Patents
Literature
109 results about "Botanical drug" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A botanical drug is defined in the United States Federal Food, Drug, and Cosmetic Act as a botanical product that is marketed as diagnosing, mitigating, treating, or curing a disease; a botanical product in turn, is a finished, labeled product that contains ingredients from plants. Chemicals that are purified from plants, like paclitaxel, and highly purified products of industrial fermentation, like biopharmaceuticals, are not considered to be botanical products.
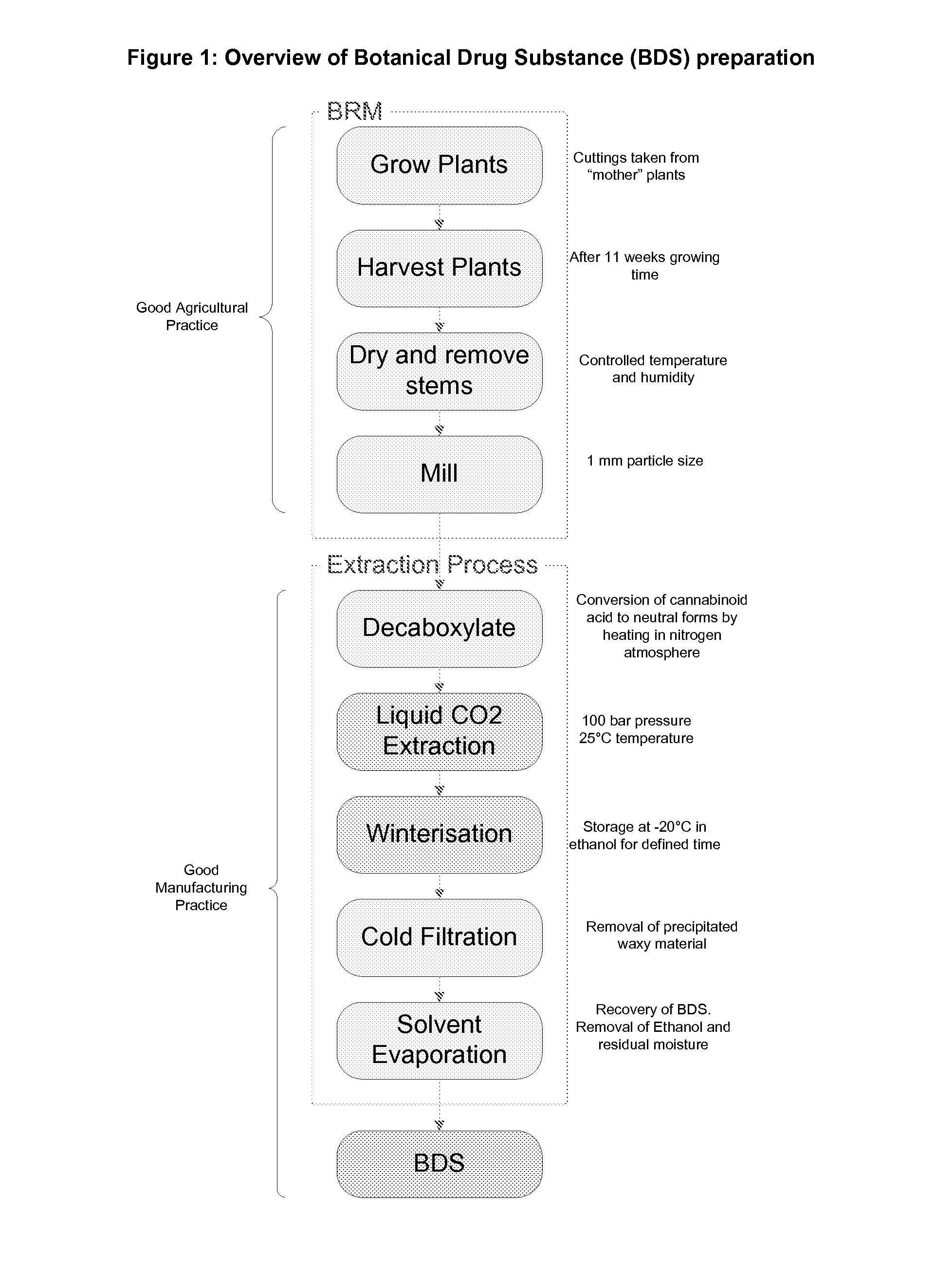
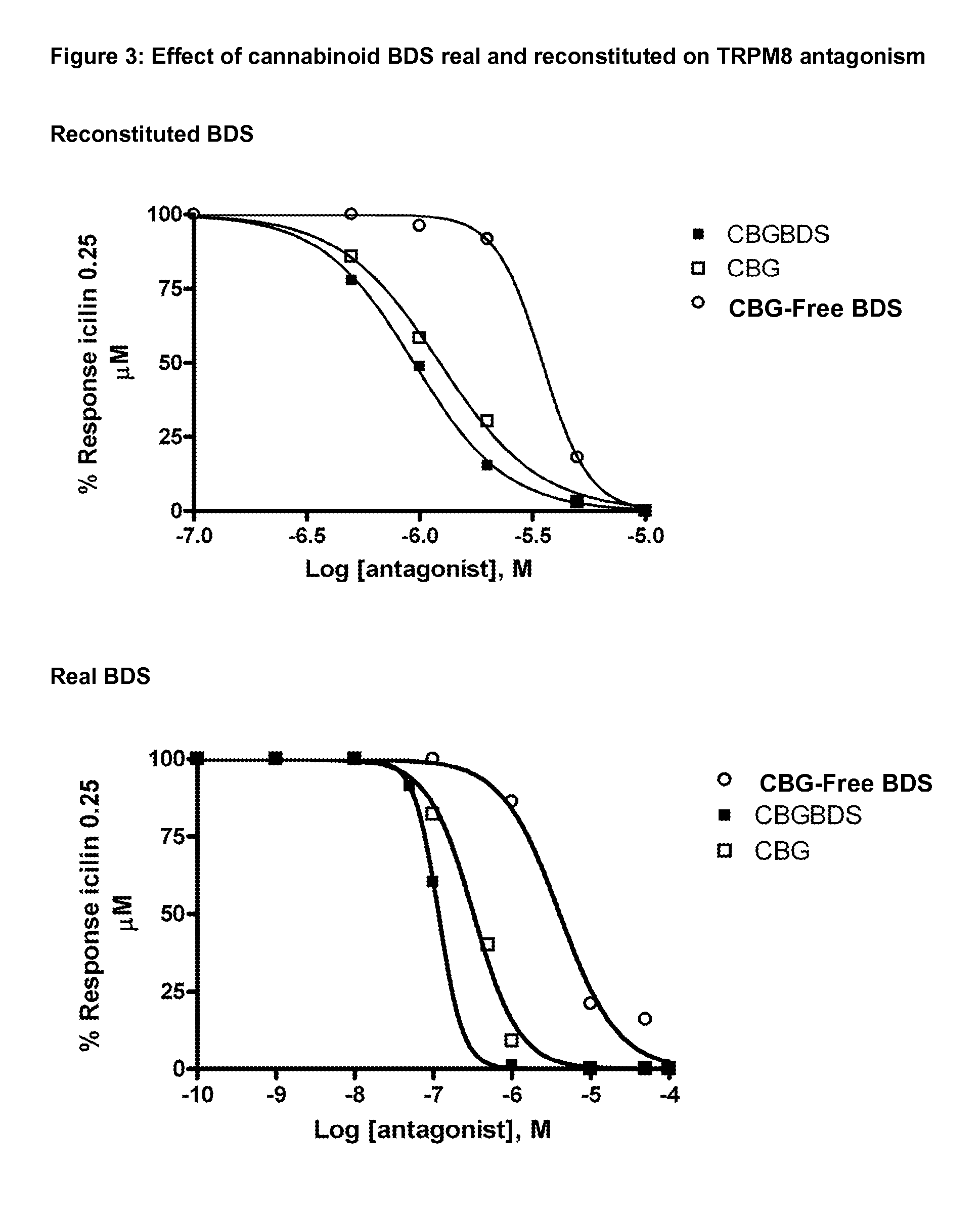
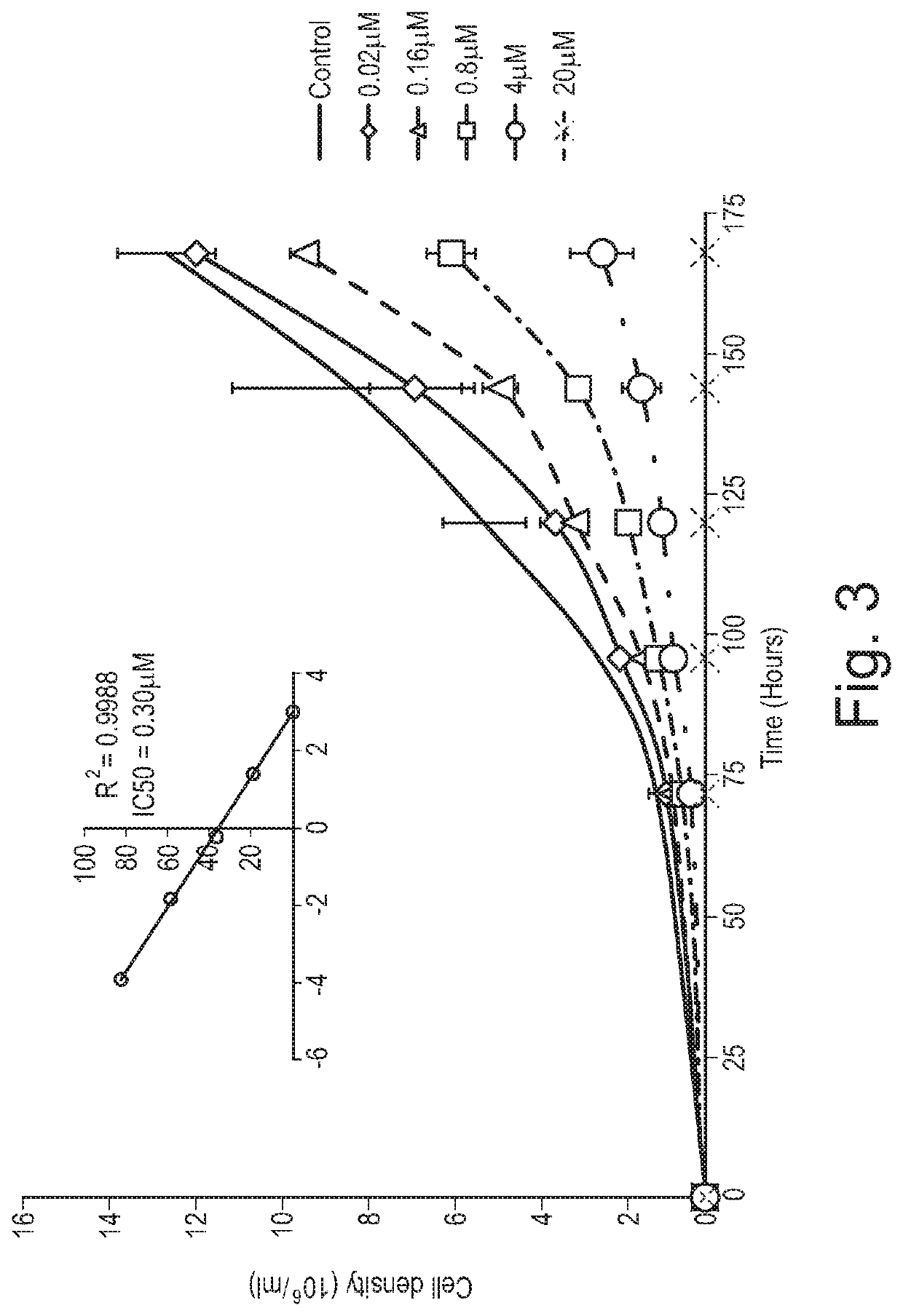
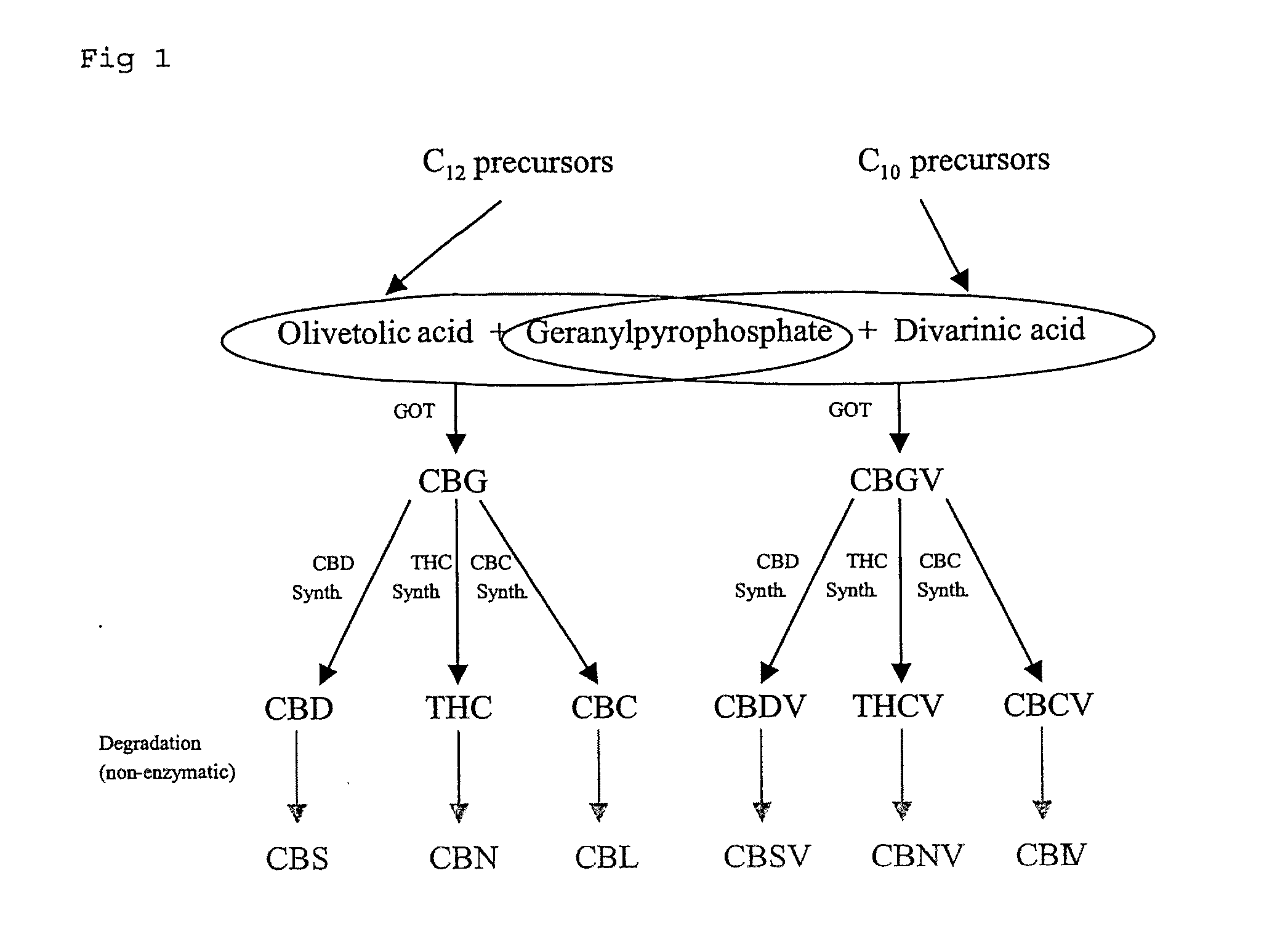
Cannabis extracts and methods of preparing and using same
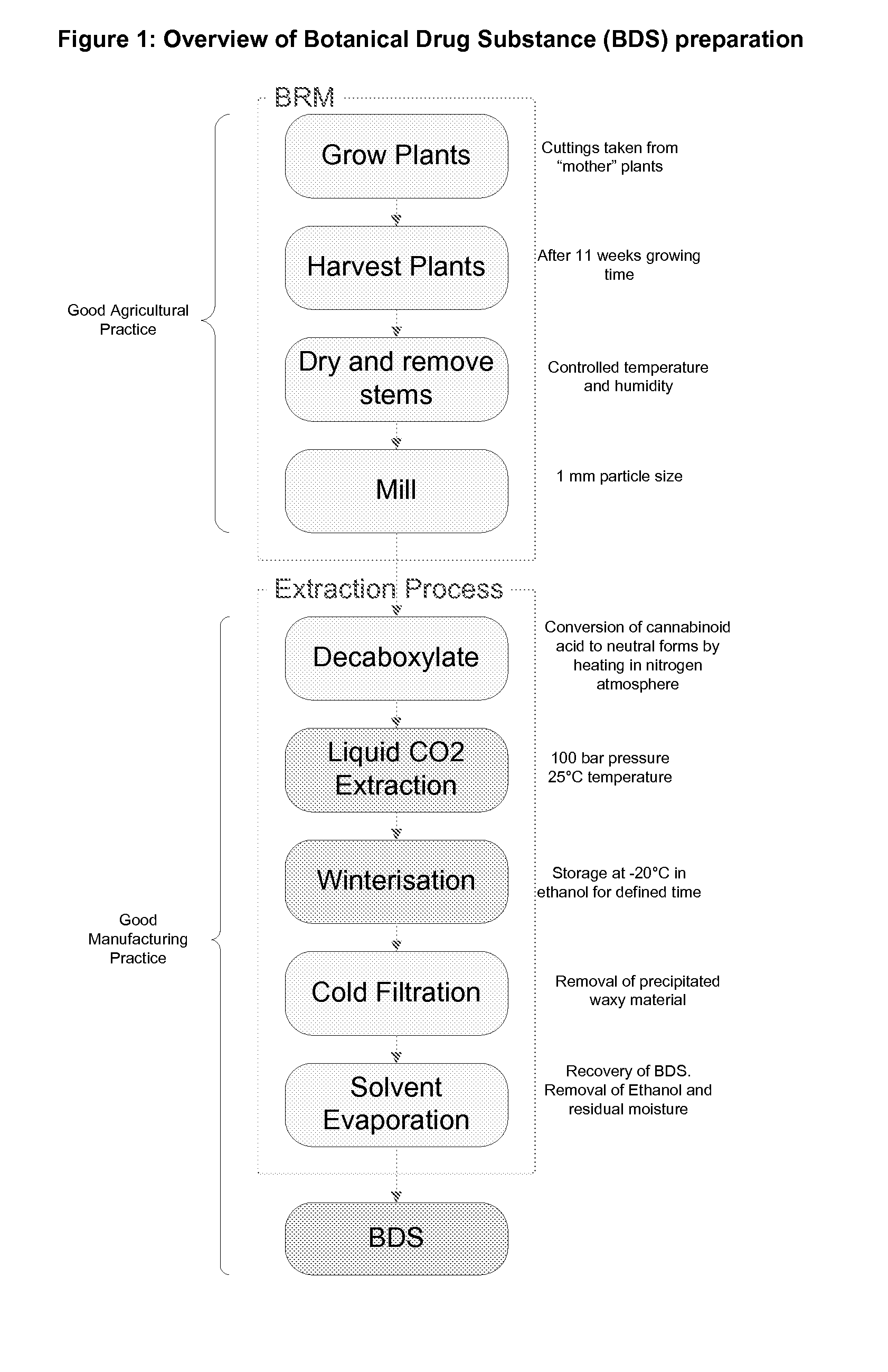
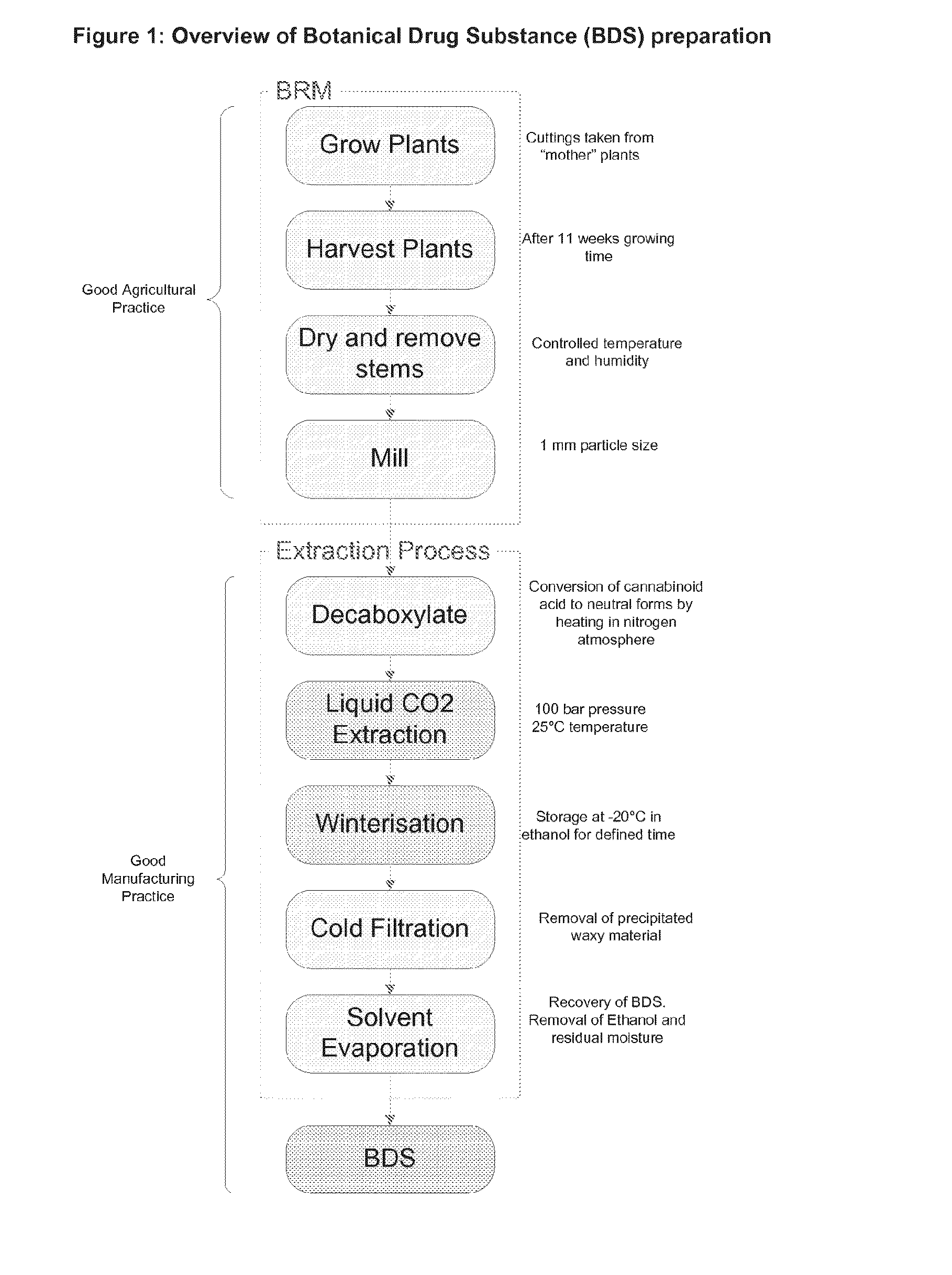
The invention relates to the extraction of pharmaceutically active components from plant materials, and more particularly to the preparation of a botanical drug substance (BDS) for incorporation in to a medicament. It also relates to a BDS, for use in pharmaceutical formulations. In particular it relates to BDS comprising cannabinoids obtained by extraction from cannabis
Owner:BLACKMON EARNEST VINSON MR
Cannabis extracts and methods of preparing and using same
The invention relates to the extraction of pharmaceutically active components from plant materials, and more particularly to the preparation of a botanical drug substance (BDS) for incorporation in to a medicament. It also relates to a BDS, for use in pharmaceutical formulations. In particular it relates to BDS comprising cannabinoids obtained by extraction from cannabis.
