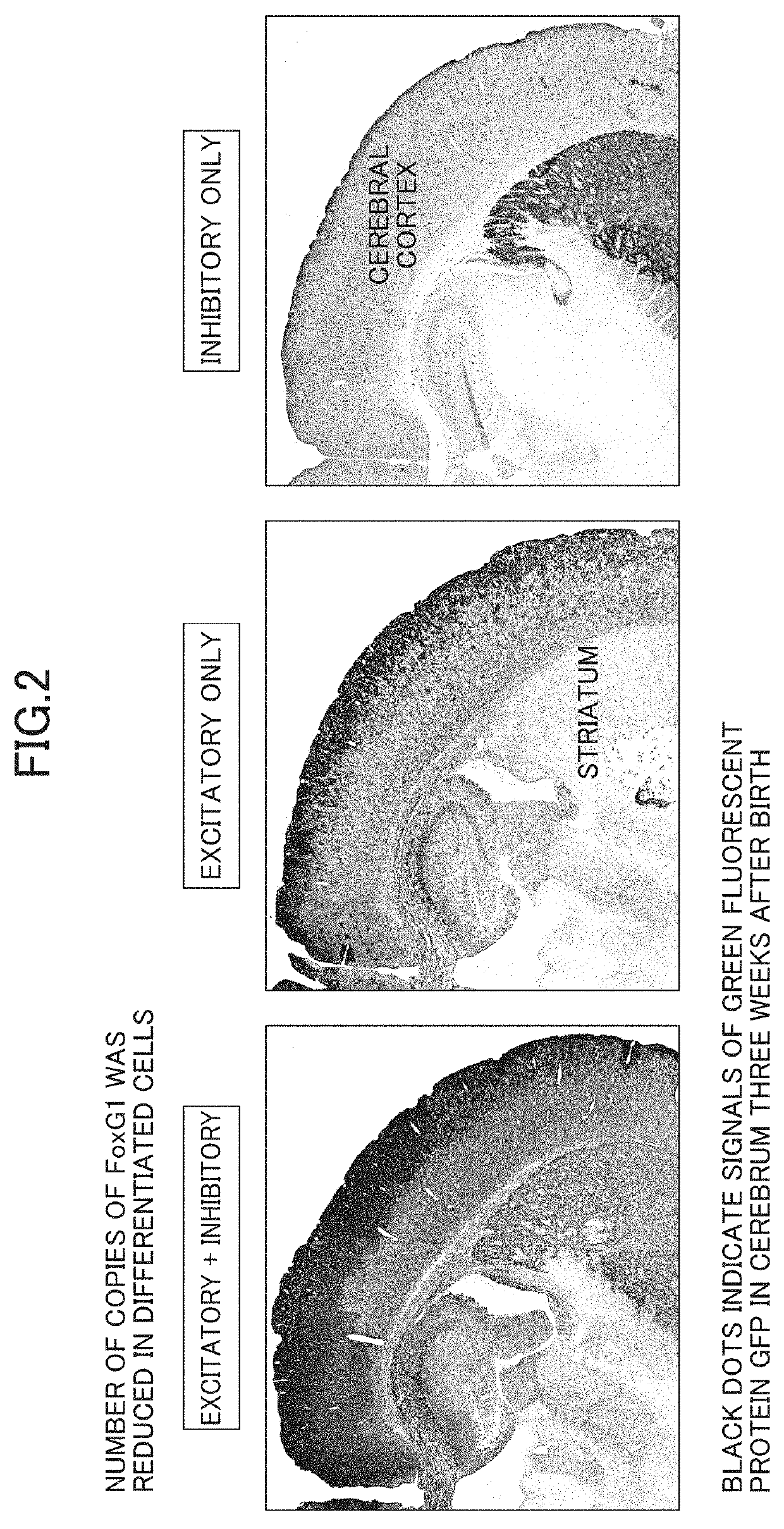
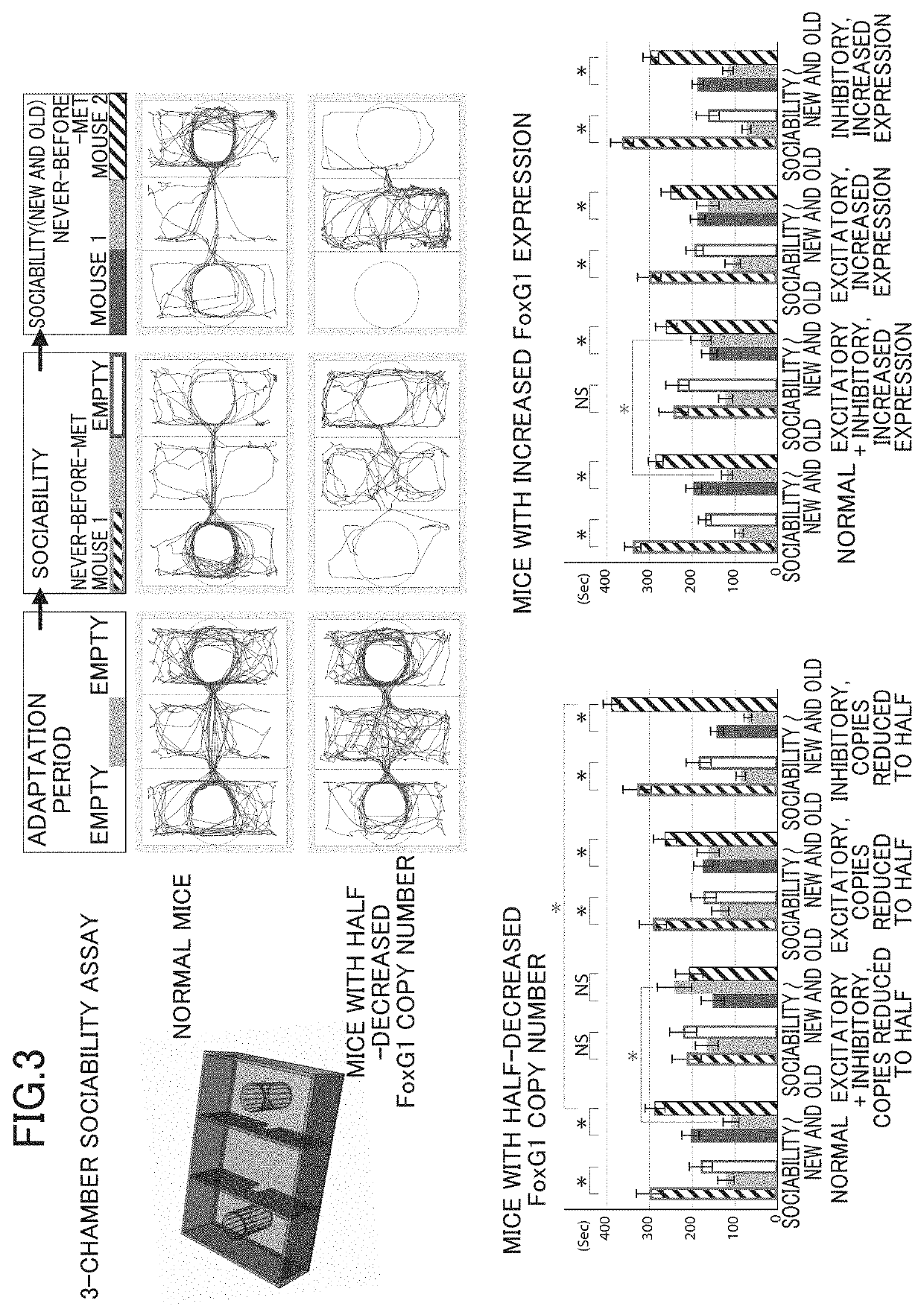
InactiveUS20190343092A1Improve expression levelNucleic acid vectorDisease diagnosisRodent modelWild type
The present invention (1) performs an operation of decreasing the number of copies of a FoxG1 gene from two copies to one copy in both differentiated excitatory neurons and differentiated inhibitory neurons present in a brain, or (2) performs, seven or more days after birth, an operation of increasing expression levels of a FoxG1 gene in both differentiated excitatory neurons and differentiated inhibitory neurons present in a brain to 1.2 to 2.0 times that of a wild type animal.
