Patents
Literature
38 results about "Angelman syndrome" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A genetic disorder that causes developmental and neurological disabilities.
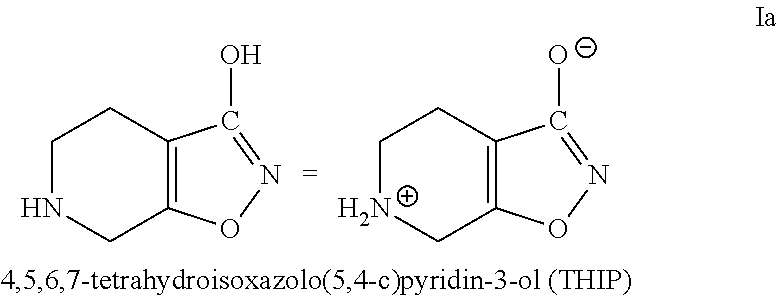
Methods of increasing tonic inhibition and treating secondary insomnia
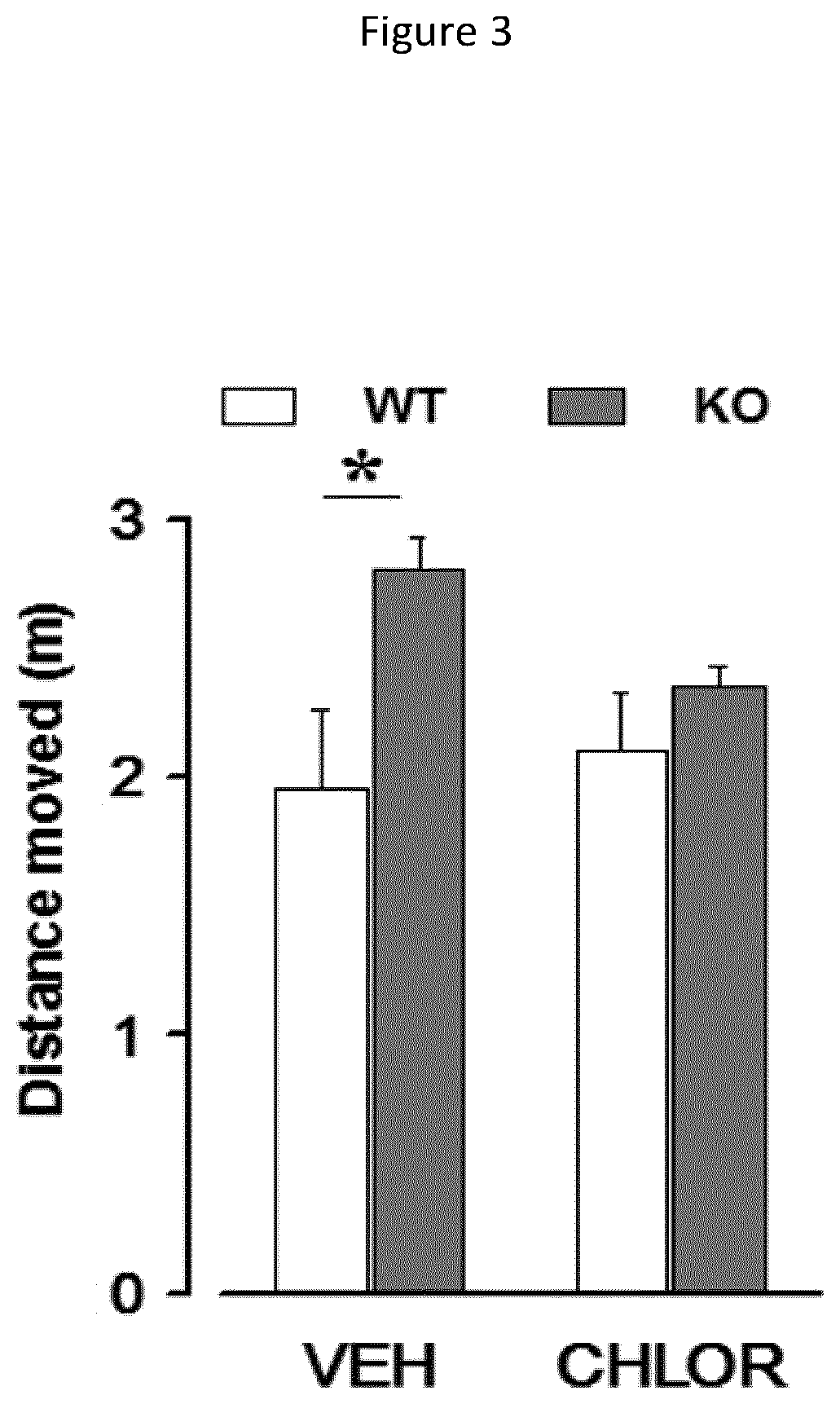
InactiveUS20150352085A1Increasing tonic inhibitionNormalize sleep architectureBiocideNervous disorderSleep architectureDisease
Methods of increasing tonic inhibition in a subject in need thereof, for example a subject with Fragile X syndrome or Angelman syndrome are disclosed. Methods of treating secondary insomnia in a subject with a neurodegenerative disease or disorder are also disclosed. The methods can include administering the subject an effective amount of 4,5,6,7-tetrahydroisoxazolo(5,4-c)pyridin-3-ol (THIP) or a derivative thereof, or a pharmaceutically acceptable salt thereof, increase tonic inhibition in neurons of the subject; to increase slow wave sleep (SWS) and / or slow wave activity (SWA), normalize sleep architecture, reduce secondary insomnia, increase non-rapid eye movement (NREM) sleep, increase sleep continuity, enhance delta activity within NREM, increase or improve total sleep time (TST), increase or improve sleep efficiency, reduce total time awake (TAA), reduce number of awakenings (NWA), reduce latency to persistent sleep (LPS), or to reduce wake after sleep onset (WASO), in the subject, or any combination thereof.