Owner:BLACKMON EARNEST VINSON MR
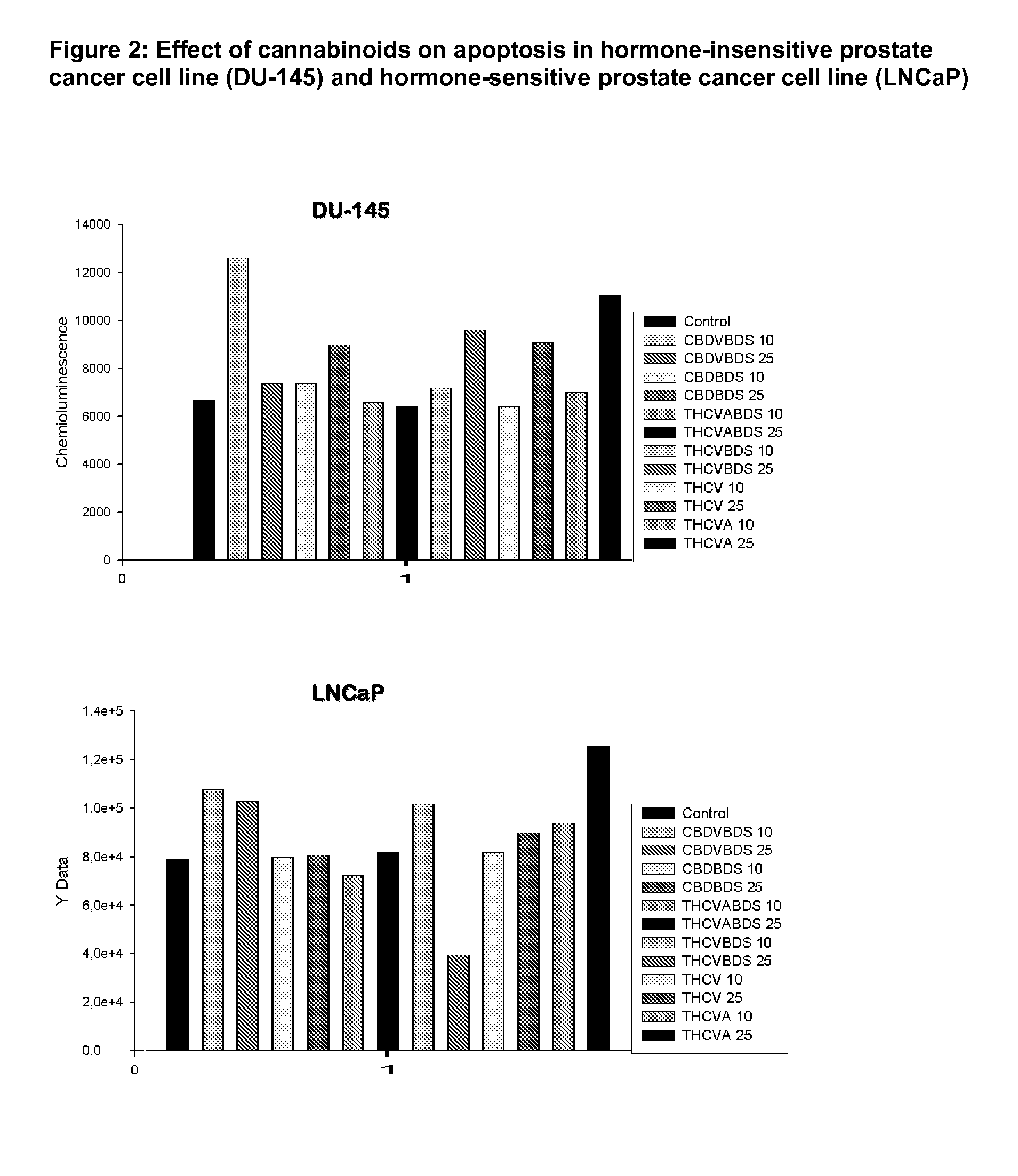
Phytocannabinoids in the treatment of cancer
ActiveUS20130059018A1Slowing down growthLower the volumeBiocideHydrocarbon active ingredientsCannabinoidProstate cancer
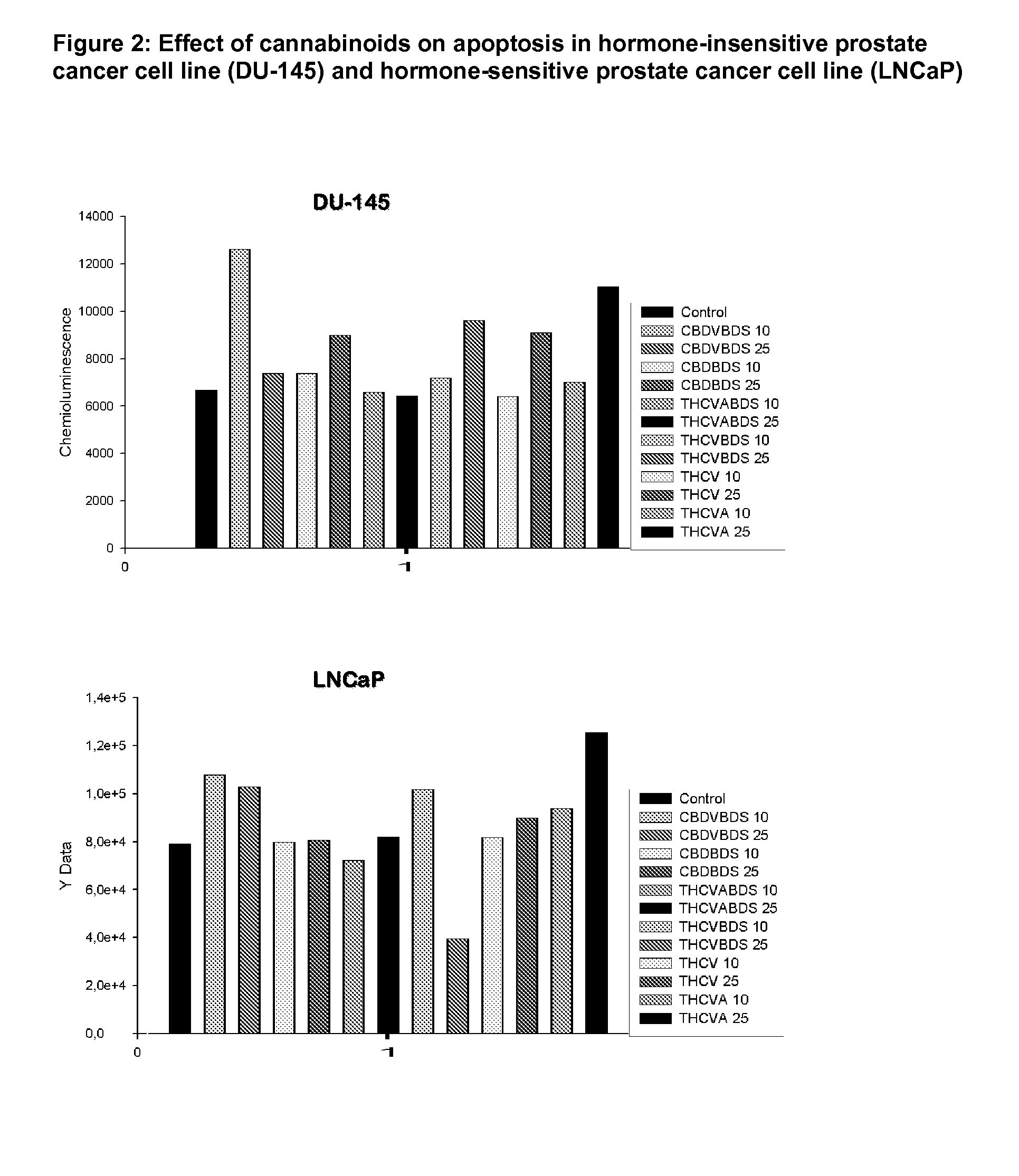
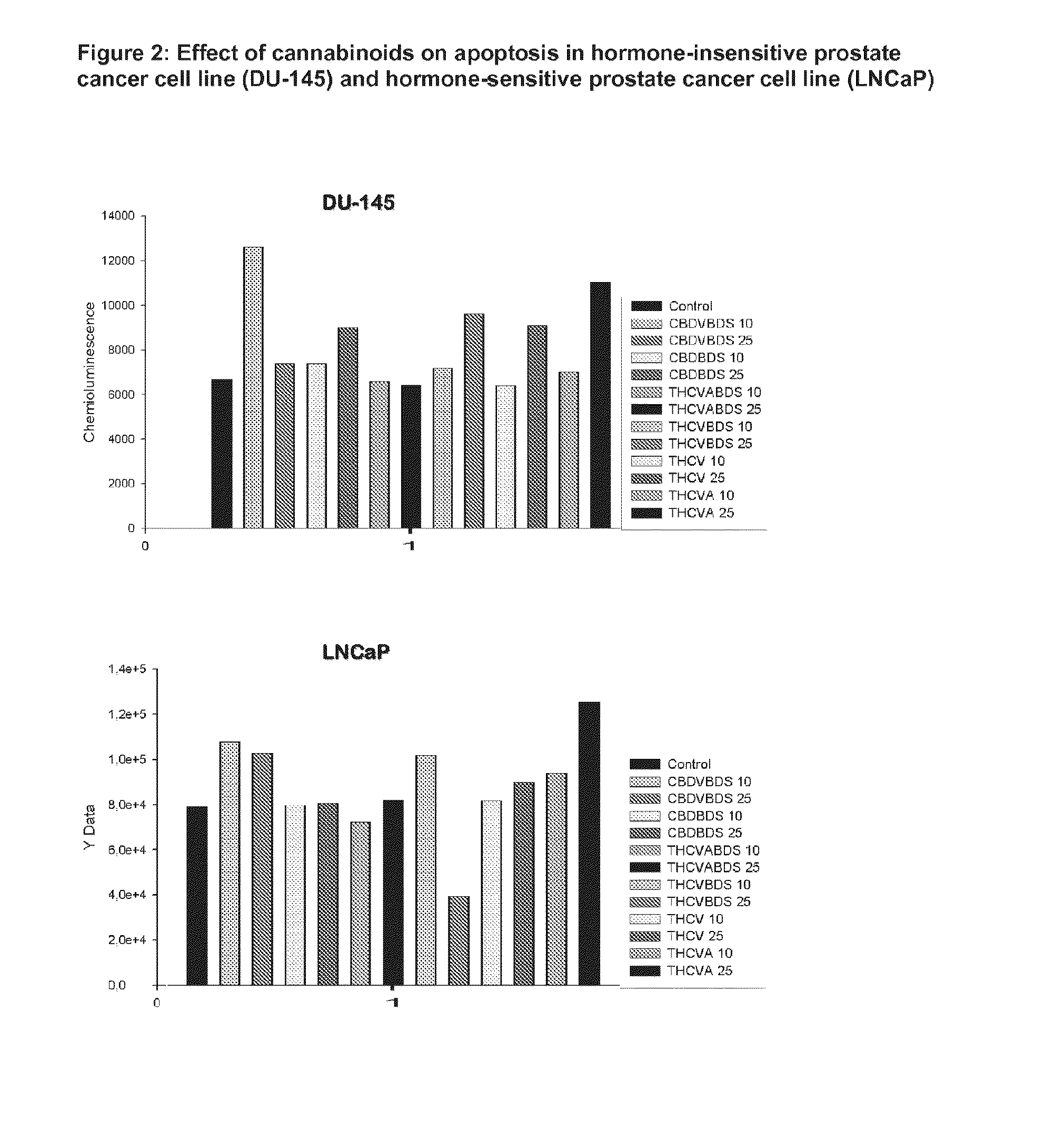
This invention relates to the use of phytocannabinoids, either in an isolated form or in the form of a botanical drug substance (BDS) in the treatment of cancer. Preferably the cancer to be treated is cancer of the prostate, cancer of the breast or cancer of the colon.
Owner:GW PHARMA LTD
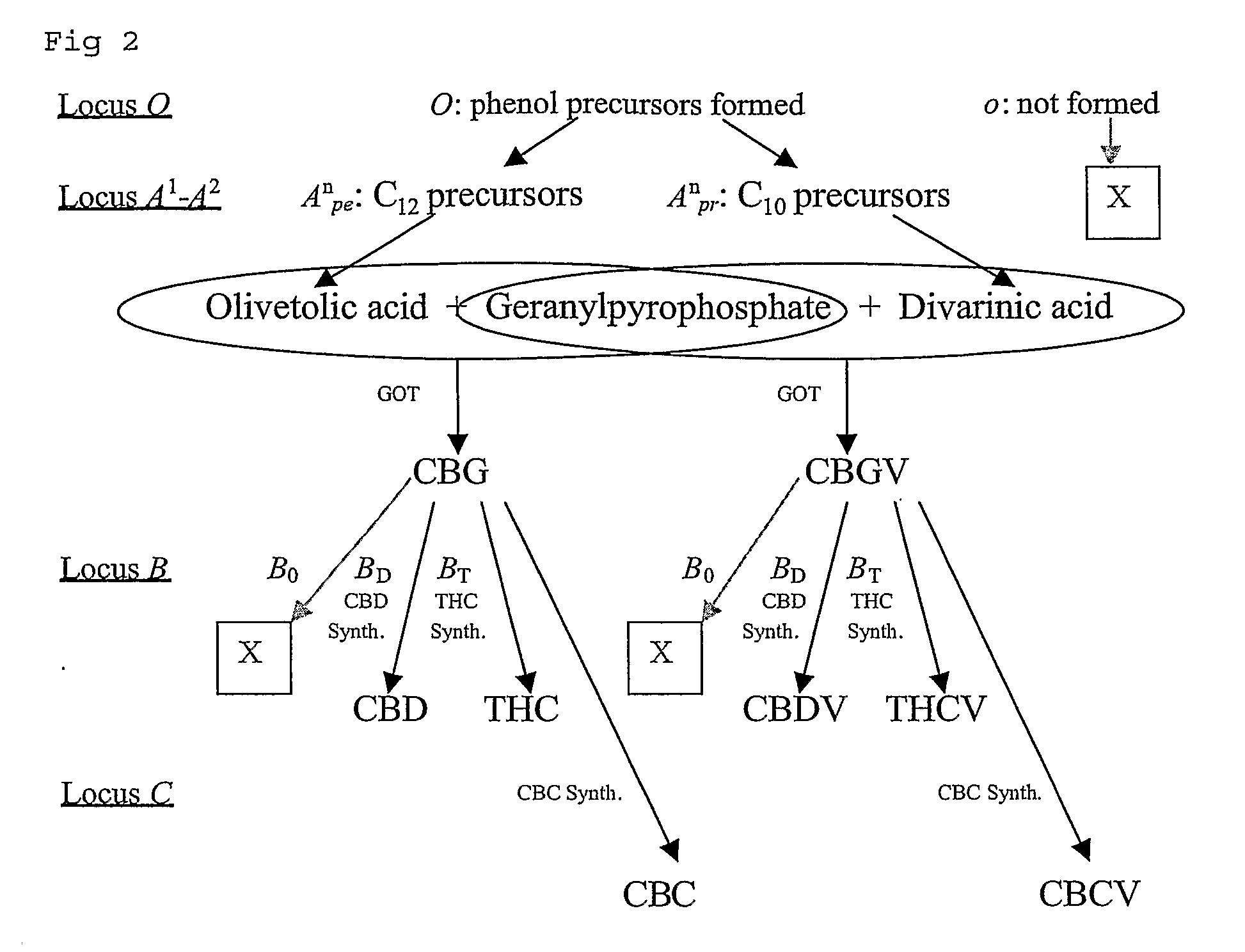
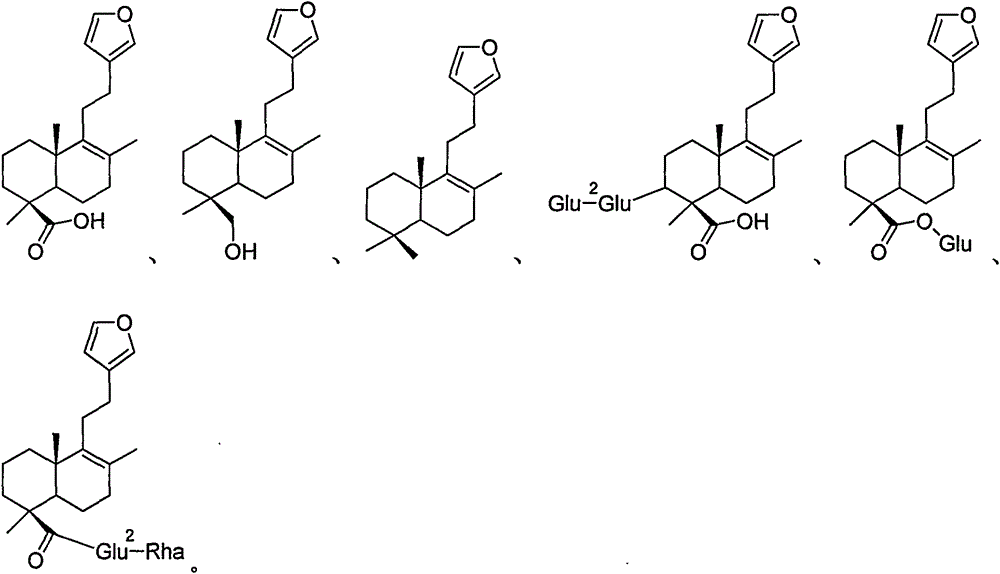
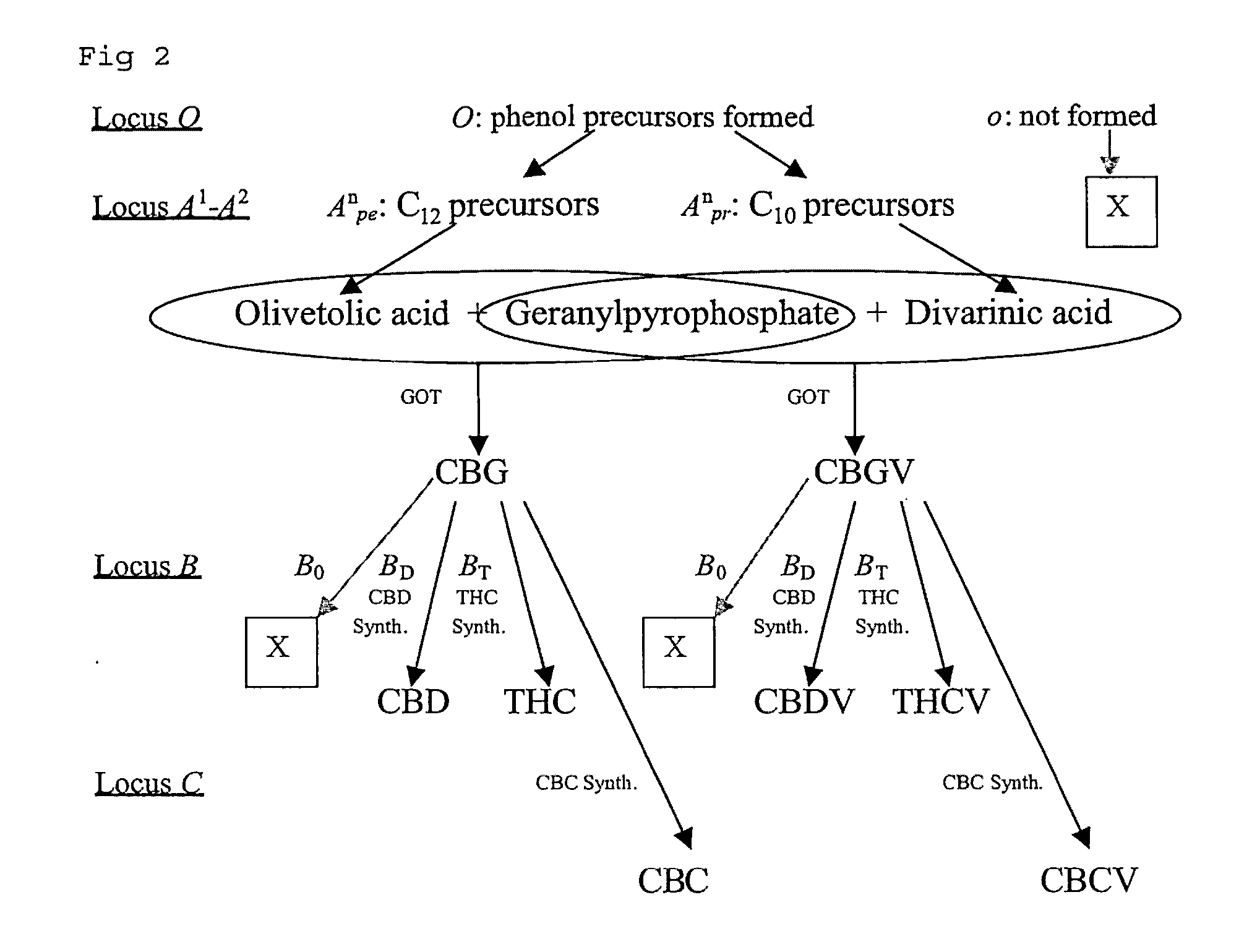
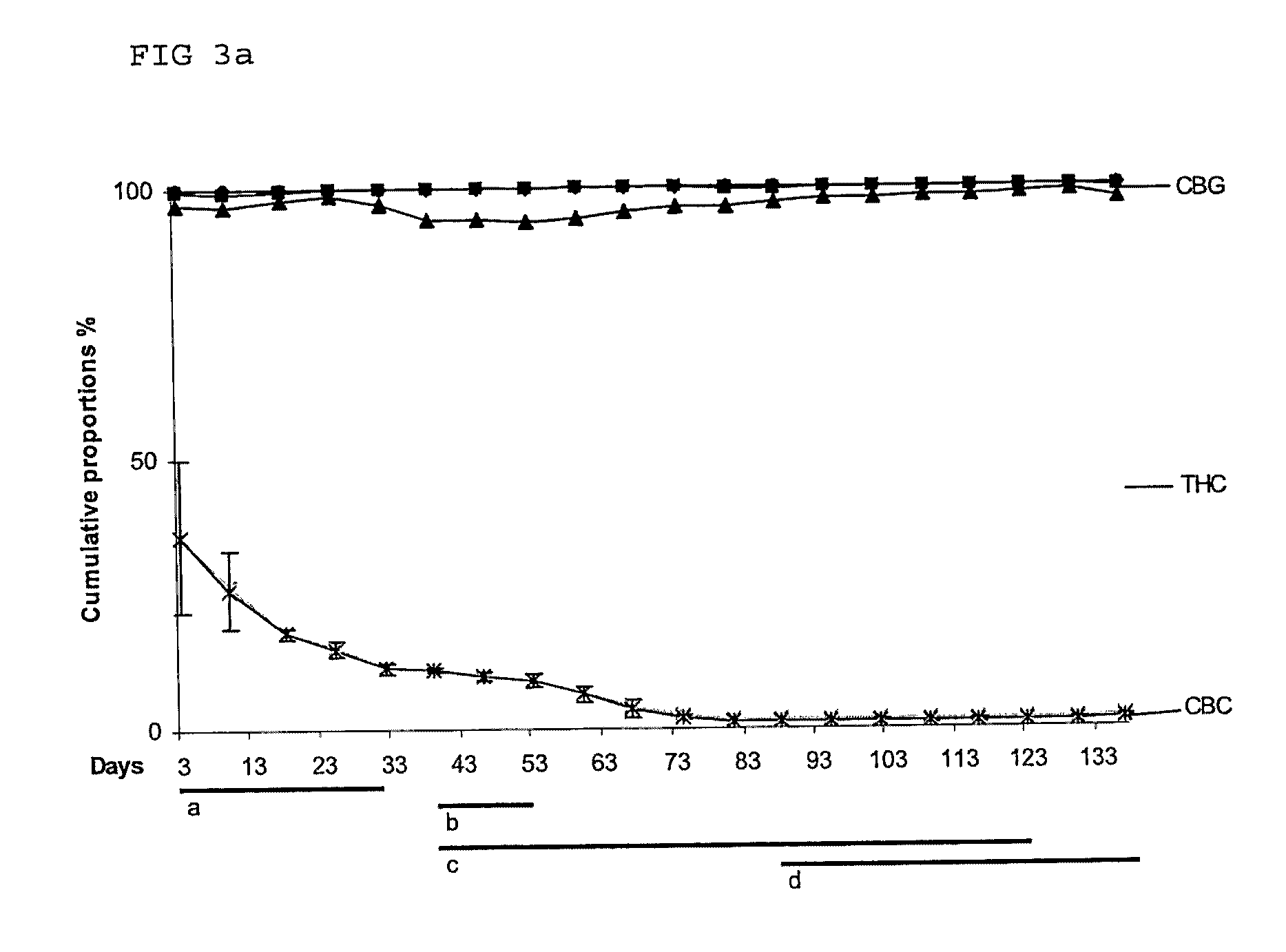
Cannabis sativa plants rich in cannabichromene and its acid, extracts thereof and methods of obtaining extracts therefrom
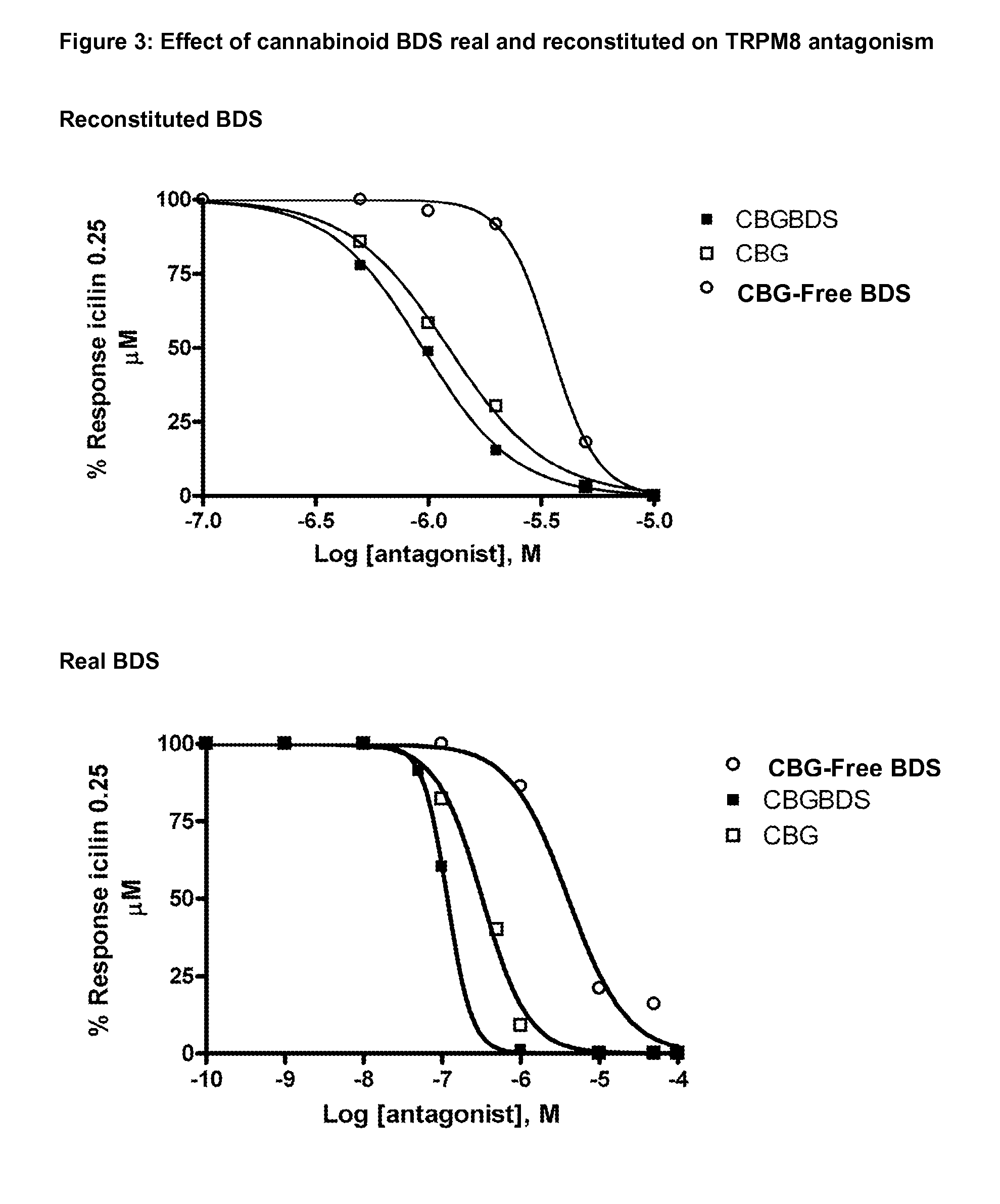
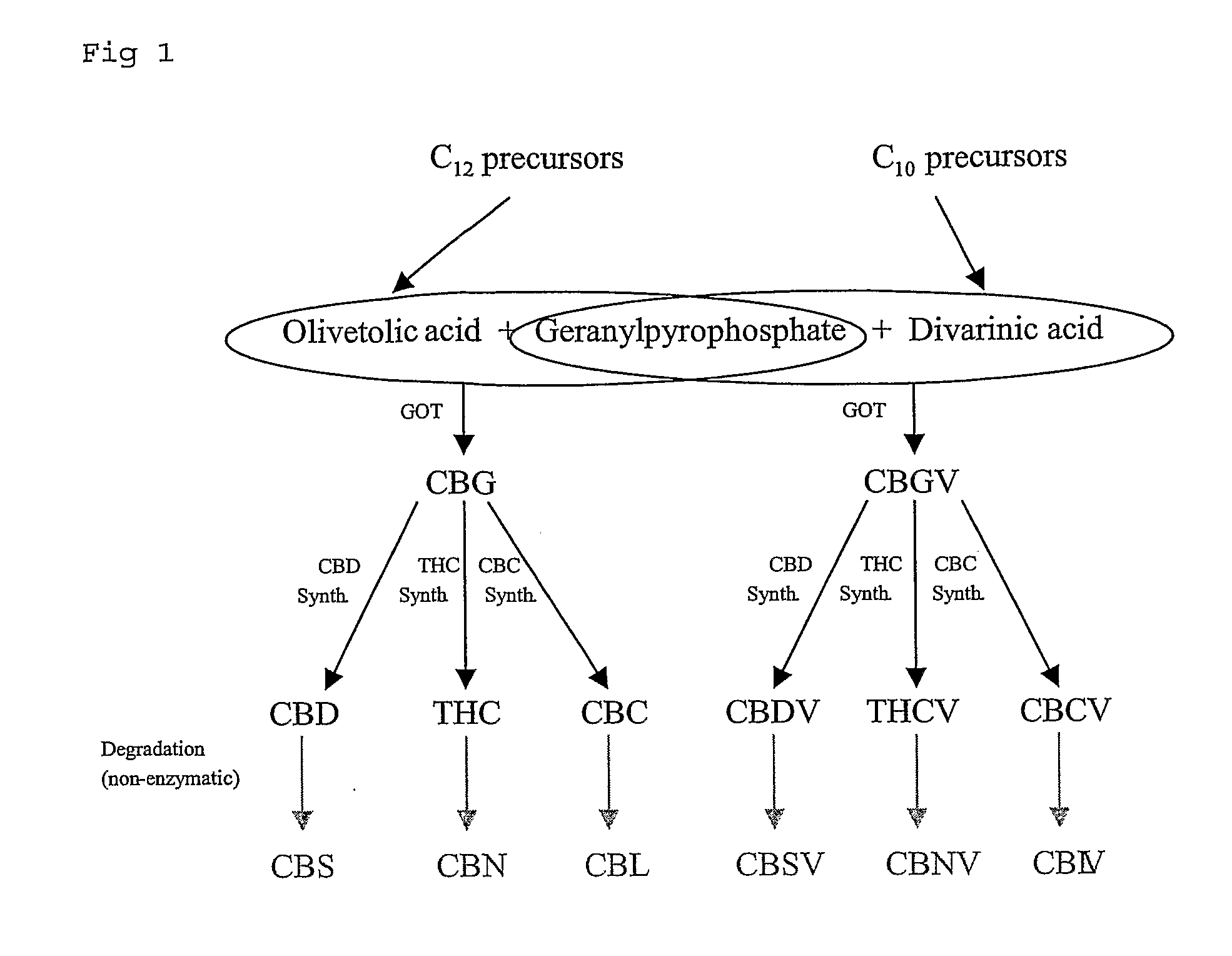