Owner:TOKYO WOMENS MEDICAL UNIV
Neuroblastoma treatment with taurolidine hydrolysis products
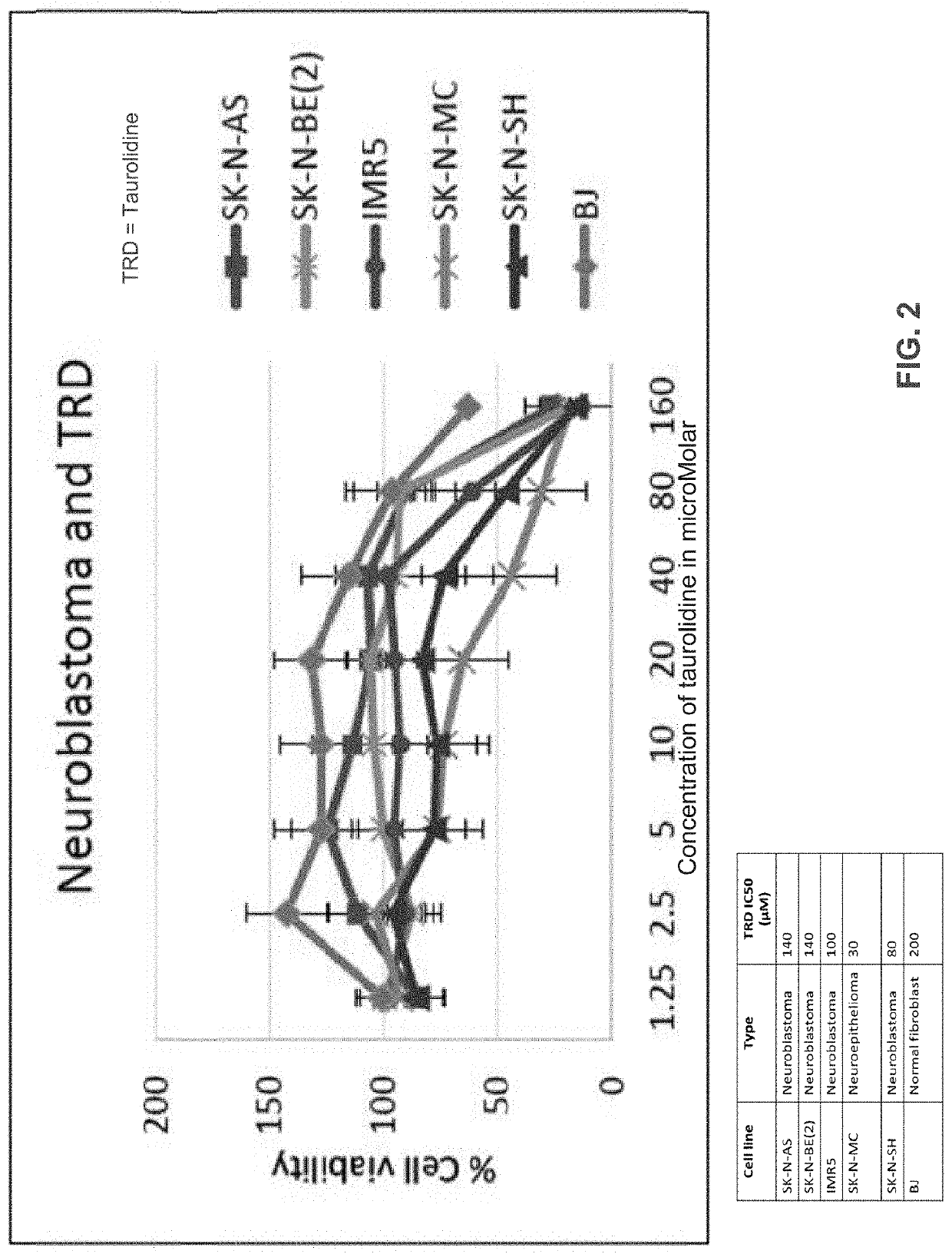
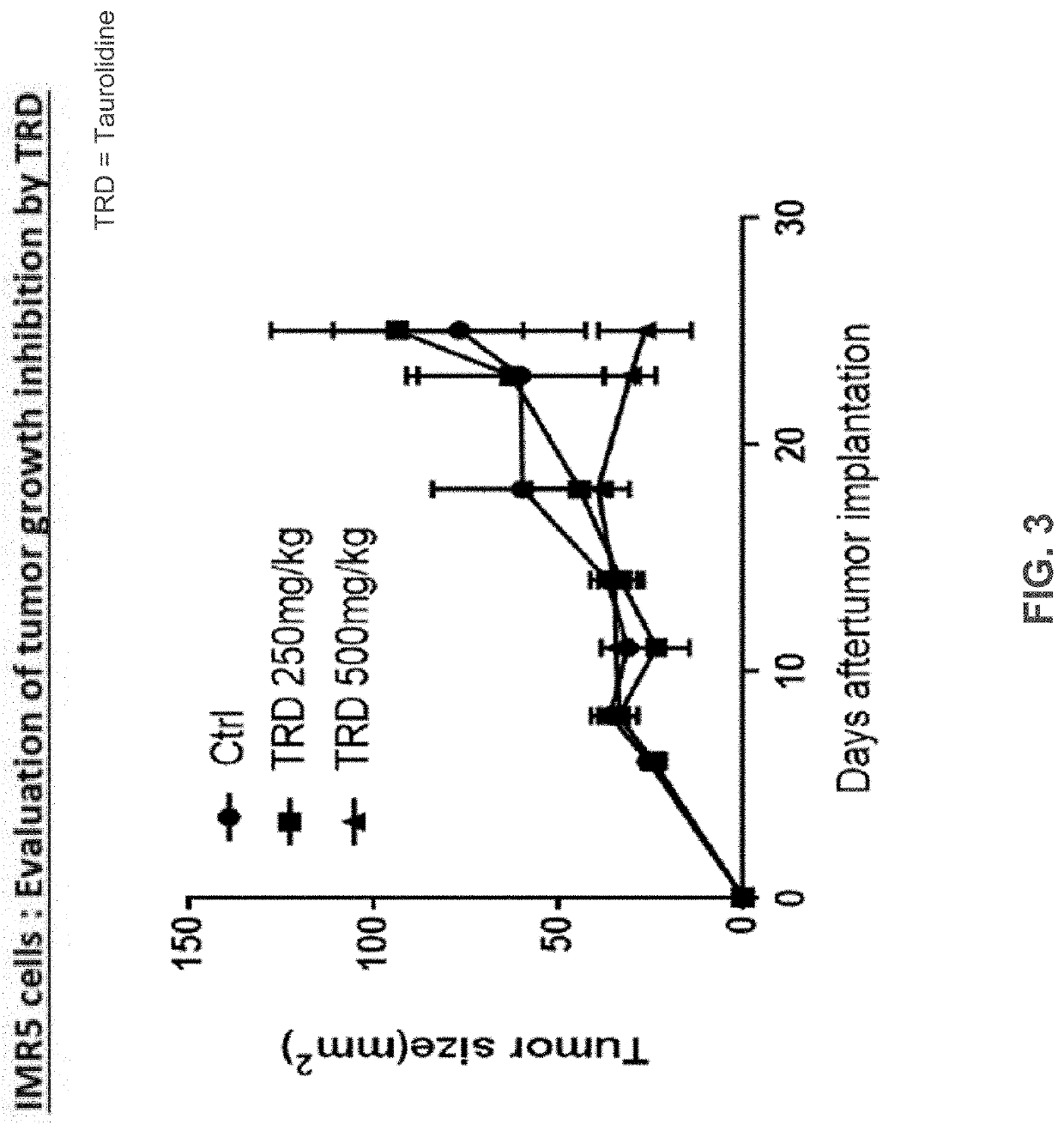
ActiveUS20190381059A1Reduce delaysAvoid rapid degradationPowder deliveryHydroxy compound active ingredientsLower riskHigh survival rate
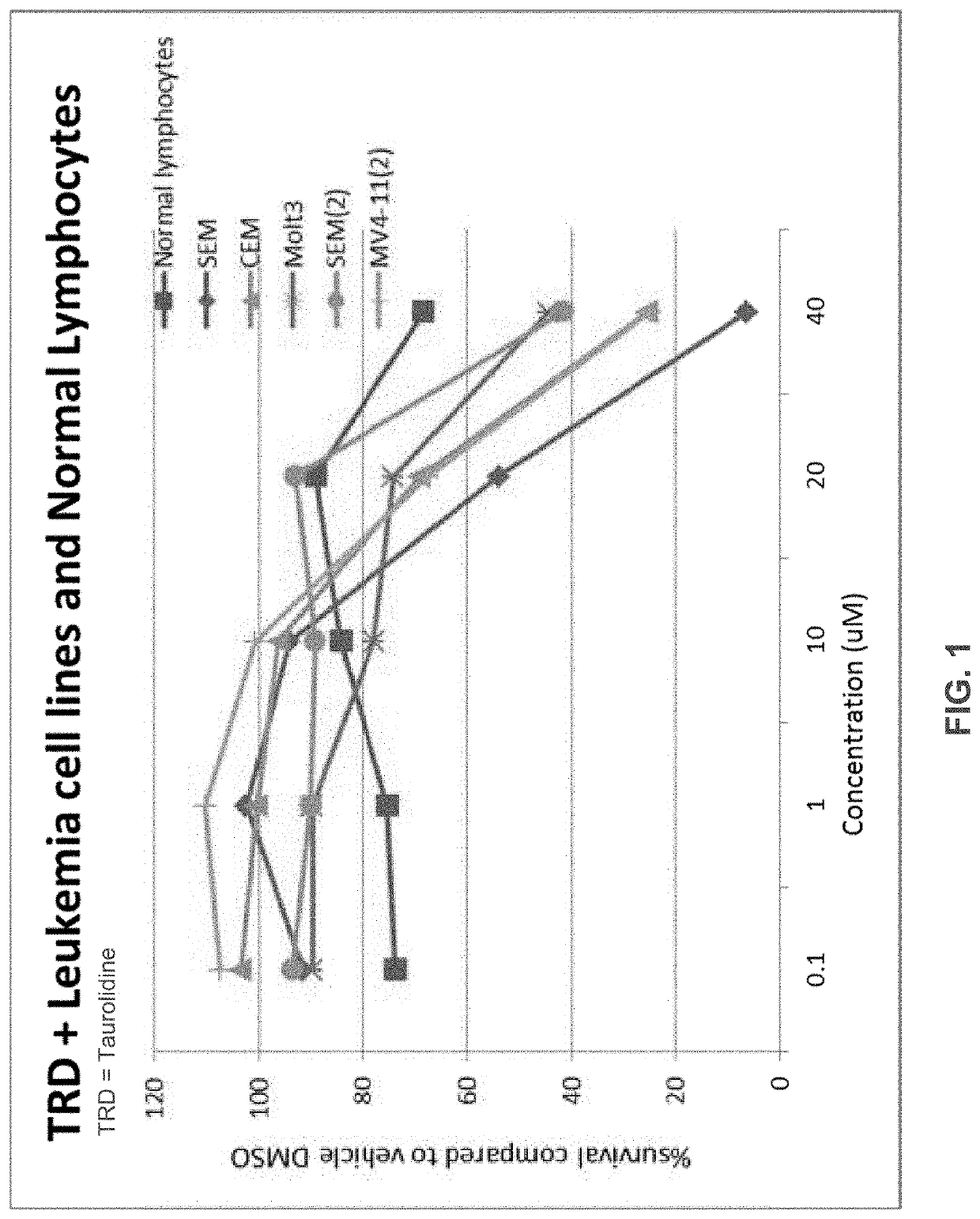
Neuroblastoma is a tumor primarily affecting children. The current standard of care is not curative except in the rare case of a surgically-resectable lesion, although very high survival rates have been documented for low-risk neuroblastoma and moderate-risk neuroblastoma. Taurolidine was developed as an anti-infective, but it has been found to have surprising oncolytic activity in cell cultures and now in a rodent cancer model. The efficacy in rodent model is superior to the efficacy in cell culture. This invention relates to the use of taurolidine hydrolysis products (tarultam and / or taurinamide and / or methylene glycol and / or selected combinations thereof) for the treatment of neuroblastoma in juvenile mammals.
Owner:CORMEDIX
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com