Owner:OVID THERAPEUTICS
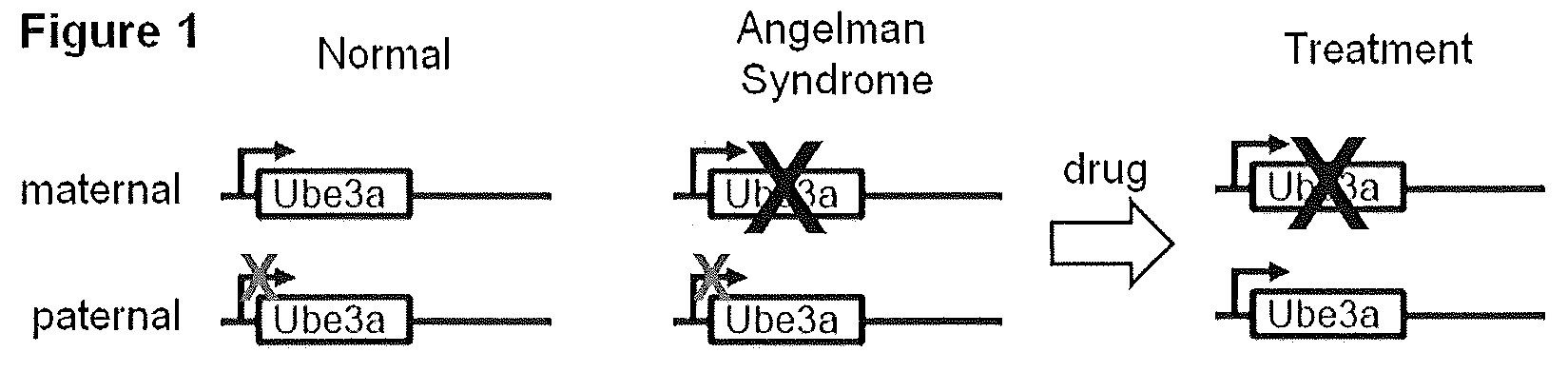
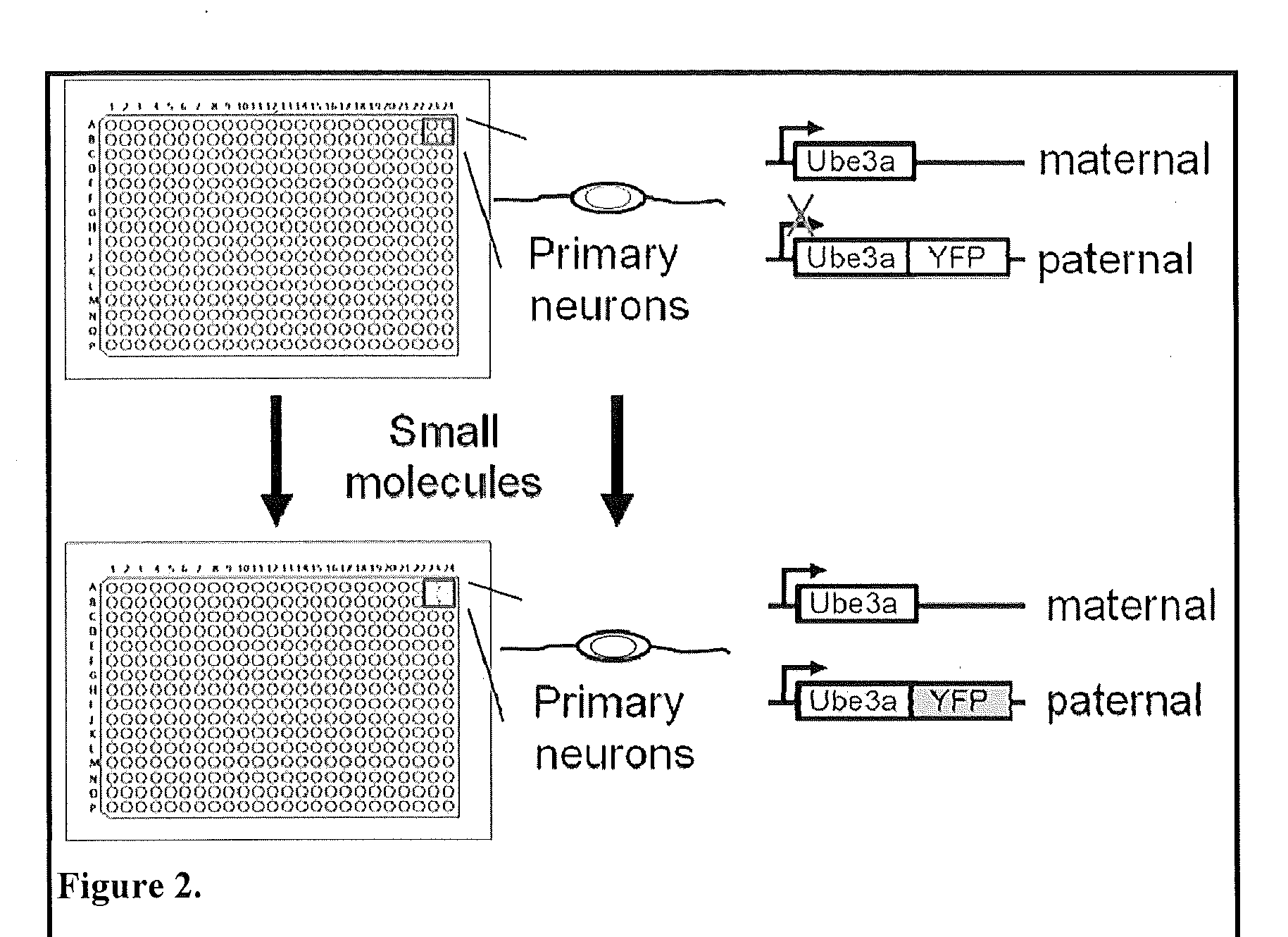
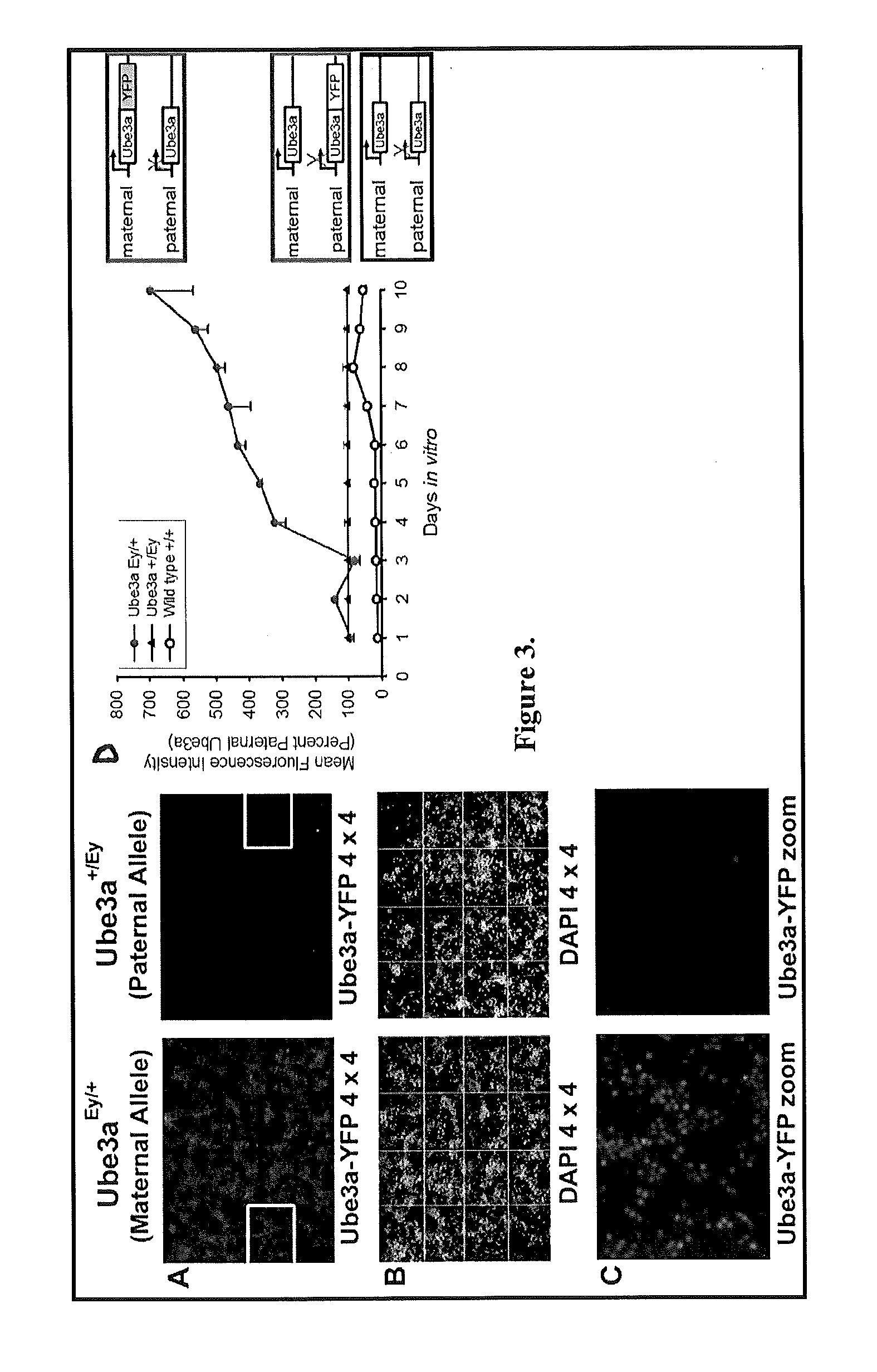