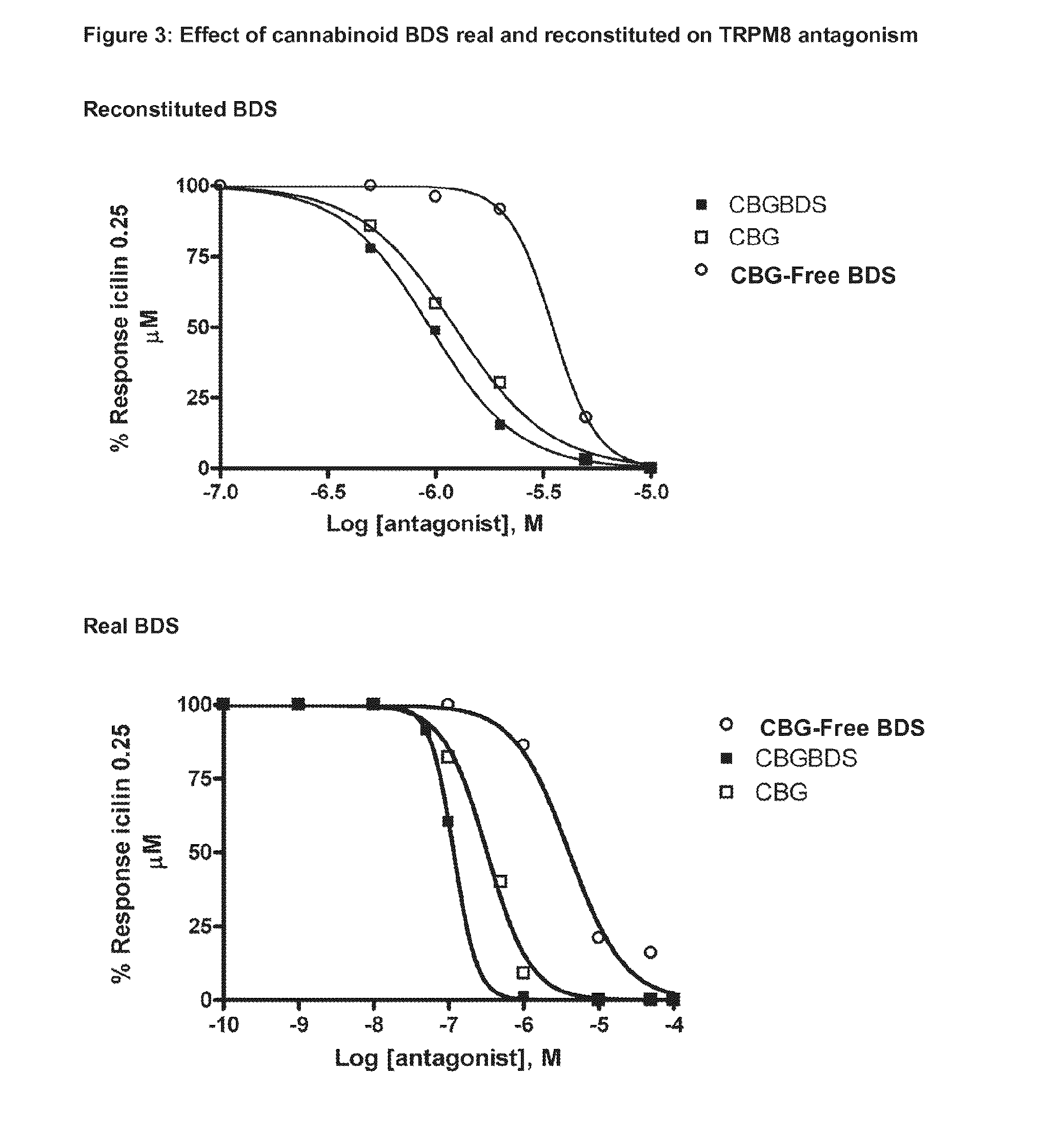
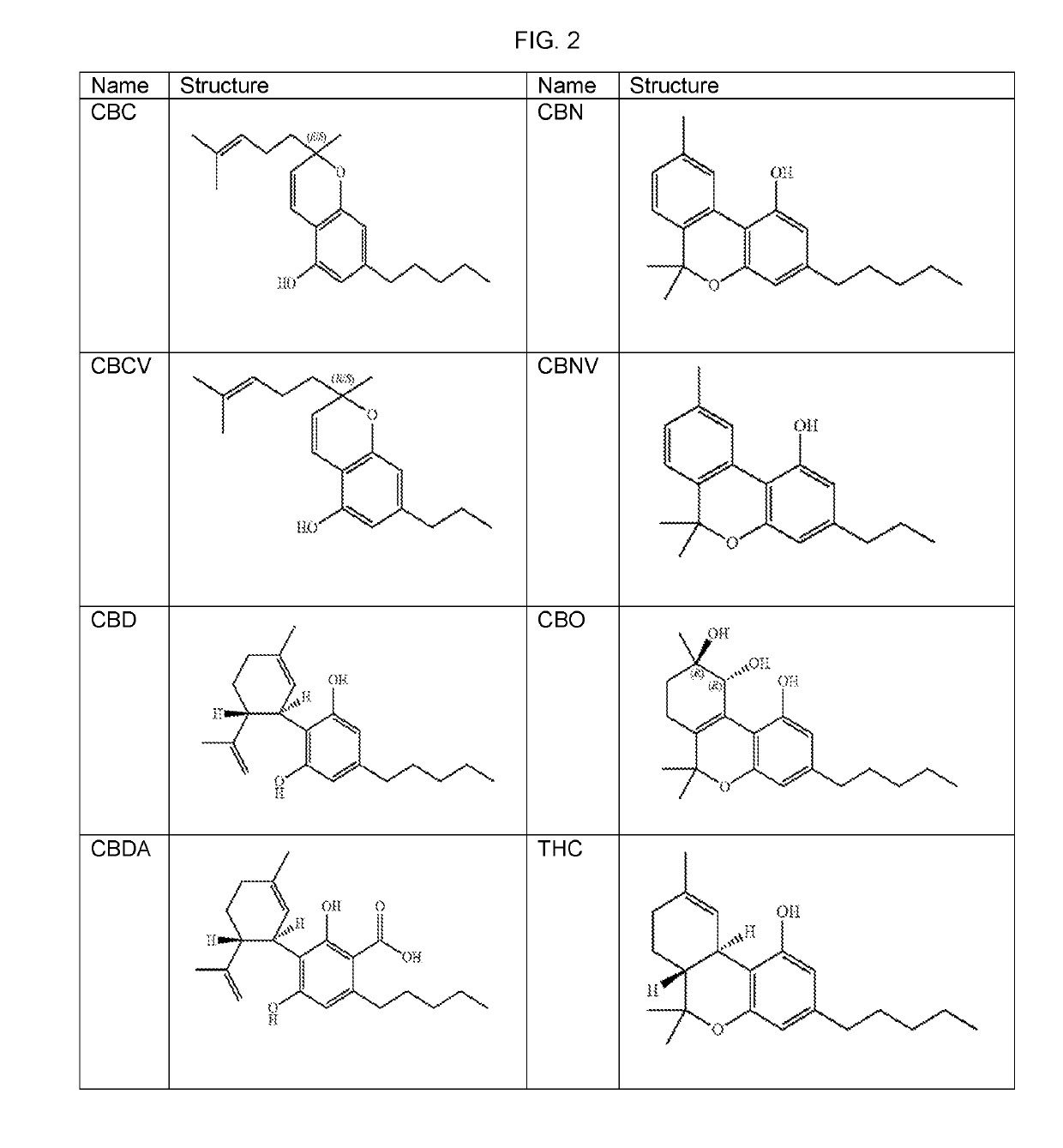
The present invention relates to plants producing, as their major cannabinoid cannabichromenic acid (CBCA) or its neutral (decarboxylated) form cannabichromene (CBC), hereafter jointly referred to as CBC(A). It additionally relates to: • A botanical material obtainable from said plants; • A botanical raw material (BRM), • An extract including a botanical drug substance (BDS) and a purified BDS; • A formulation comprising the BRM, BDS, purified BDS or other extract; • The use of the BRM, BDS, purified BDS or other extract in the manufacture of a medicament; • A method of deriving plants yielding a high proportion of the cannabinoid CBC (A) at the expense of other cannabinoids; • A method of cultivating plants such that they yield a high proportion of the cannabinoid CBC(A) at the expense of other cannabinoids; and • A method of extracting CBC(A) from said plants.