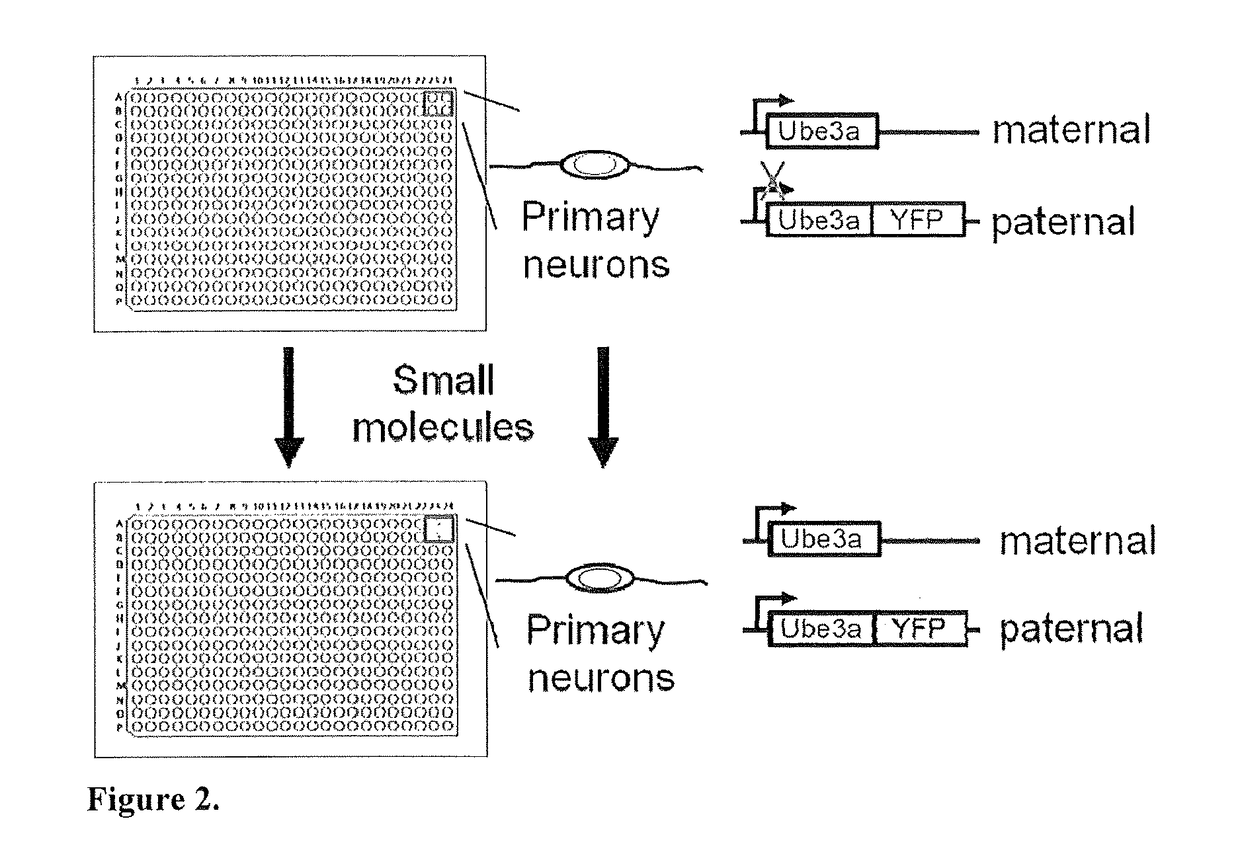
Methods and Compositions for Unsilencing Imprinted Genes
ActiveUS20130317018A1Induce