Owner:GW PHARMA LTD
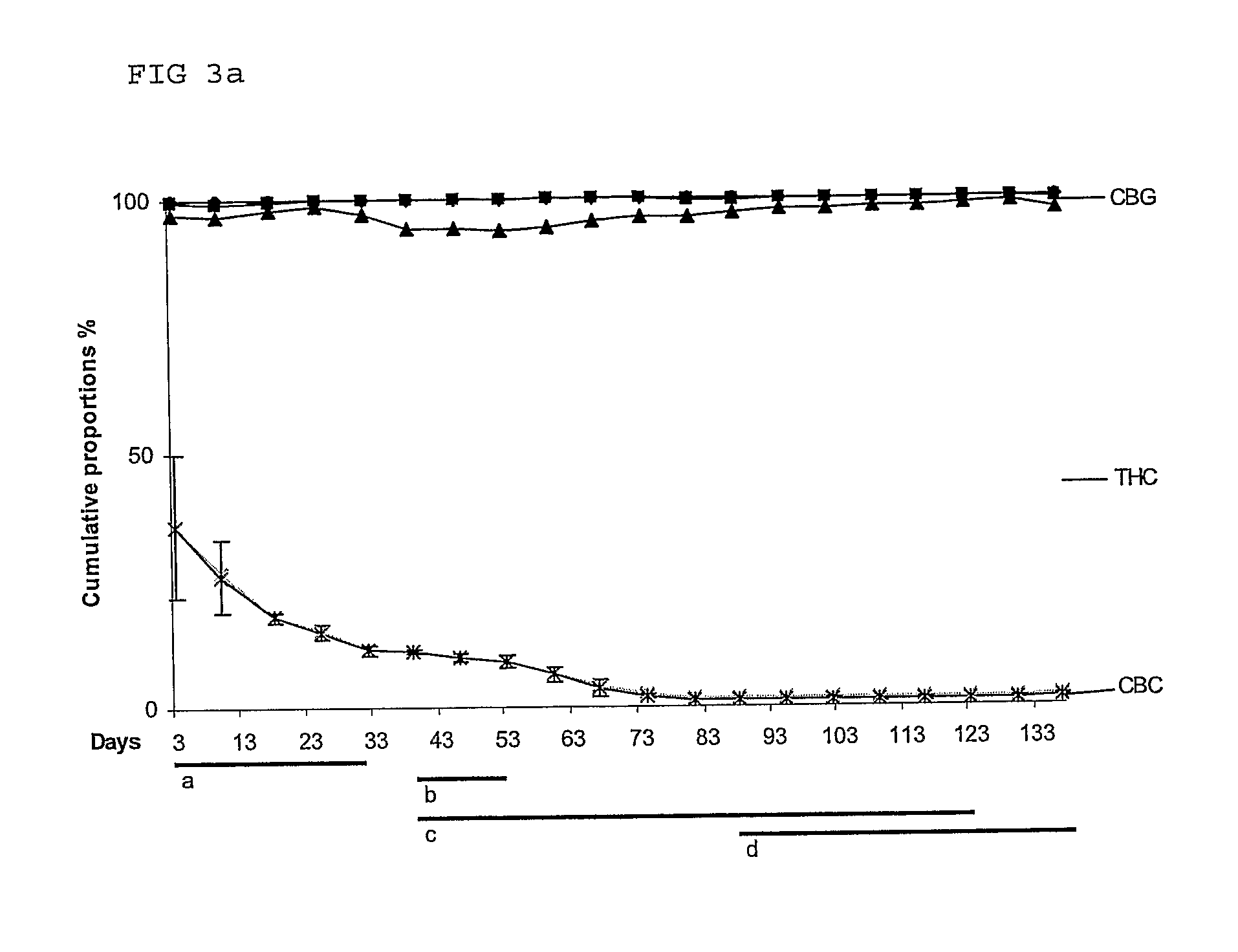
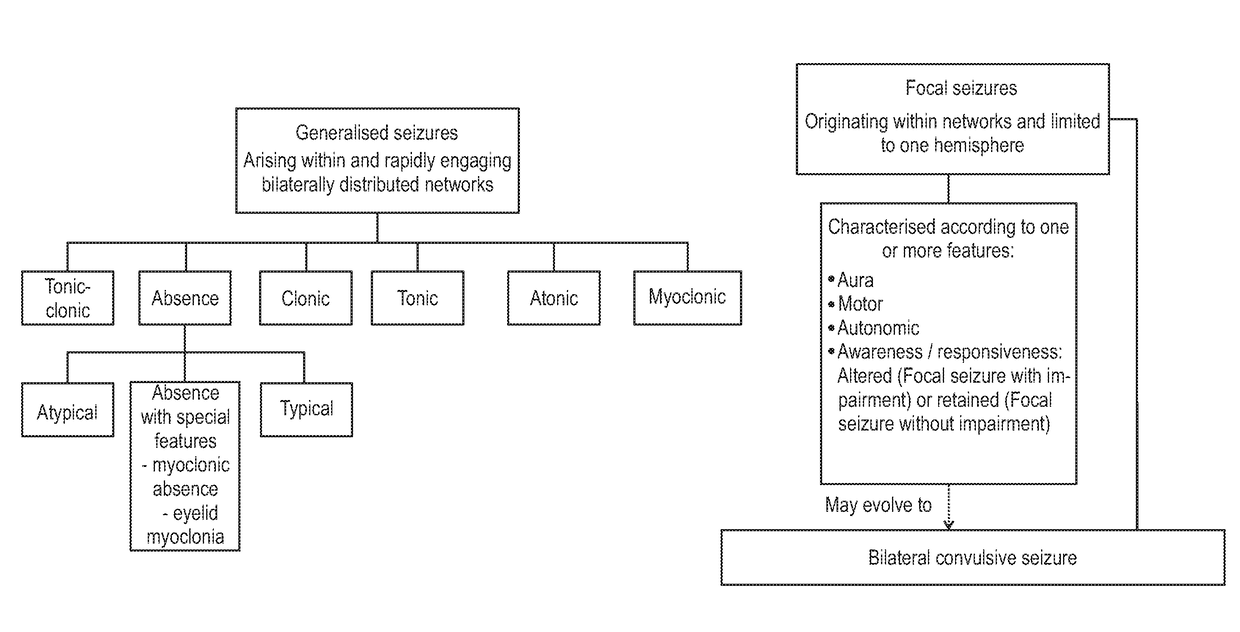
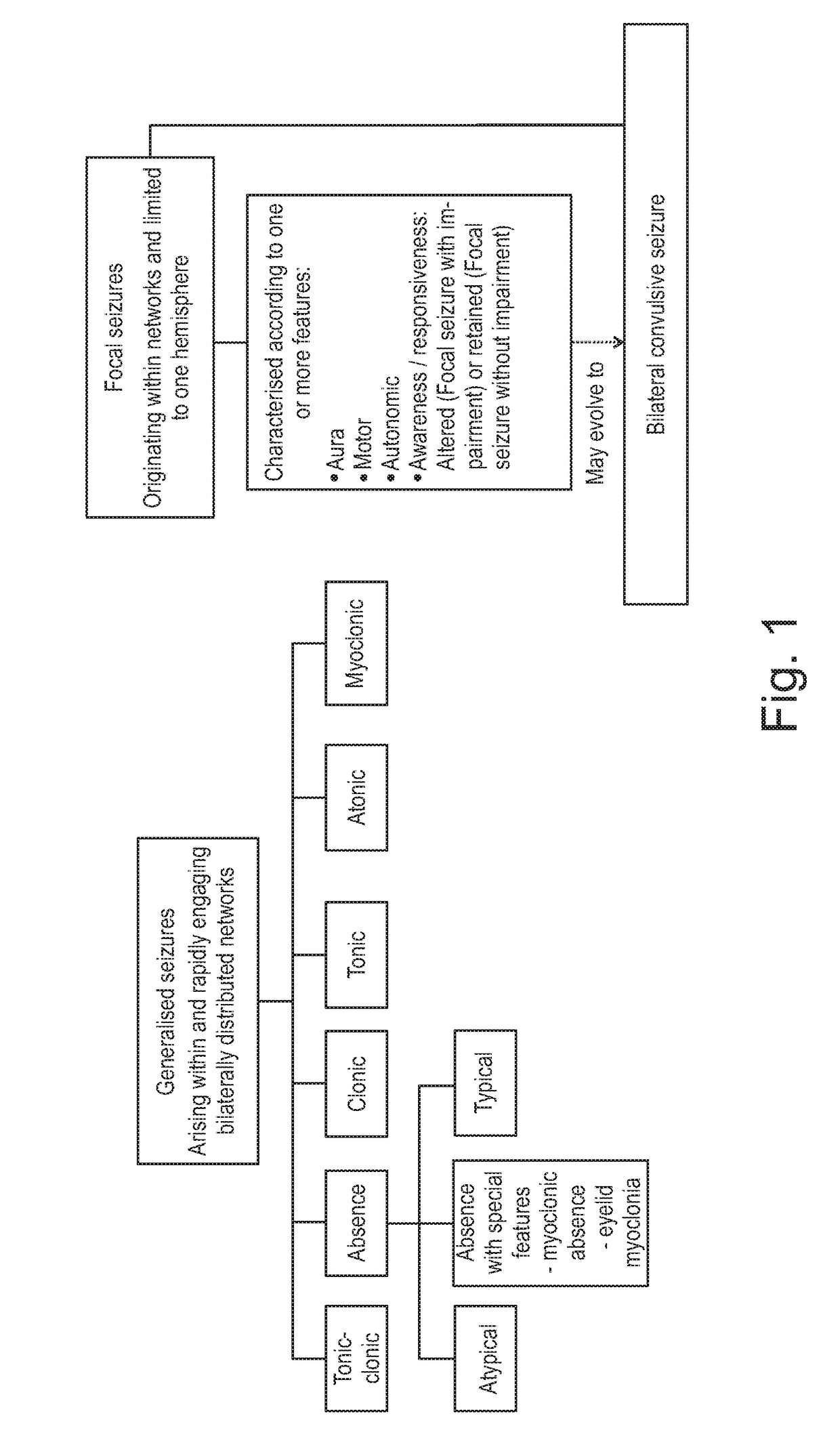
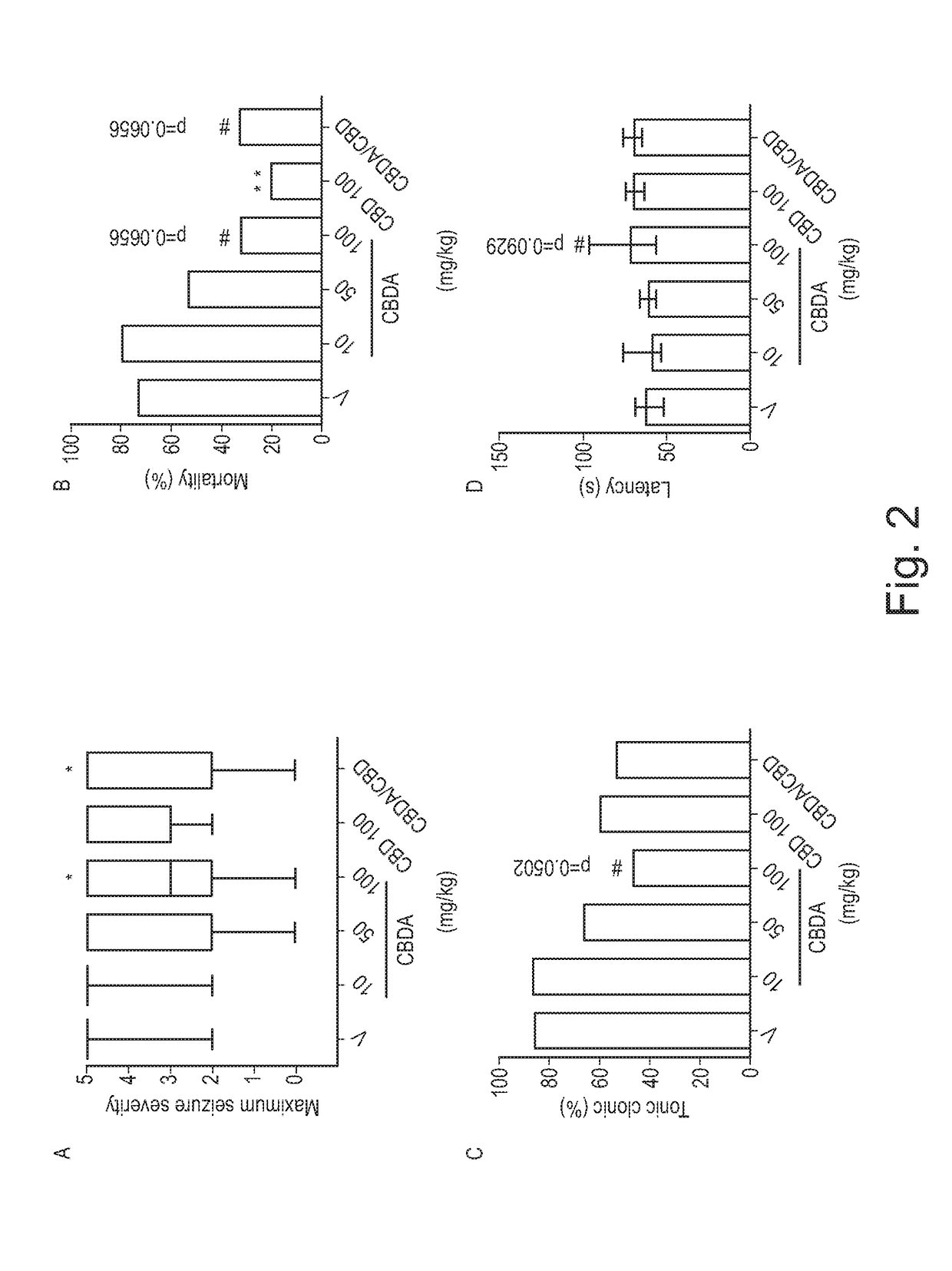
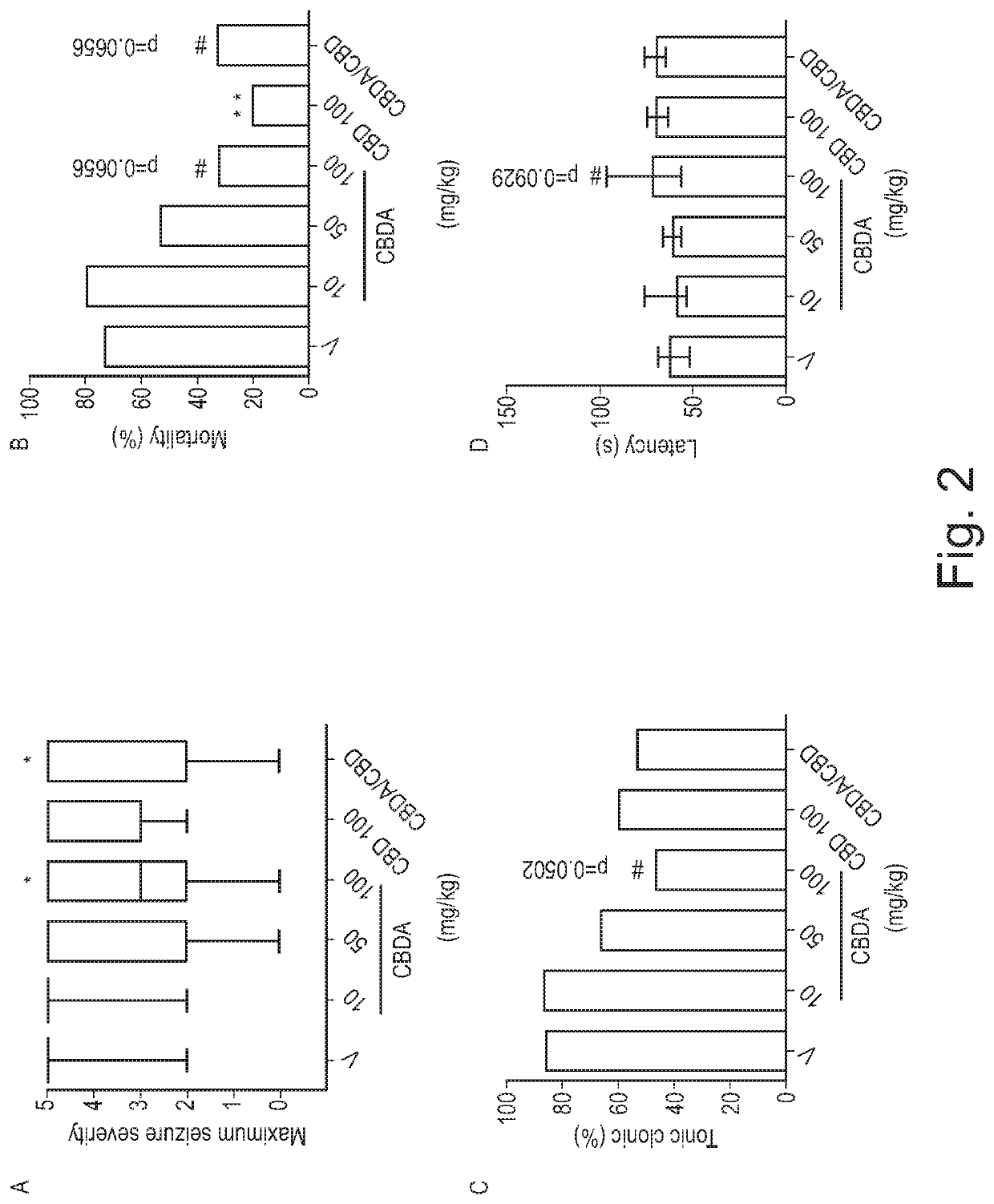
Use of cannabinoids in the treatment of epilepsy
ActiveUS20180228751A1Reduce doseReduced dosNervous disorderHydroxy compound active ingredientsTonic-clonic seizuresEpileptic seizure
The present invention relates to the use of a therapeutically effective amount of cannabidiolic acid (CBDA) in the treatment of epilepsy. In one embodiment the CBDA is used in the treatment of generalised seizures, preferably tonic-clonic seizures. Preferably the CBDA used is in the form of a botanical drug substance in which the CBDA content is greater than 60%, and most preferably, it is a highly purified extract of cannabis such that the CBDA is present at greater than 95%, through 96% and 97% to most preferably, greater than 98% of the total extract (w / w) and the other components of the extract are characterised. In particular the cannabinoids tetrahydrocannabinol (THC) or tetrahydrocannabinol acid (THCA) have been substantially removed. Alternatively, the CBDA may be synthetically produced.
Owner:GW RES LTD
Phytocannabinoids in the treatment of cancer
This invention relates to the use of phytocannabinoids, either in an isolated form or in the form of a botanical drug substance (BDS) in the treatment of cancer. Preferably the cancer to be treated is cancer of the prostate, cancer of the breast or cancer of the colon.
Owner:GW PHARMA LTD
Phytocannabinoids in the treatment of cancer
ActiveUS20150086653A1Slowing down growthLower the volumeBiocideHydrocarbon active ingredientsCannabinoidProstate cancer
This invention relates to the use of phytocannabinoids, either in an isolated form or in the form of a botanical drug substance (BDS) in the treatment of cancer. Preferably the cancer to be treated is cancer of the prostate, cancer of the breast or cancer of the colon.