expressionBiocideCarbohydrate active ingredientsTopoisomerase inhibitorGenome
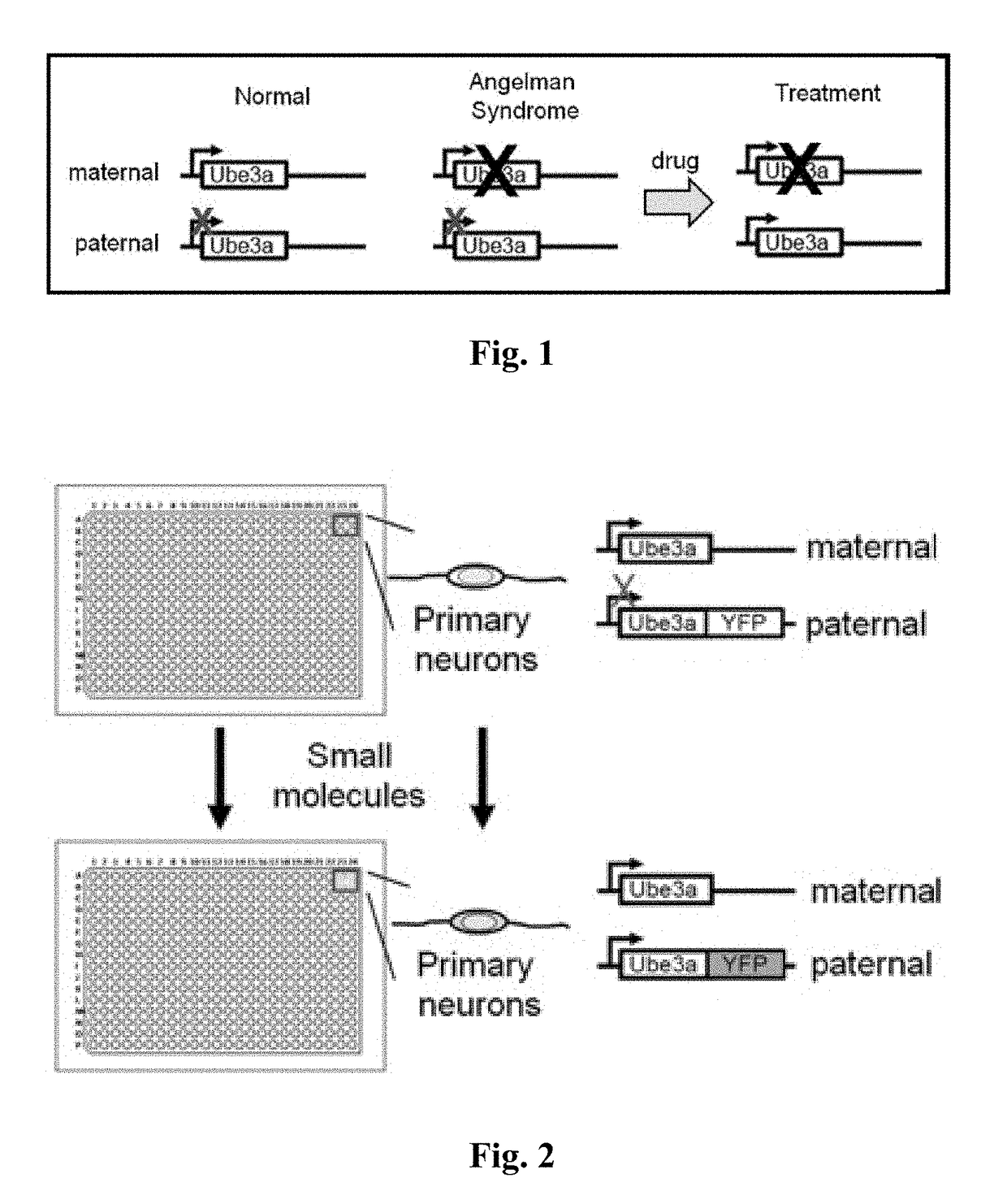
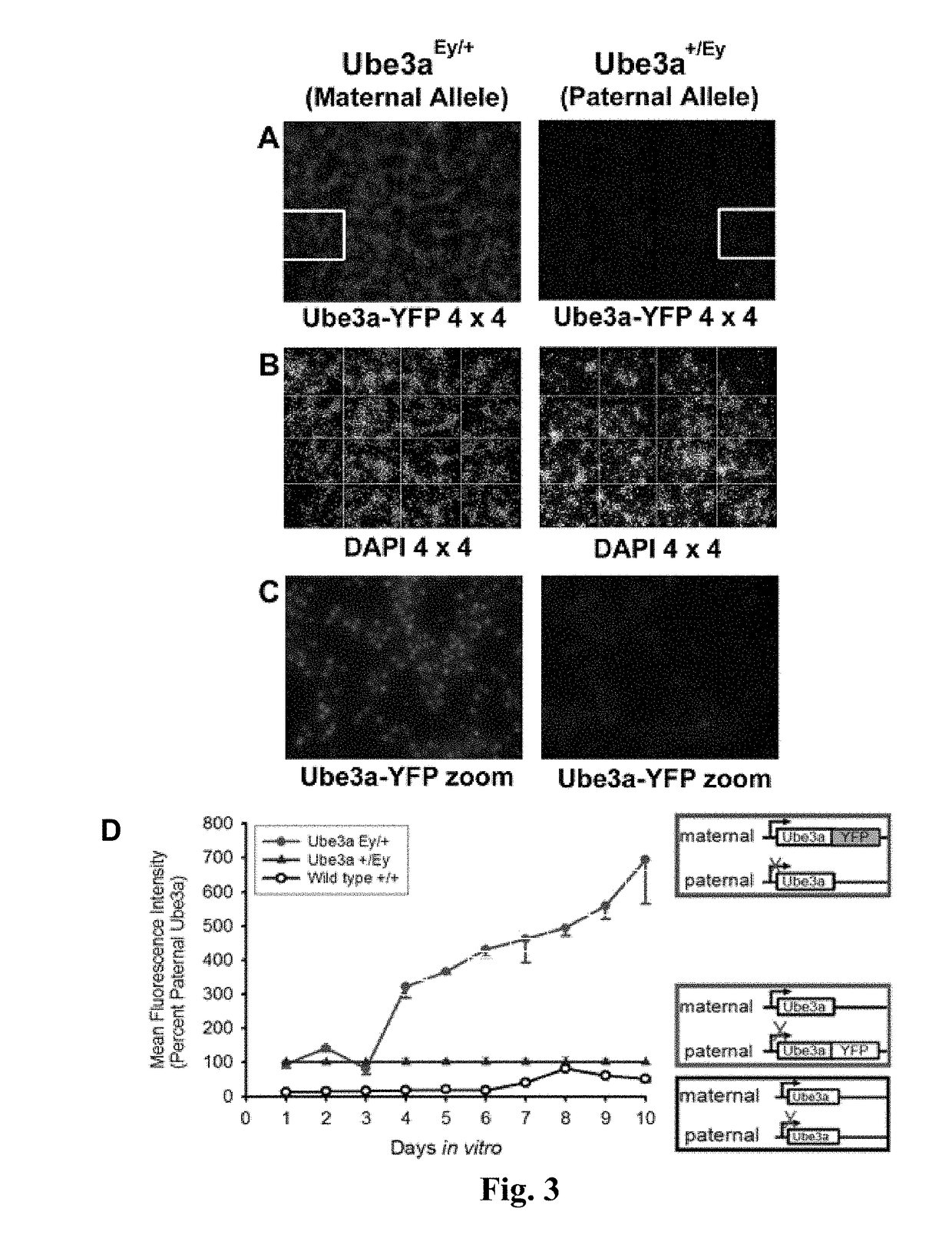