Owner:GW PHARMA LTD
Method for preparing flavonoid glycoside and stibene glucoside type compound by separating from fenugreek
InactiveCN103304605AEfficient separationOvercome the disadvantages of dead adsorption loss and low yieldSugar derivativesEther separation/purificationSeparation technologyRhapontigenin
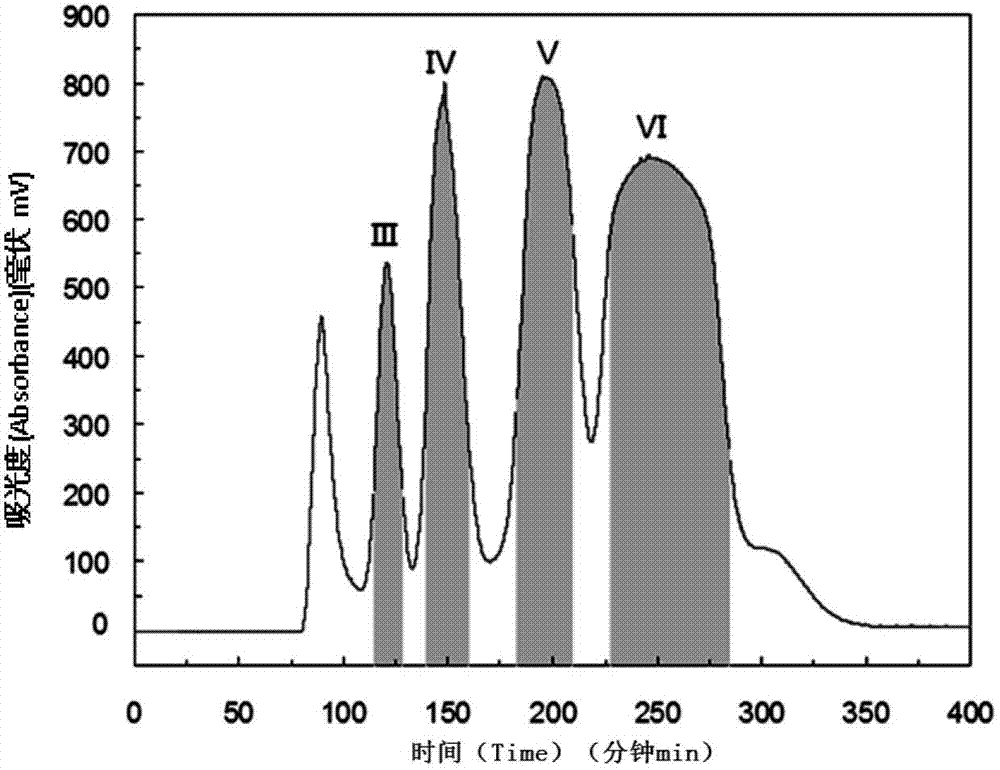
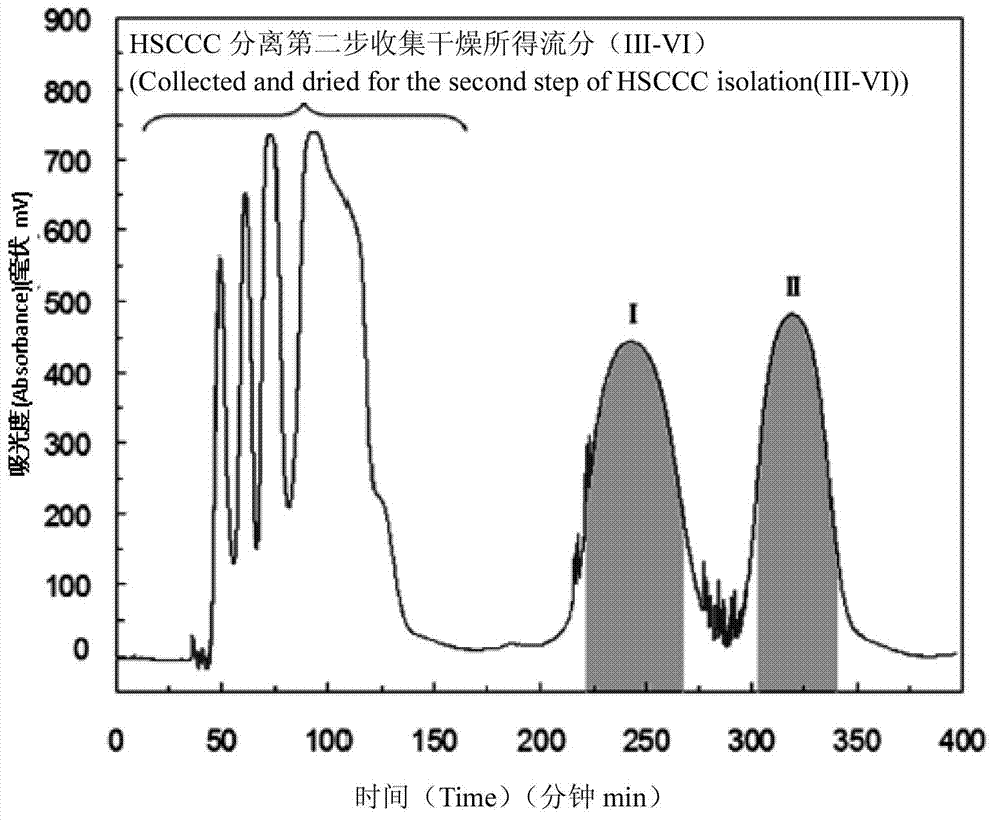
The invention discloses a method for preparing a flavonoid glycoside and stibene glucoside type compound by separating from fenugreek. By virtue of a high-speed countercurrent chromatography (HSCCC) separation technology, isomerides such as vitexin and isovitexin and stibene glucoside type compounds such as rhaponticin, deoxyrhapontin and rhapontigenin which have the similar polarity to that of the isomerides in the fenugreek are subjected to separation preparation, so that the separation time is effectively shortened, and the shortcomings of complicated operation, sample die-adsorption loss, low yield and the like of the conventional preparation method are overcome. According to the method, the technology is simple, the reproducibility is high, and the separation efficiency is high; the obtained monomer content is higher than 96.0 percent and can meet the requirements of a type of botanical drugs and standard contrasts; and the method is suitable for industrial production of medical medicines, standard substances, health protection food, health protection medicines and the like.
Owner:湖州藏源生物科技有限公司
Fingerprint pattern quality control method for cordyceps sinensis bacterium powder raw material in herbs medicaments for strengthening the body resistance and activating blood and dissolving stasis
ActiveCN101293002AGuarantee normal implementationHigh sensitivityFungiComponent separationHplc fingerprintRetention time
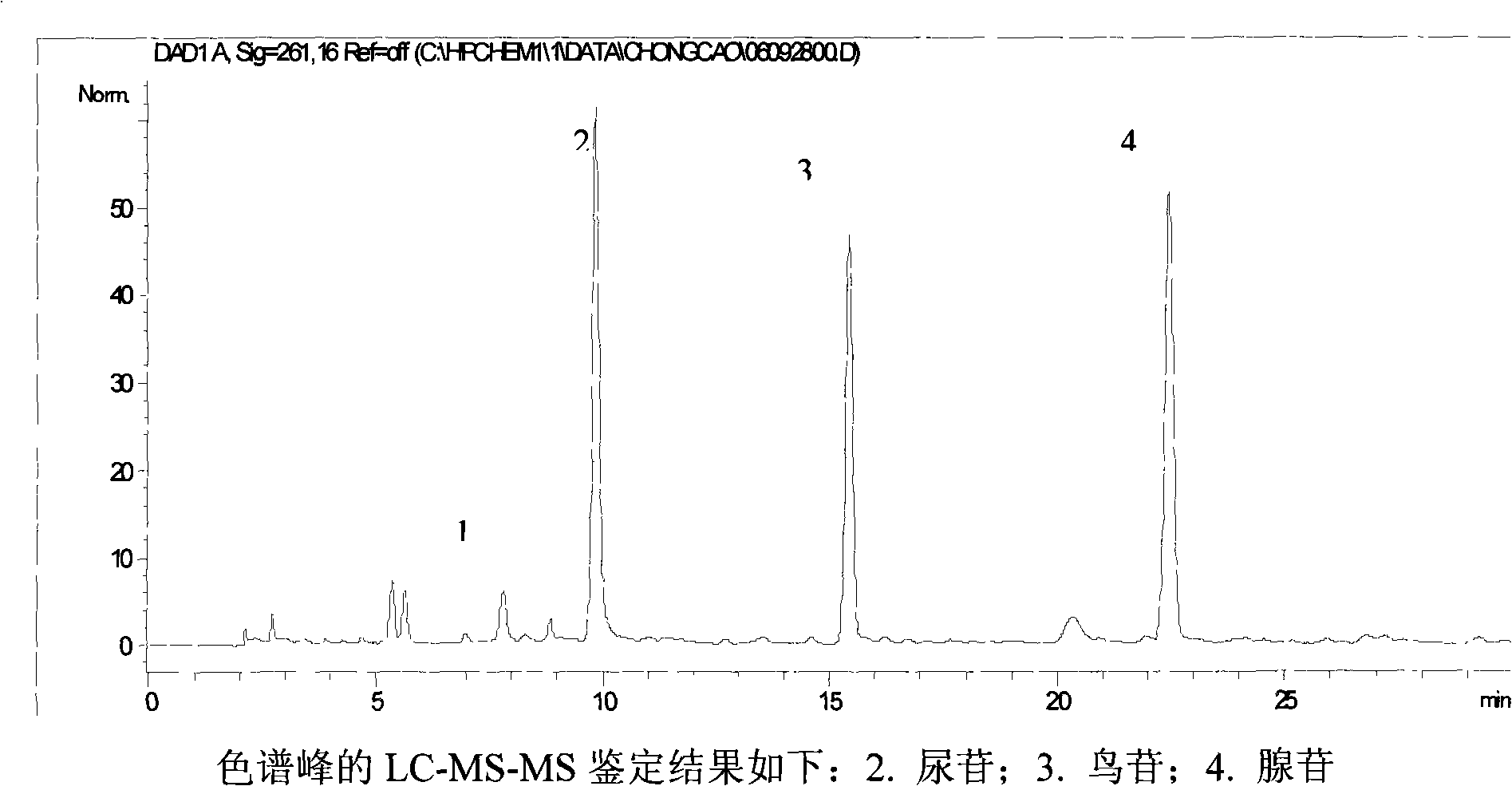
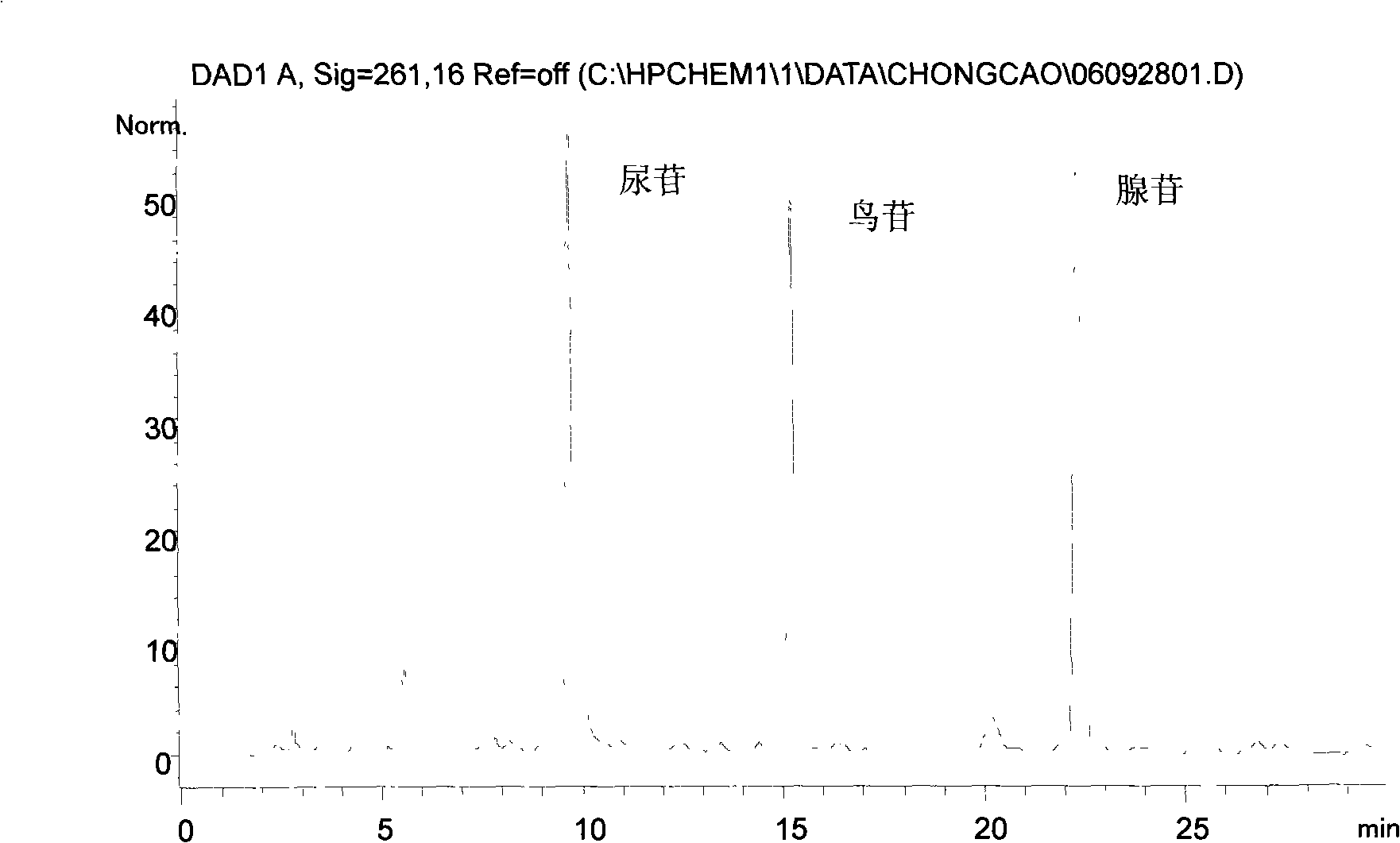
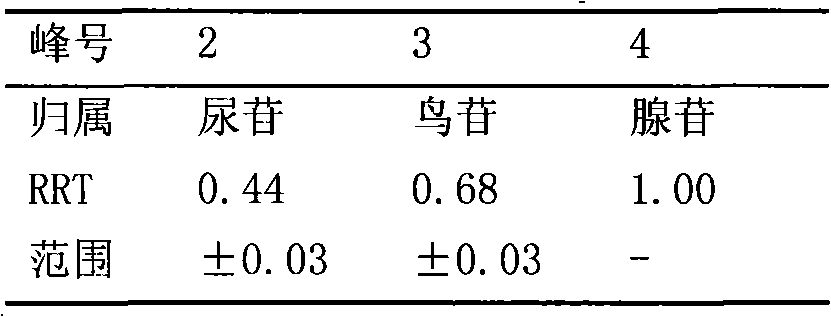
The invention relates to a control method of the fingerprint spectrum quality of cordyceps sinensis powder raw material in botanical drug for strengthening vital qi and removing blood stasis, comprising the steps that: (1) cordyceps sinensis powder is extracted: 0.100g of cordyceps sinensis powder is taken, purified water is added, the ultrasonic extraction, the filtration and the sample injection are carried out; (2) the gradient elution with mobile phase is carried out: octadecyl silane bonded silica gel is taken as a filler, water and acetonitrile are taken as mobile phase to carry out the gradient elution for 0 to 30min and 0 to 7 percent B; (3) a standard fingerprint spectrum is established: the HPLC standard fingerprint spectrum of the cordyceps sinensis powder is determined, and 3 characteristic peaks are selected; (4) the quality control of the fingerprint spectrum is carried out: the relative retention time of No.2 peak uridine, No.3 peak guanosine and No.4 peak adenosine are 0.44 plus or minus 0.03, 0.68 plus or minus 0.03 and 1.00 respectively; the HPLC fingerprint spectrum of the sample is compared with the contrast fingerprint spectrum. The similarity calculated by the 5 common peaks is not less than 0.9, (5) the preparation of the cordyceps sinensis powder raw material is carried out; the control method has good repetitivity and can fully reflect the basic characteristics of nucleoside ingredients of the cordyceps sinensis powder.
Owner:SHANGHAI MODERN CHINESE TRADITIONAL MEDICINE TECH DEV
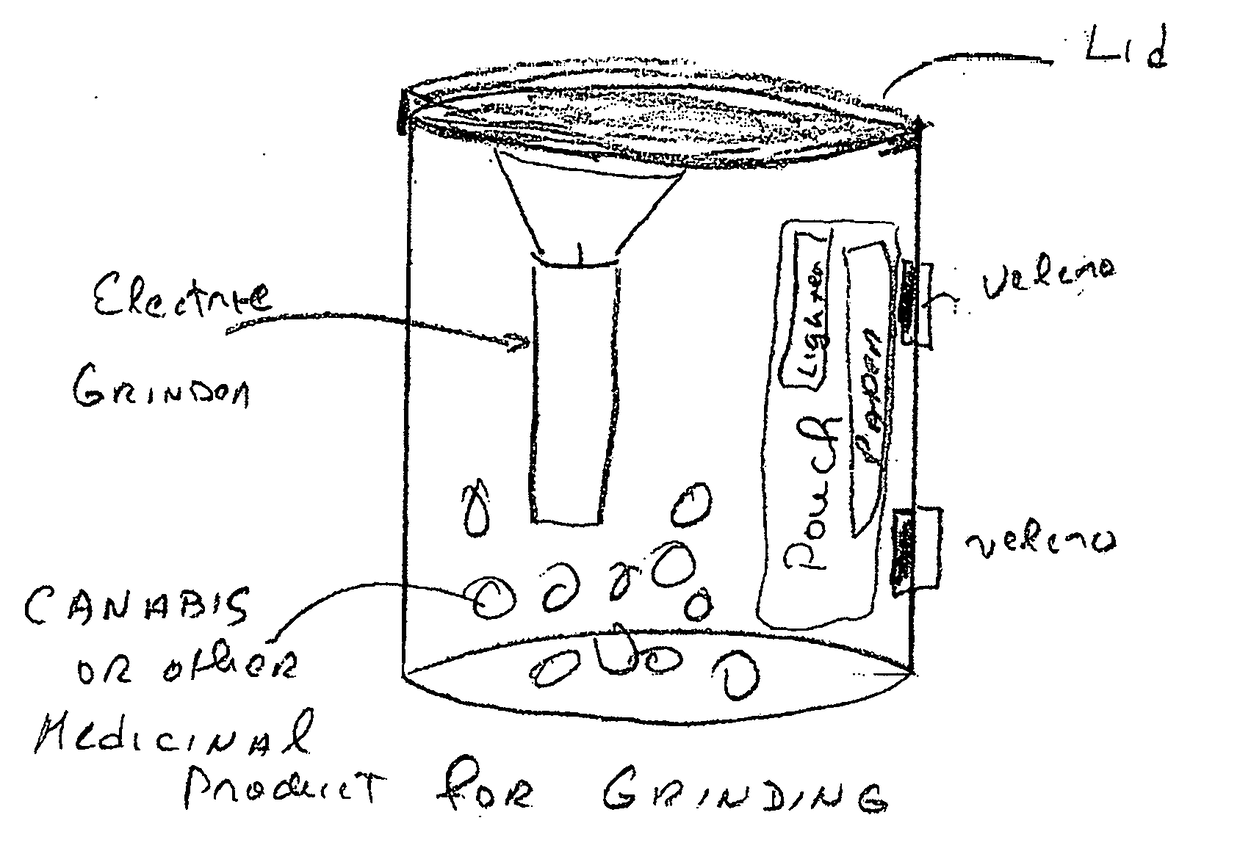
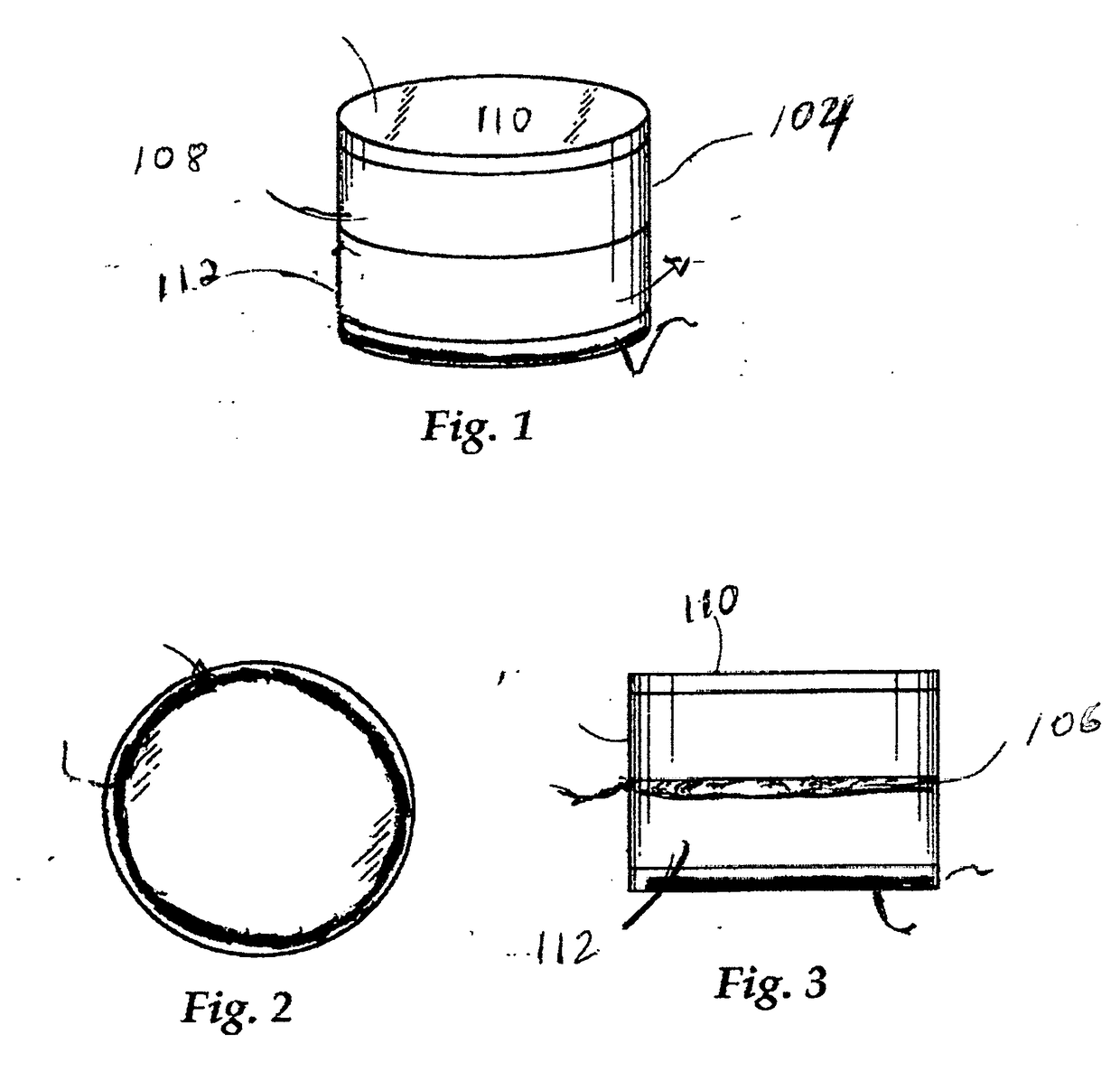
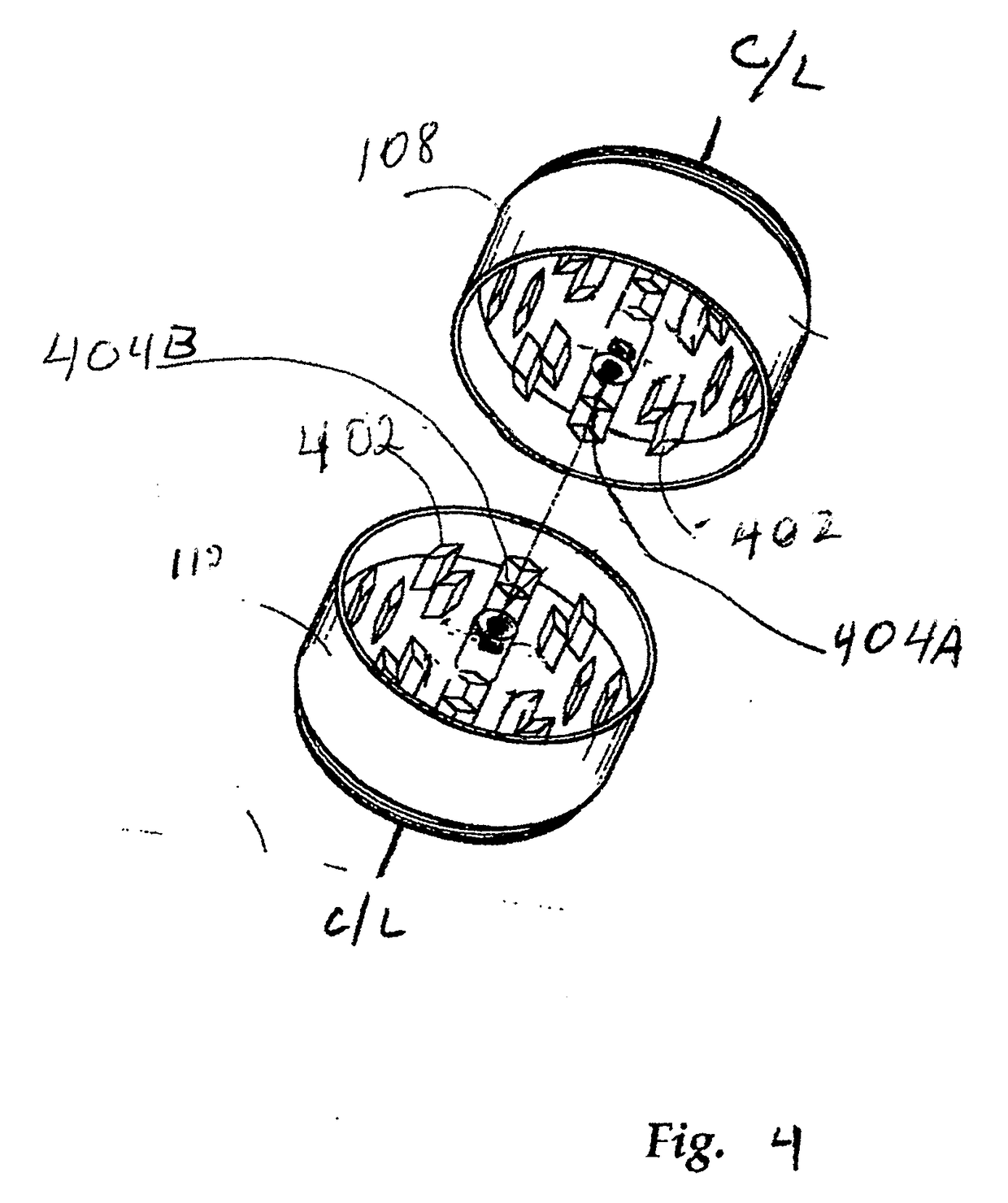
Integrated grinding and storage system for optimizing and enhancing plant performance of plant-based medical therapies and related cannabis usage
InactiveUS20170368554A1Easy and convenient accessReduce the possibilityCoffee millsSpice millsCannabisEngineering
An integrated grinding and storage system for optimizing and enhancing plant performance for plant-based medical therapies and related uses of cannabis including an apparatus for grinding that includes a head operable to be removably attached to a receptacle or container. The head includes a magnet, an upper, inner grinding element, a lower grinding element, a shaft, and a neck sleeve. The upper, inner grinding element is in communication with the lower grinding element within the head. The grinding elements are operable to rotate relative to each other. The shaft extends between the upper and lower sections and magnetically couples with the grinding elements and orients them in relation to one another. The head may be manually or electrically activated.
Owner:NICHOLS WILLIAM
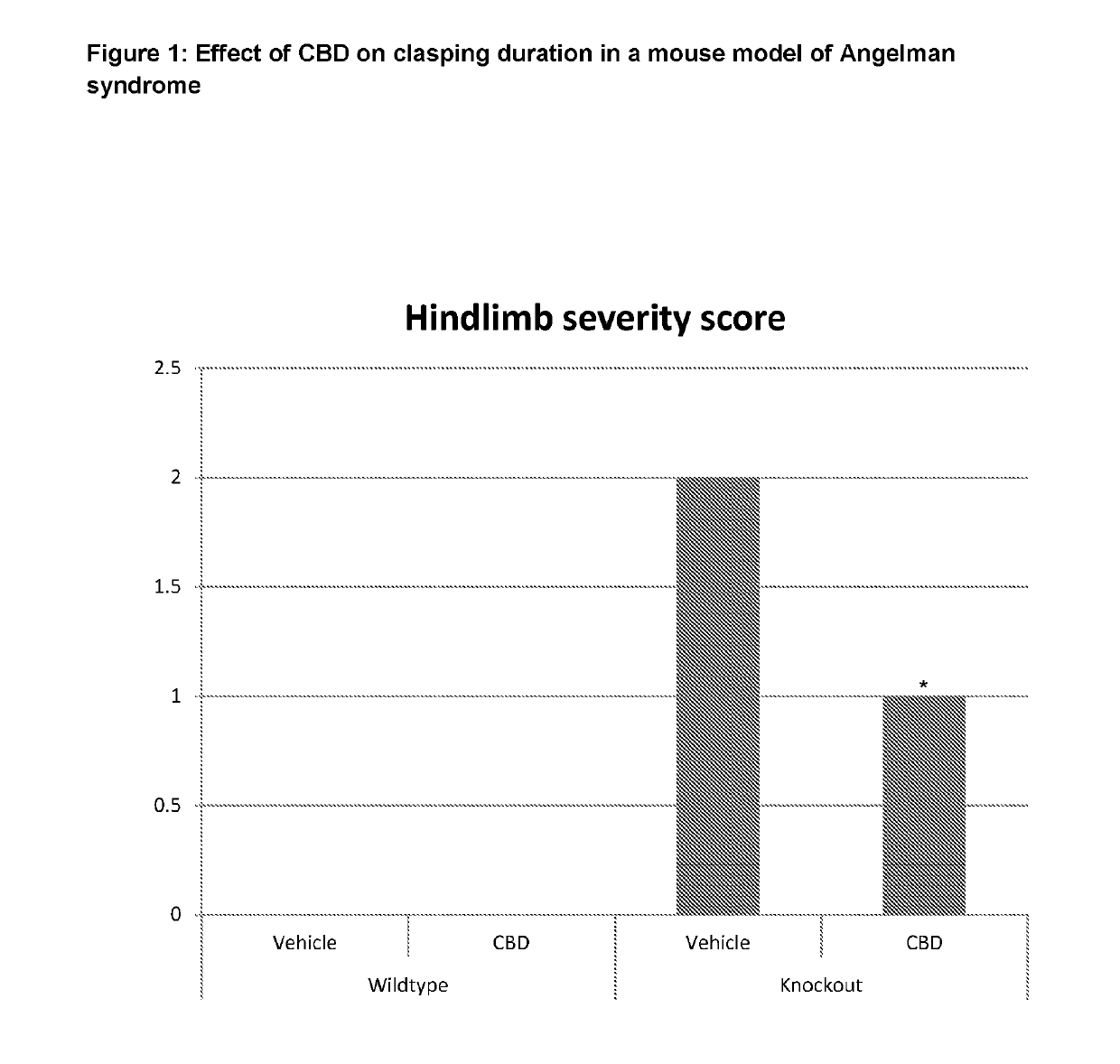
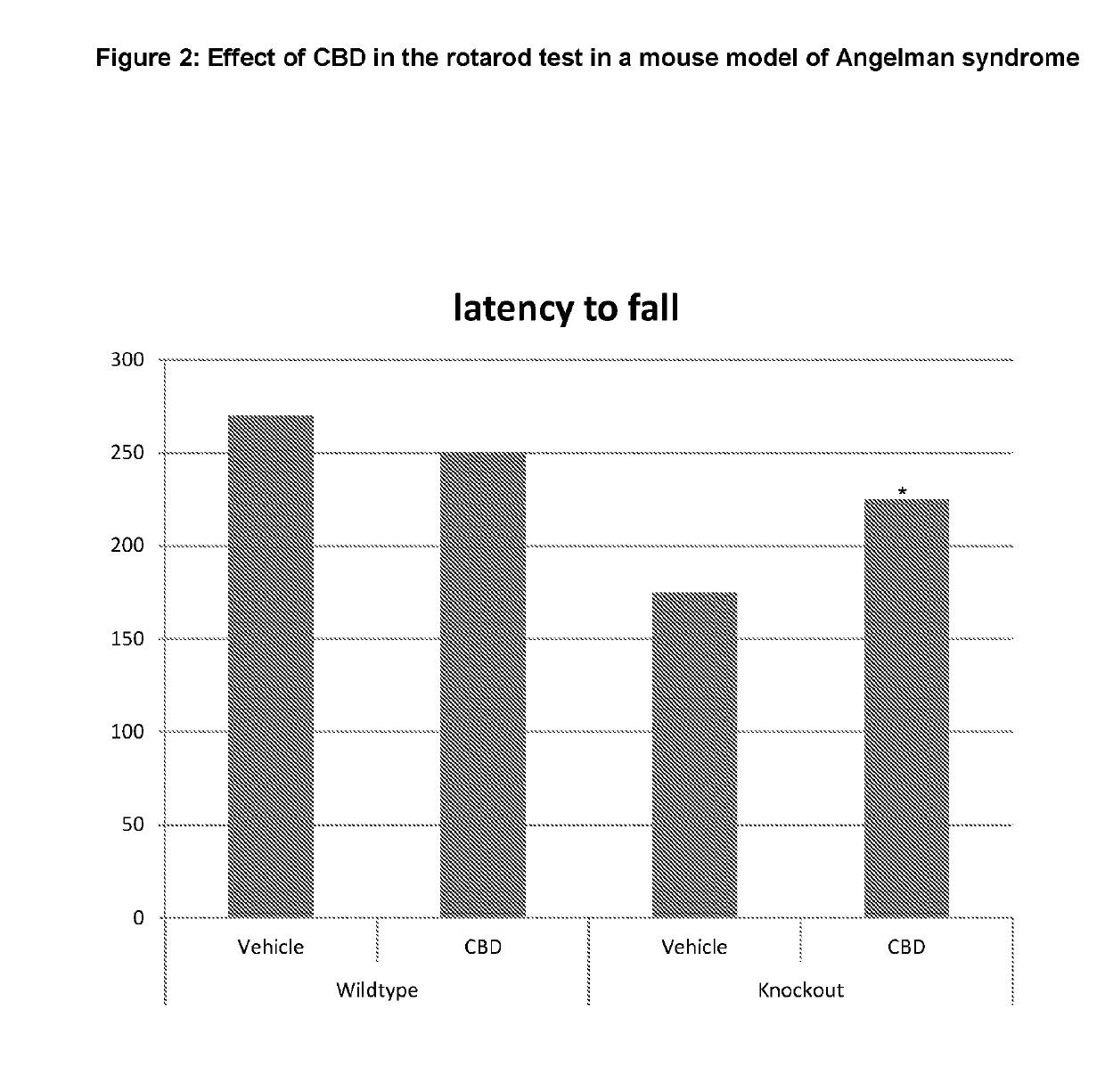
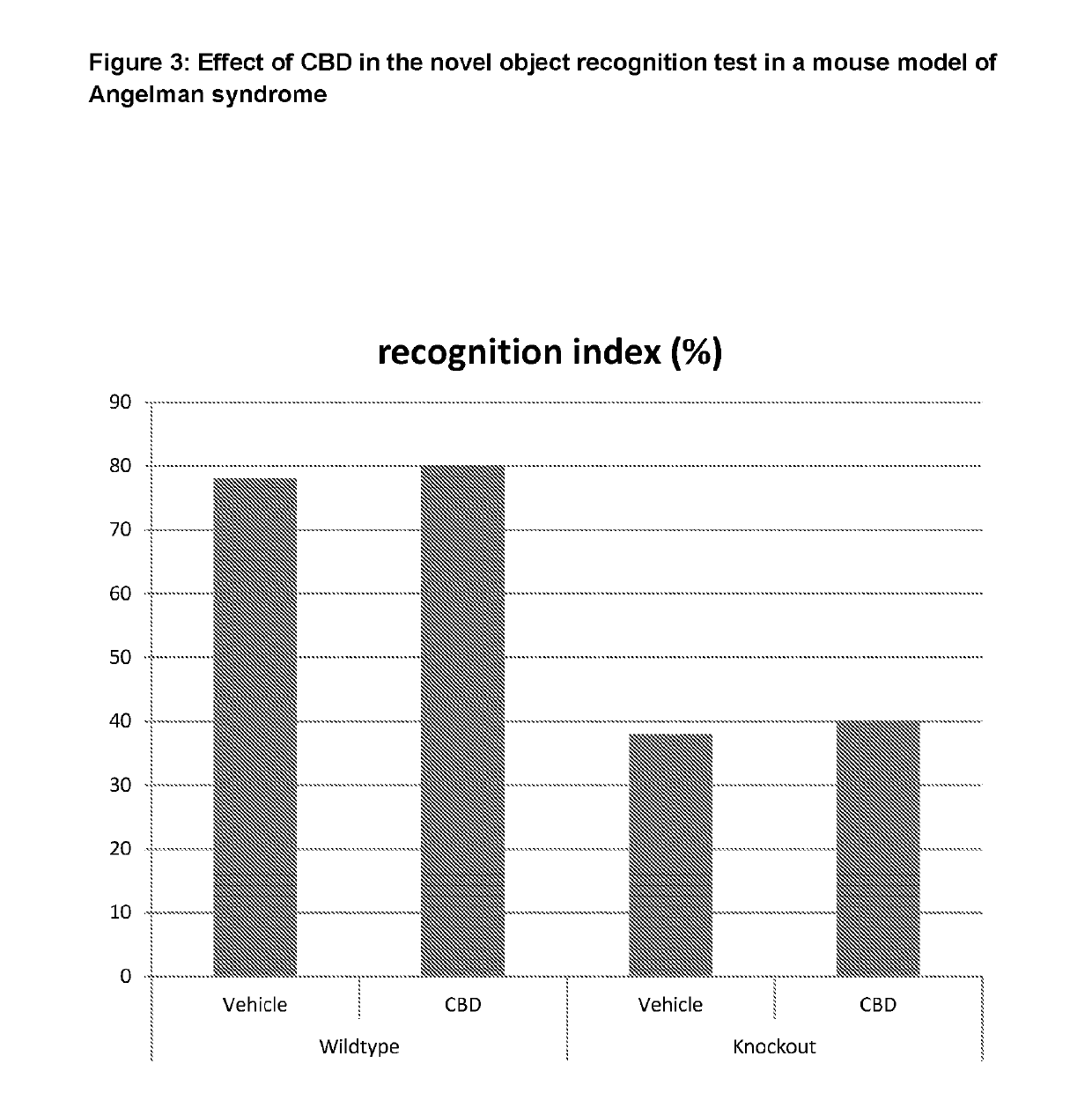
Use of cannabinoids in the treatment of angelman syndrome
ActiveUS20190321307A1Symptoms improvedNervous disorderHydroxy compound active ingredientsRodent modelAnxiety
The present invention relates to the use of cannabidiol (CBD) in the treatment of Angelman syndrome (AS). CBD has It been shown to be particularly effective in improving anxiety in rodent models of AS. The CBD is preferably substantially pure. It may take the form of a highly purified extract of Cannabis such that the CBD is present at greater than 98% of the total extract (w / w) and the other components of the extract are characterised. Alternatively, the CBD is synthetically produced. Alternatively, the CBD may be used as a botanical drug substance (BDS) from a Cannabis plant in which CBD is the predominant cannabinoid. The CBD may also be present in combination with other cannabinoids and non-cannabinoid components such as terpenes. In use the CBD may be used concomitantly with one or more other medicaments. Alternatively, the CBD may be formulated for administration separately, sequentially or simultaneously with one or more medicaments or the combination may be provided in a single dosage form. Where the CBD is formulated for administration separately, sequentially or simultaneously it may be provided as a kit or together with instructions to administer the one or more components in the manner indicated. It may also be used as the sole medication, i.e. as a monotherapy.
Owner:GW RES LTD
Dosage delivery film
ActiveUS10058531B1Hydroxy compound active ingredientsOrganic non-active ingredientsDose deliveryVascular dilatation
A dosage delivery film composition containing a botanical drug substance formable into a bioerodible dosage delivery film, the dosage delivery film composition including: (i) one or more of: a polymer, a plasticizer, a defoamer, or an antioxidant; (ii) a cannabinoid-cyclodextrin-terpene complex or a cannabinoid-terpene-surfactant micelle, and (iii) optionally, one or more of: a taste mask, a vasodilator, or a lipophilic vehicle to transport the botanical drug substance across the mucous membrane.