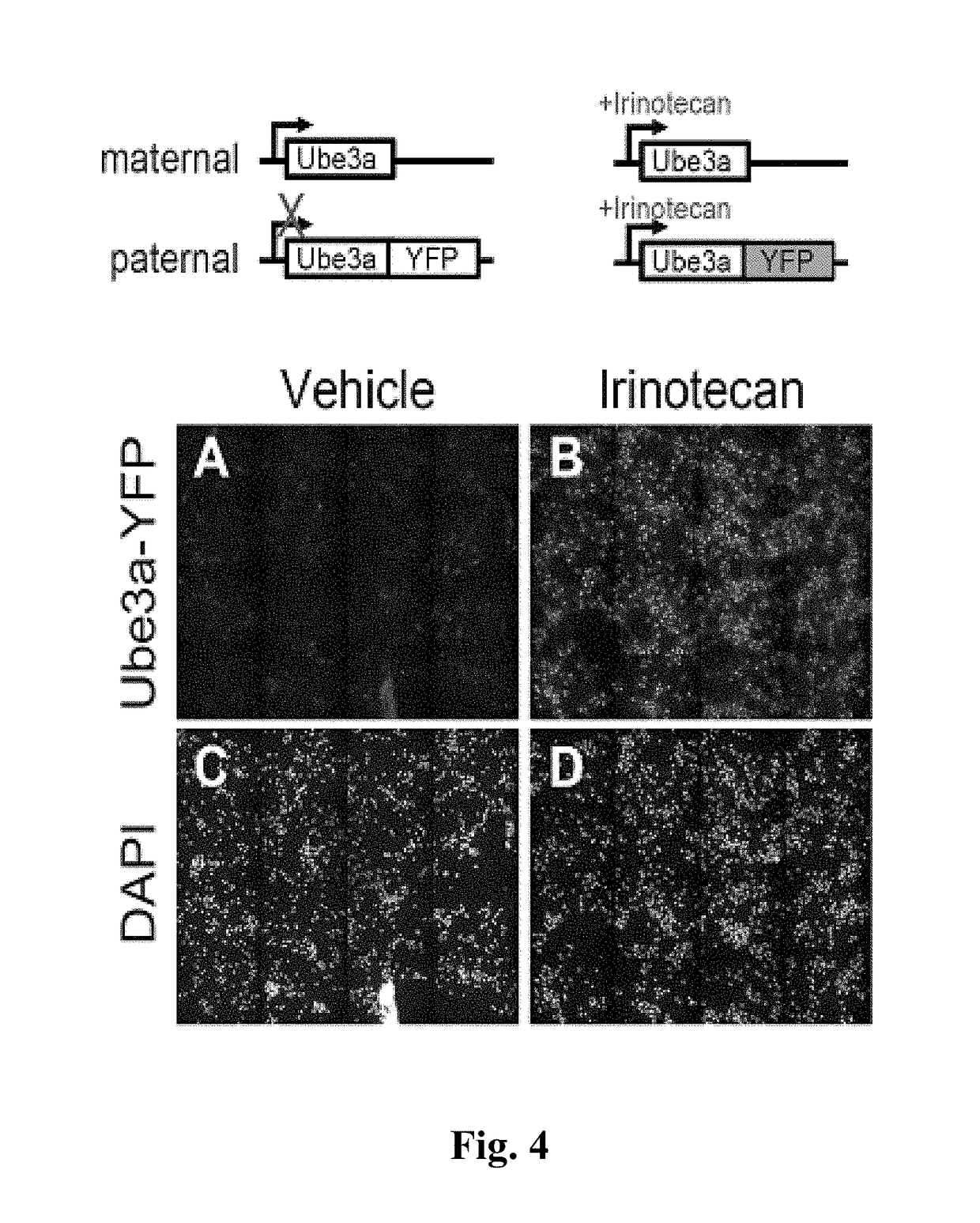
The present invention provides methods and compositions for inducing expression