Owner:SPARTAK LLC
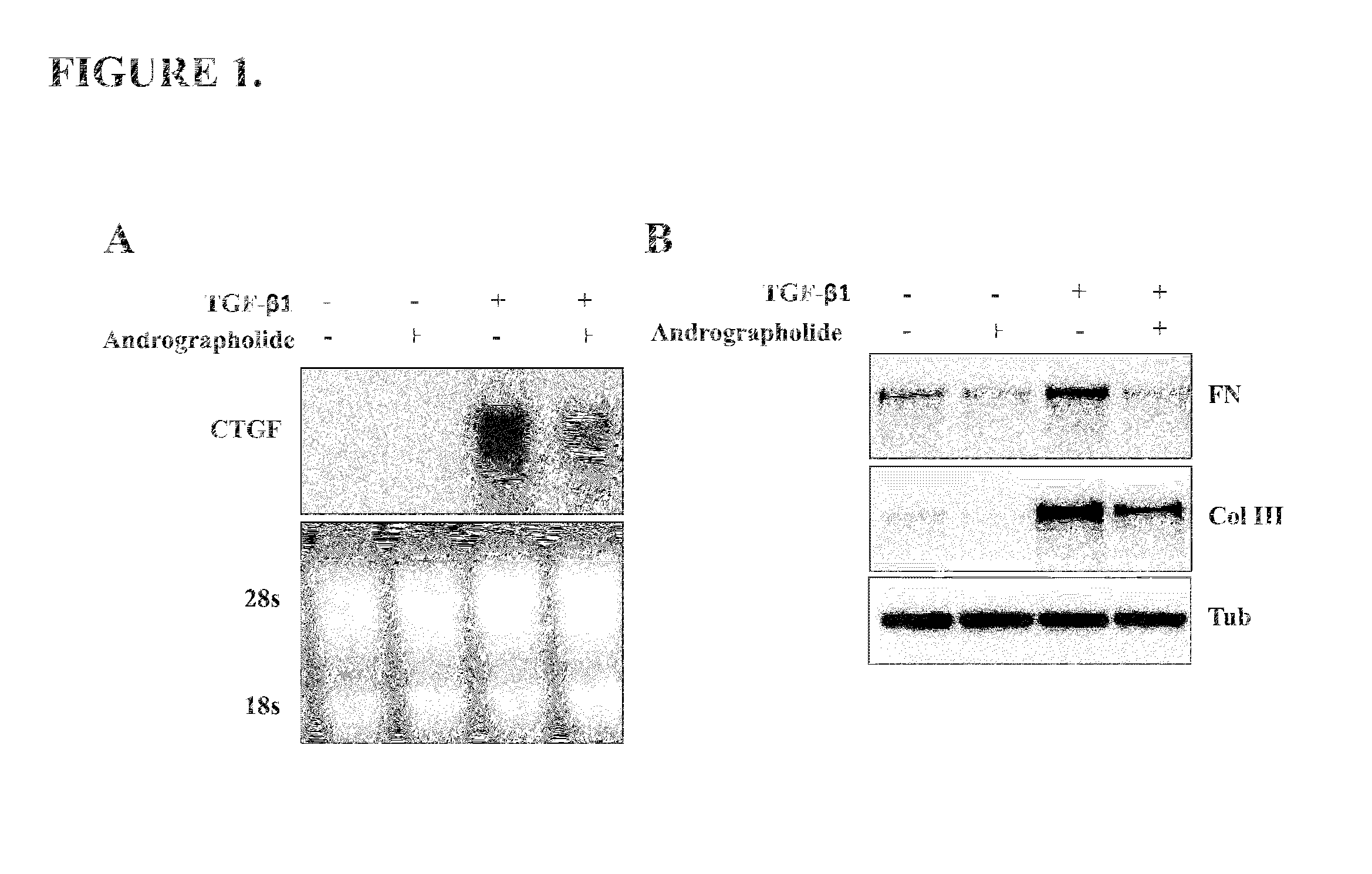
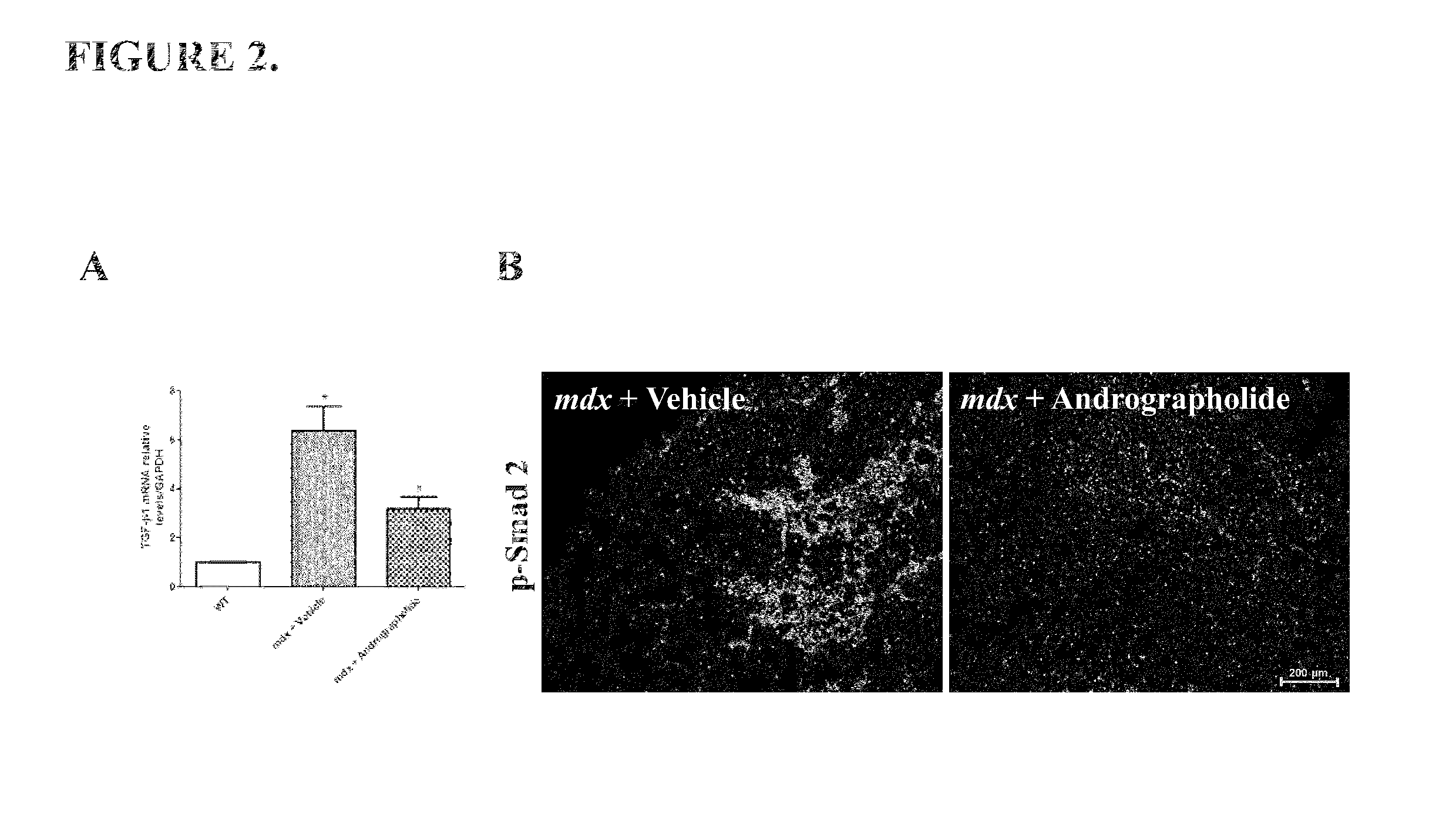
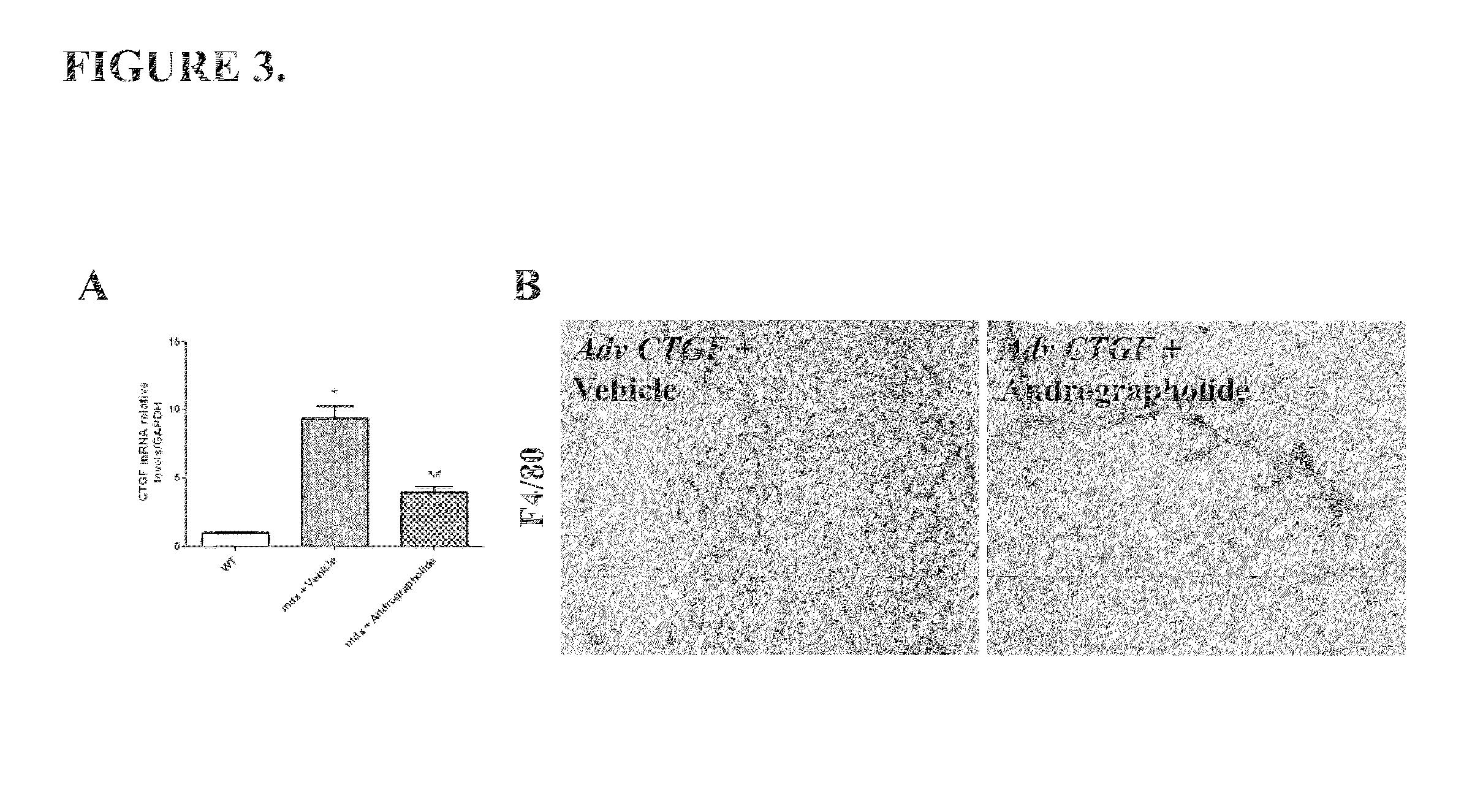
Pharmaco-cellular therapeutic method for the treatment of muscular dystrophies
A method of treating a muscular dystrophy disease in a patient includes administering an effective amount of a botanical drug isolated from Andrographis paniculata in combination with cell therapy. The method improves skeletal muscle performance.
Owner:PONTIFISIA UNIVERSIDAD KATOLIKA DE CHILE
Series product for whitening and softening skin
The invention provides a series of skin whitening and beautifying products prepared by taking natural herbal medicine extract as raw materials, adding in other base materials, and screening skin whitening and beautifying medicament combinations from natural botanical drugs according to the skin whitening theory of traditional Chinese medicine. The products can improve basically skins, adapt to natural metabolism law of skins, promote regeneration of skin cells, prolong the ageing time of skins, maintain the self protection capability of skins, promote blood circulation, absorb ultraviolet radiation, inhibit secretion of melanin and make skins more white, tender and lustrous.
Owner:北京蓝邦生物医学工程科技有限公司
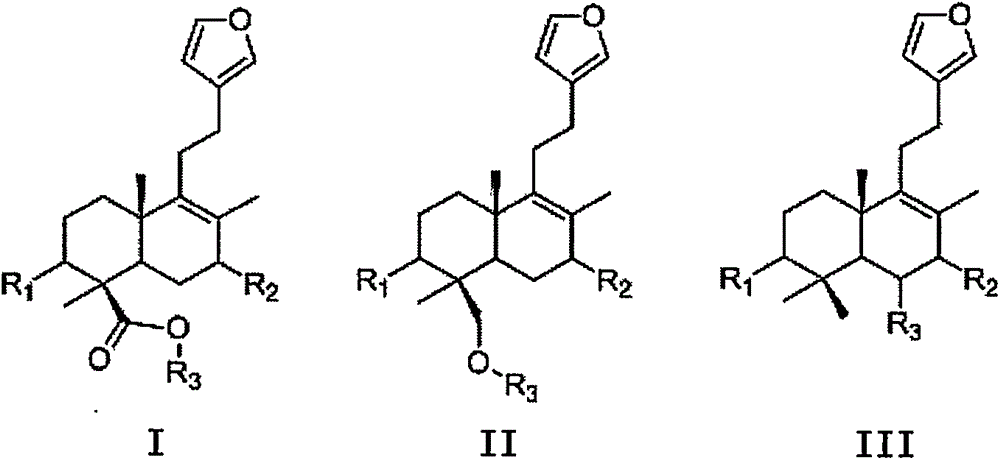
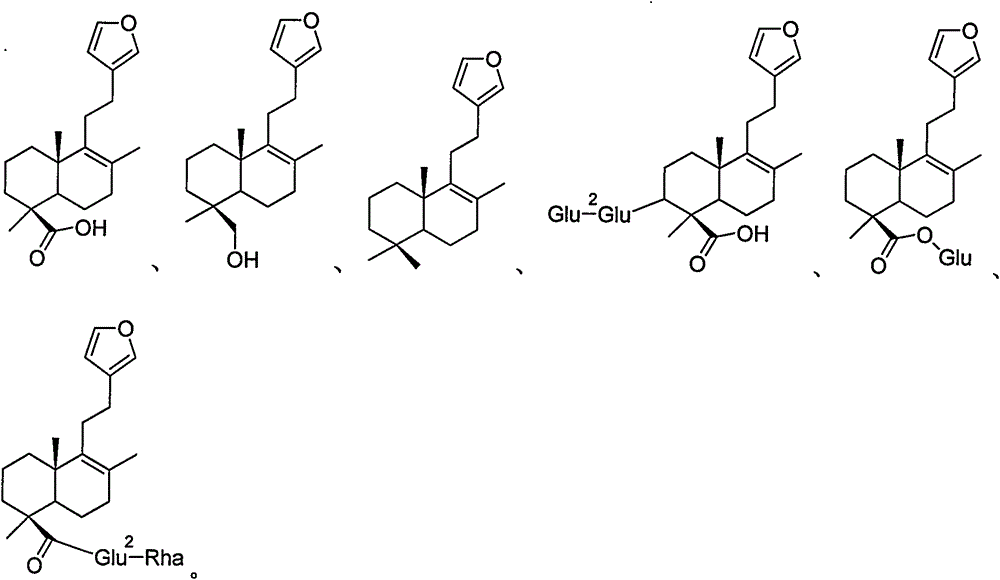
Labdane type diterpene derivatives and preparation method and application thereof
ActiveCN105085449AInhibits α-glucosidase activityEsterified saccharide compoundsSugar derivativesPhlomis umbrosaOxygen
The invention provides a series of labdane type diterpene derivatives and a preparation method and application thereof. The derivatives are compounds containing structures as shown in general formula I, II or III or addition salts formed from compounds and pharmaceutically acceptable acids or alkalis. R1 is hydrogen, hydroxy or C1-C3 alkyloxy or acyloxy; R2 is C1-C3 saturated or unsaturated alkyl, C1-C3 oxygen-containing alkyl or C1-C3 acid and ester thereof or salt. The derivatives employ Labiatae Phlomis plant Phlomis umbrosa as a raw material, and solvent extraction, column chromatography separation and purification are carried out for preparation. According to the invention, based on separation for preparing the labdane type diterpene derivatives, the compounds have activity for inhibiting alpha-glucosidase is verified, so that lead compounds for developing oral medicaments for reducing blood glucose are provided, and the preparation method has important meanings for exploring new purposes of natural products and botanical drugs.
Owner:SHANGHAI UNIV OF T C M
Topical treatments incorporating cannabis sp. derived botanical drug product
A topical formulation comprising a Cannabis derived botanical drug product is disclosed, wherein the concentration of tetrahydrocannabinol, cannabidiol, or both in the topical formulation is greater than 2 milligrams per kilogram. Other objects of the invention include methods of making the topical preparations and methods of using the topical preparations to treat dermatological diseases.
Owner:罗纳德 D 塞库拉 +1
Use of cannabinoids in the treatment of epilepsy
ActiveUS11147783B2Improve bioavailabilityAct more quicklyNervous disorderHydroxy compound active ingredientsClonic seizureCannabielsoin
The present invention relates to the use of a therapeutically effective amount of cannabidiolic acid (CBDA) in the treatment of epilepsy. In one embodiment the CBDA is used in the treatment of generalised seizures, preferably tonic-clonic seizures. Preferably the CBDA used is in the form of a botanical drug substance in which the CBDA content is greater than 60%, and most preferably, it is a highly purified extract of cannabis such that the CBDA is present at greater than 95%, through 96% and 97% to most preferably, greater than 98% of the total extract (w / w) and the other components of the extract are characterised. In particular the cannabinoids tetrahydrocannabinol (THC) or tetrahydrocannabinol acid (THCA) have been substantially removed. Alternatively, the CBDA may be synthetically produced.
Owner:GW RES LTD
Botanical drug compound recipe for preventing and treating microwave radiation injury
ActiveCN102727597AGood effectEnhance immune functionDermatological disorderPlant ingredientsMetabolic enzymesTherapeutic effect
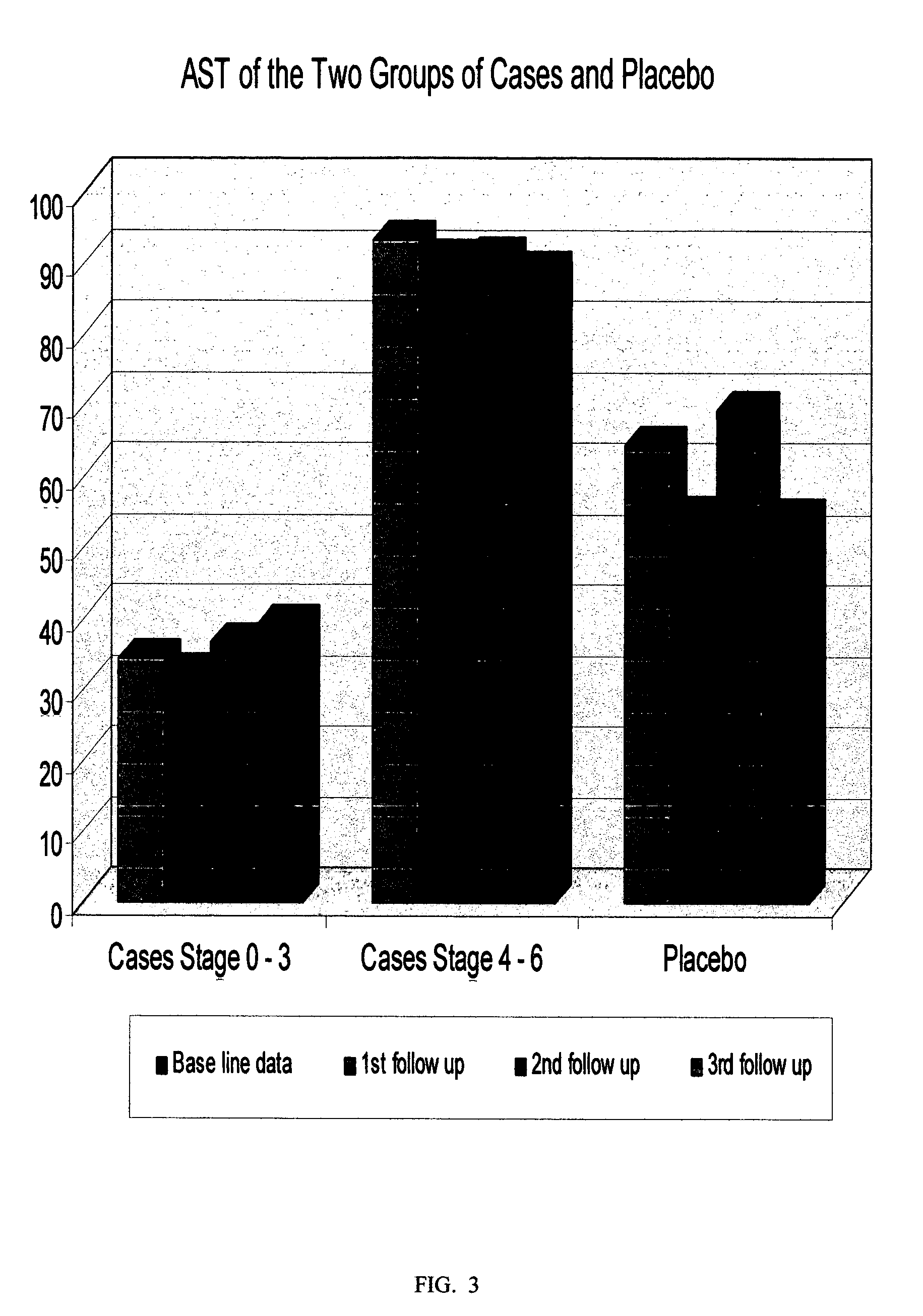
The invention relates to a botanical drug compound recipe for preventing and treating microwave radiation injury. According to the invention, it is found that botanical drugs mainly consisting of peony root, milkvetch root, duckweed and red sage root can be used for preparing a medicine used for preventing and treating microwave radiation injury. According to results of abundant experiments of detection of learning and memory capacity of rats, hippocampal amino acid neurotransmitters, sperm motility, the rate of teratosperm, myocardial metabolic enzyme (CK-MB, LDH and AST activity) and the content of Ca<2+> in serum of rats, the number of peripheral blood lymphocytes and the like, the medicine provided in the invention has an obvious treatment effect on injury to the brain, reproduction, the heart and immunity of rats caused by microwave radiation, and the effect is superior to that of medicines on the market.
Owner:INST OF RADIATION MEDICINE ACAD OF MILITARY MEDICAL SCI OF THE PLA +1
Traditional Chinese medicine face applying mud
InactiveCN102091007APromote blood circulationImprove refreshmentCosmetic preparationsToilet preparationsSide effectAngelica Sinensis Root
The invention discloses traditional Chinese medicine mineral face applying mud comprising the following components: pearls, white poria, radix sileris, medical stones, honey, angelica sinensis, peppermint, folium ginkgo, flos rosae rugosae, vitamin E and dextrin. A preparation method of the traditional Chinese medicine mineral face applying mud comprises the following steps of: firstly respectively selecting, decontaminating and cleaning the botanical drugs; then soaking the botanical drugs in clear water for 20-60 minutes; decocting for 40-80 minutes; filtering and removing slag; then crushing the medical stones together with the pearls to 800-1000 meshes in an ultramicro way; sufficiently mixing the decoction of the decocted botanical drugs with medical stone powder, pearl powder, the honey, the vitamin E and the dextrin into paste face mud; and sterilely and quantificationally packaging so as to obtain the traditional Chinese medicine mineral face applying mud. The traditional Chinese medicine mineral face applying mud is simple and easy to prepare, materials are easy to obtain, and the traditional Chinese medicine mineral face applying mud can effectively promote the metabolism function of skin cells, enhance the refreshment, the tenderness and the elasticity of facial skin and inhibit the skin pigments and has no toxic and side effect when being used for a long time.
Owner:蒋健
Chinese medicine color spectrum fingerprint pattern characteristic digitalization and full-qualitative full-quantitative quality control method
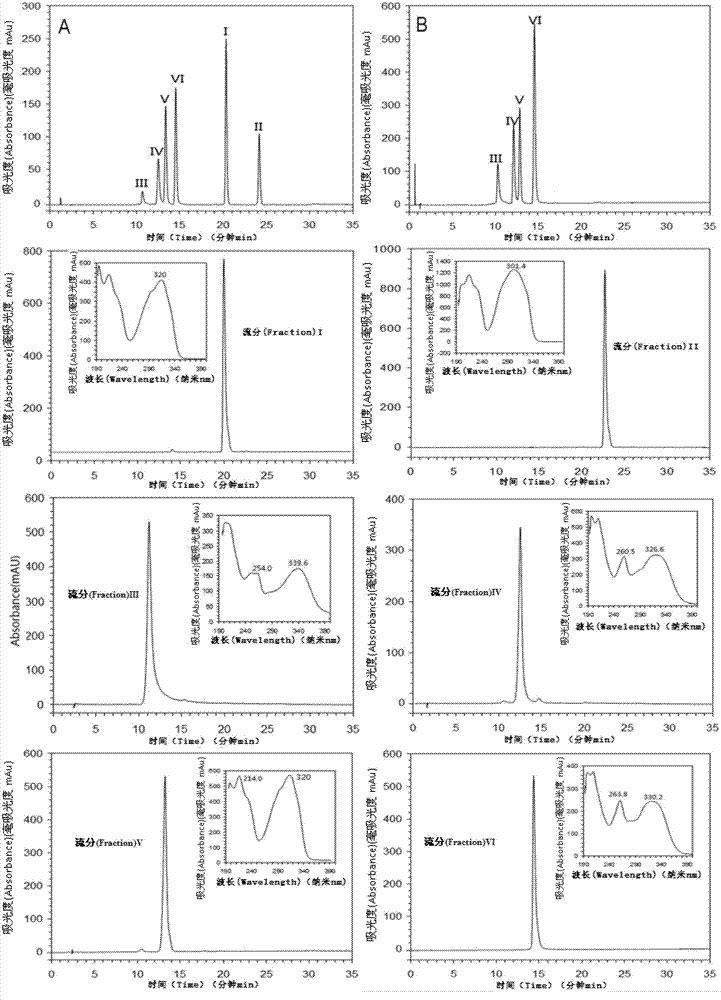
The invention discloses a digital control method of super-information characteristics of traditional Chinese medicine chromatographic fingerprint, and 37 characteristic indicators are combined with the computer software technology to be used for the production quality control of traditional Chinese medicine materials, traditional Chinese medicine extracts and traditional Chinese medicine preparations. The invention simultaneously discloses an overall qualitative and overall quantitative quality control method of the traditional Chinese medicine chromatographic fingerprint, which uses the ratio qualitative similarity SF', Q percent of the content similarity, QF percent of the correction content similarity, C percent of the projection content similarity, P percent of the quantitative similarity, W percent of the norm length percentage, M percent of the average weight percentage, MF percent of the correction average weight percentage, d percent of the Euclidean distance percentage, Delta C percent of the projection content similarity error and other indicators and is further combined with the computer software technology to be used for the production quality control of the traditional Chinese medicine materials, the traditional Chinese medicine extracts, the traditional Chinese medicine preparations and botanical drugs. The digital control method is used for the evaluation of the fingerprint of the traditional Chinese medicine or the traditional Chinese medicine preparations and the confirmation and the evaluation of the test conditions, thus leading the test results to have quantitative reference indicators under the different conditions, which has great practicality.
Owner:SHENYANG PHARMA UNIVERSITY
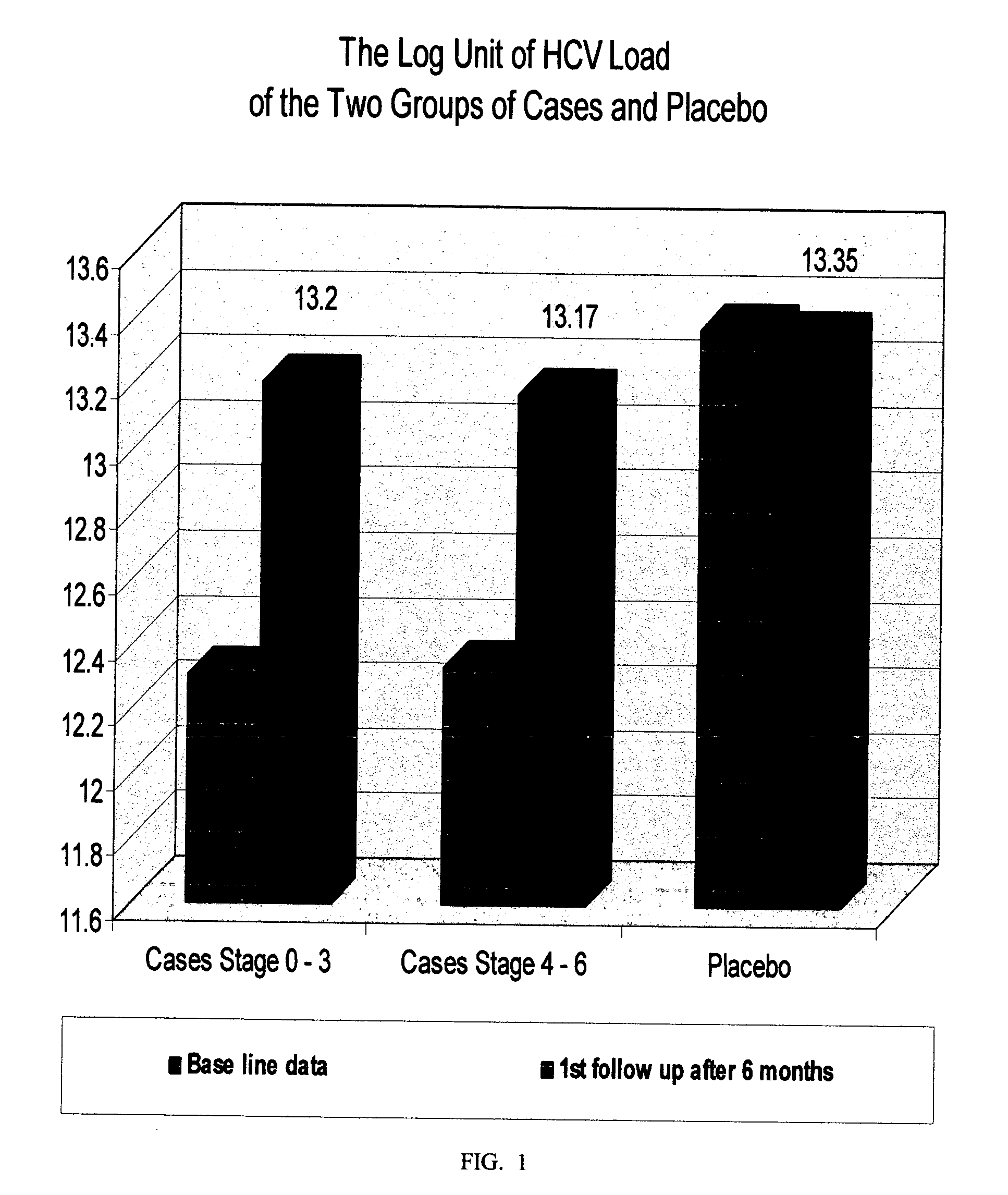
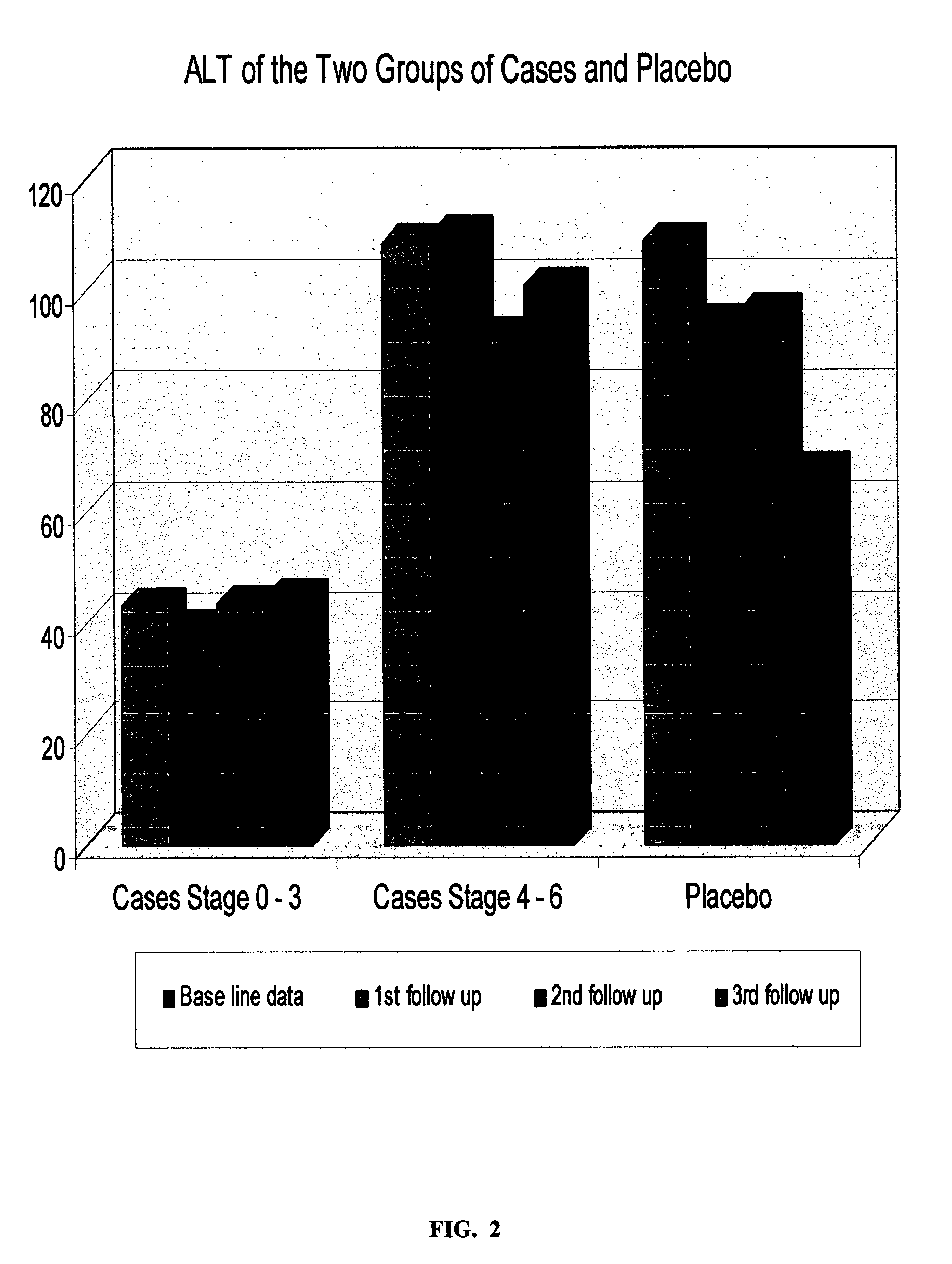
Plant-based medicament for the treatment of hepatitis c
InactiveUS20070160693A1Reducing and alleviating symptomLiver functionBiocideAntiviralsAdditive ingredientDietary supplement
The present invention relates to a botanical drug or dietary supplement for use in the treatment of patients suffering from Hepatitis C virus infection. More particularly, it relates to a botanical drug consisting essentially of four botanical drug substances, optionally formulated with excipients, for use either in alleviating the symptoms of Hepatitis, particularly chronic Hepatitis C, and / or inhibiting the activity of the causative Hepatitis C virus. The botanical raw -materials, botanical drug substances or botanical ingredients used are from a species of each of the genera: (a) Silybum; (b) Astragalus or Hedysarum; (c) Salvia; and (d) Schisandra.
Owner:PHYNOVA LTD
Plant-based medicament for the treatment of liver disease
The present invention relates to further medical uses for a botanical drug or dietary supplement consisting essentially of four botanical drug substances, optionally formulated with excipients. The botanical raw materials, botanical drug substances or botanical ingredients used are from a species of each of the genera: (a) Silybum; (b) Astragalus or Hedysarum; (c) Salvia; and (d) Schisandra.
Owner:PHYNOVA LTD
Cannabis sativa plants rich in cannabichromene and its acid, extracts thereof and methods of obtaining extracts therefrom
InactiveUS20160360721A1Flowers cultivationOrganic active ingredientsCannabis sativa plantCannabichromene
The present invention relates to plants producing, as their major cannabinoid cannabichromenic acid (CBCA) or its neutral (decarboxylated) form cannabichromene (CBC), hereafter jointly referred to as CBC(A). It additionally relates to; a botanical material obtainable from said plants; a botanical raw material (BRM), an extract including a botanical drug substance (BDS) and a purified BDS; a formulation comprising the BRM, BDS, purified BDS or other extract; the use of the BRM, BDS, purified BDS or other extract in the manufacture of a medicament; a method of deriving plants yielding a high proportion of the in cannabinoid CBC(A) at the expense of other cannabinoids; a method of cultivating plants such that they yield a high proportion of the cannabinoid CBC(A) at the expense of other cannabinoids; and a method of extracting CBC(A) from said plants.
Owner:GW PHARMA LTD
Botanical drug compositions for treatments of liver and immunological disorders
InactiveUS20050058735A1Increase the number ofHigh activityBiocideUnknown materialsDiseaseAdditive ingredient
The present invention relates to compositions comprising the botanicals from the Family Ranunculaceae, Subclass: Dicotyledonae; Crassinucelli, Superorder: Ranunculales. Examples of these botanicals include but are not limited to; Actaea, Anemone, Ranunculus, and Nigella, or extracts thereof, which are useful in treating liver diseases, particularly those with viral etiology. More specifically, the compositions of the present invention are derived from various botanicals or medicinal plants. The compositions of the invention have demonstrated outstanding efficacy for treatment of patients with hepatic disorders. Compositions of the present invention have also exhibited immunomodulatory activities. The preferred compositions contain the botanical ingredients in concentrations of not less than 20% w / v. The treatment can be therapeutic or prophylactic and may be administered orally, parenterally, as suppository or via nasal mucosa. The treatment may be delivered in a single dose, multi-doses or via a slow release mechanism.
Owner:AMBOTAN PHARMA
Cannabis extracts and methods of preparing and using same
The invention relates to the extraction of pharmaceutically active components from plant materials, and more particularly to the preparation of a botanical drug substance (BDS) for incorporation in to a medicament. It also relates to a BDS, for use in pharmaceutical formulations. In particular it relates to BDS comprising cannabinoids obtained by extraction from cannabis
Owner:NASCENT PHARMA LLC
Safe botanical drug for treating malignant pleural effusion and cancer and increasing immune function
InactiveUS20030152647A1Specific activityInhibit oncogeneBiocideUnknown materialsAbnormal tissue growthAnticarcinogen
Three new safe pharmaceutical compositions in accordance with the present invention are for treatment and prevention of malignant pleural effusion and increase immune function comprise Lan Xiang Xi (LX) and polysaccharide of Dang Gui. Methods of treating and preventing cancer cells include inhibiting oncogenes, increasing activity of tumor suppressor, inducing differentiation of cancer cells, inhibiting cancer cells proliferation, inducing apoptosis of cancer cells, inhibiting growth of transplanted cancer and inhibiting cancer incidence, etc. In general, anticancer drug always decreases immune function. However, LX and LX+PDG can inhibit cancer also increase immune function at the same time. Also, LX and DG containing soybean-liposomes is safer than LX and DG.
Owner:LIU YAGUANG
Preparation of clathrate compound containing isoflavone and cyclodextrin of chickpea
InactiveCN101757640ALow toxicityImprove bioavailabilityOrganic active ingredientsPharmaceutical non-active ingredientsSolubilityWater baths
The invention relates to preparation of clathrate compound containing isoflavone and cyclodextrin of chickpea, comprising the following steps: grinding the chickpea into powder, drying, adding petroleum ether, backflowing for 1.5 hours in water bath with the temperature of 70 DEG C, filtering and drying; adding 65% alcohol, putting the obtained mixture in water bath with the temperature of 85 DEG C, backflowing for 1.5 hours, carrying out backflowing for two times, and filtering; adding distilled water, pouring the distilled water into a macroporous absorbing column, adjusting the flow rate to be 1ml / min, using a beaker to receive samples passing the column; adding 70% alcohol, adjusting the flow rate to be 0.5ml / min, collecting flow liquid, and distilling to obtain pure flavone; charging cyclodextrin and pure isoflavone with the mass ratio of 4-6:1, adding 2-4 times of water under normal temperature, grinding the mixture to be pasty, vacuum-drying, cleaning with alcohol, and drying to obtain the clathrate compound. The preparation has simple method, low cost, little toxicity of selected microcapsule wall materials, can increase the solubility of botanical drug after clathrate, improve the bioavailability of isoflavone and has good application prospect.
Owner:DONGHUA UNIV
Food product & process for manufacturing same
InactiveUS20080050479A1Preserve the natural botanical functional additive's activityEasily broken into pieceReady-for-oven doughsMechanical apparatusManufacturing technologyFood flavor
There is provided a commercially packaged mammal pet food product that includes a manufactured, shelf-life stable food substrate and a combination of functional additives. The functional additives include at least one non-palatable plant-based remedy and / or dietary fiber source that are present to strengthen and / or maintain a specified health indicator of a mammal pet animal. The food product is portioned and packaged with the functional additives being present in predetermined concentrations and amounts sufficient to be effective in achieving said indications on regular feeding of the pet animal with said food product. The food substrate is present in a proportion sufficient to mask the flavor and / or odor of the non-palatable additive and is made-up of a unique combination of materials that are able to be processed at lower temperatures to preserve the natural botanical functional additive's activity.
Owner:HODGE JASON +5
Fast-acting plant-based medicinal compounds and nutritional supplements
ActiveUS20190117778A1Increase usefulnessImprove practicalitySenses disorderNervous disorderPlant basedPenetration enhancer
Plant-based medicinal compounds or nutritional supplements in various carrier combinations are described. The carriers can include N-acylated fatty amino acids, penetration enhancers, and / or various other beneficial carriers. The plant-based composition / carrier combinations can create administration benefits.
Owner:RECEPTOR HLDG INC
Safe medicine for treating and preventing cancer and method of use
InactiveUS20070166415A1Growth inhibitionEnhanced inhibitory effectBiocideAnimal repellantsCancer preventionCancer cell
The present invention provides a new method for extracting Homoharringtonine (HHT) by culture cells or culture plant tissues. Also, disclosed are methods of obtaining HHT from semi-synthesis and biosynthesis. The present invention disclosed that HHT combined with some botanical drugs could induce cancer cells to resemble normal cells. To add some botanical drugs combined with HHT can significantly increase anticancer effects of HHT. These drugs include Matrine (MAT), Guanzhongsu (GU), Maidongsu (MU), and Indirubin (IND). The experimental data showed that above drugs have strong synergisms effects for treating leukemia and other cancer cells and more safe.
Owner:LIU YAGUANG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com