of Ube3a in a cell by contacting the cell with a topoisomerase inhibitor. Particular embodiments include a method of treating a genomic imprinting disorder, such as Angelman syndrome, in a subject by administering to the subject an effective amount of a topoisomerase inhibitor.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
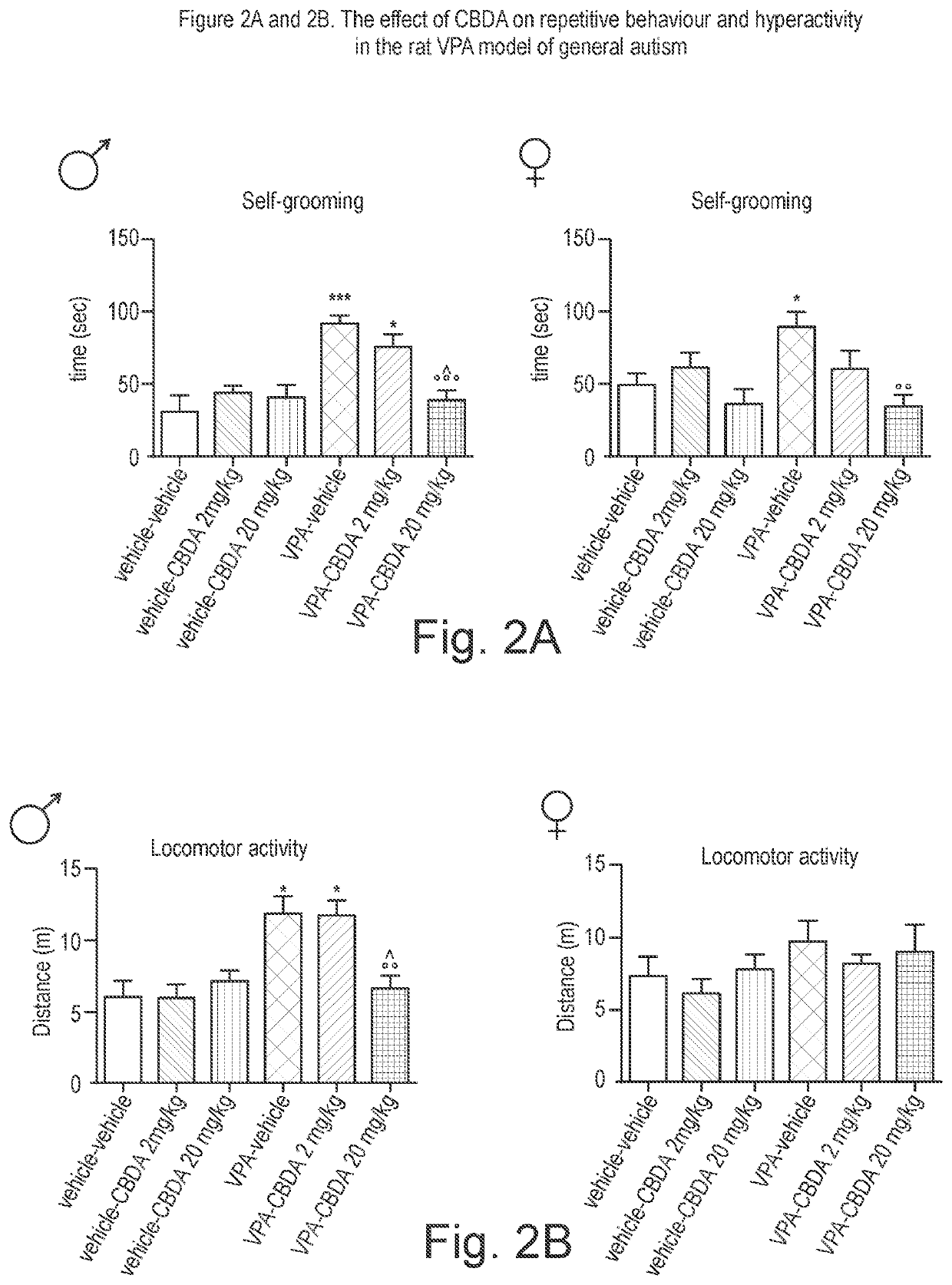
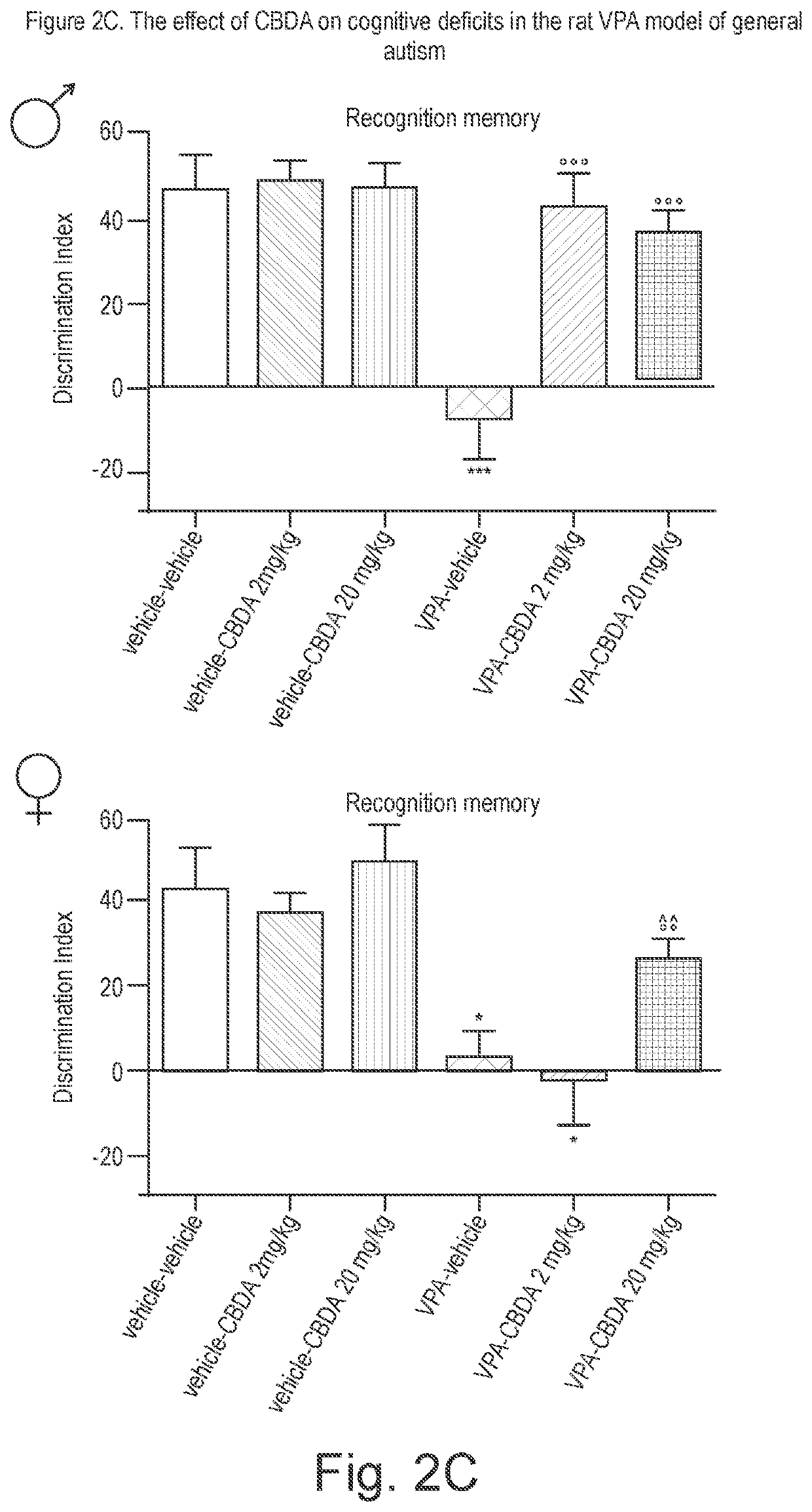
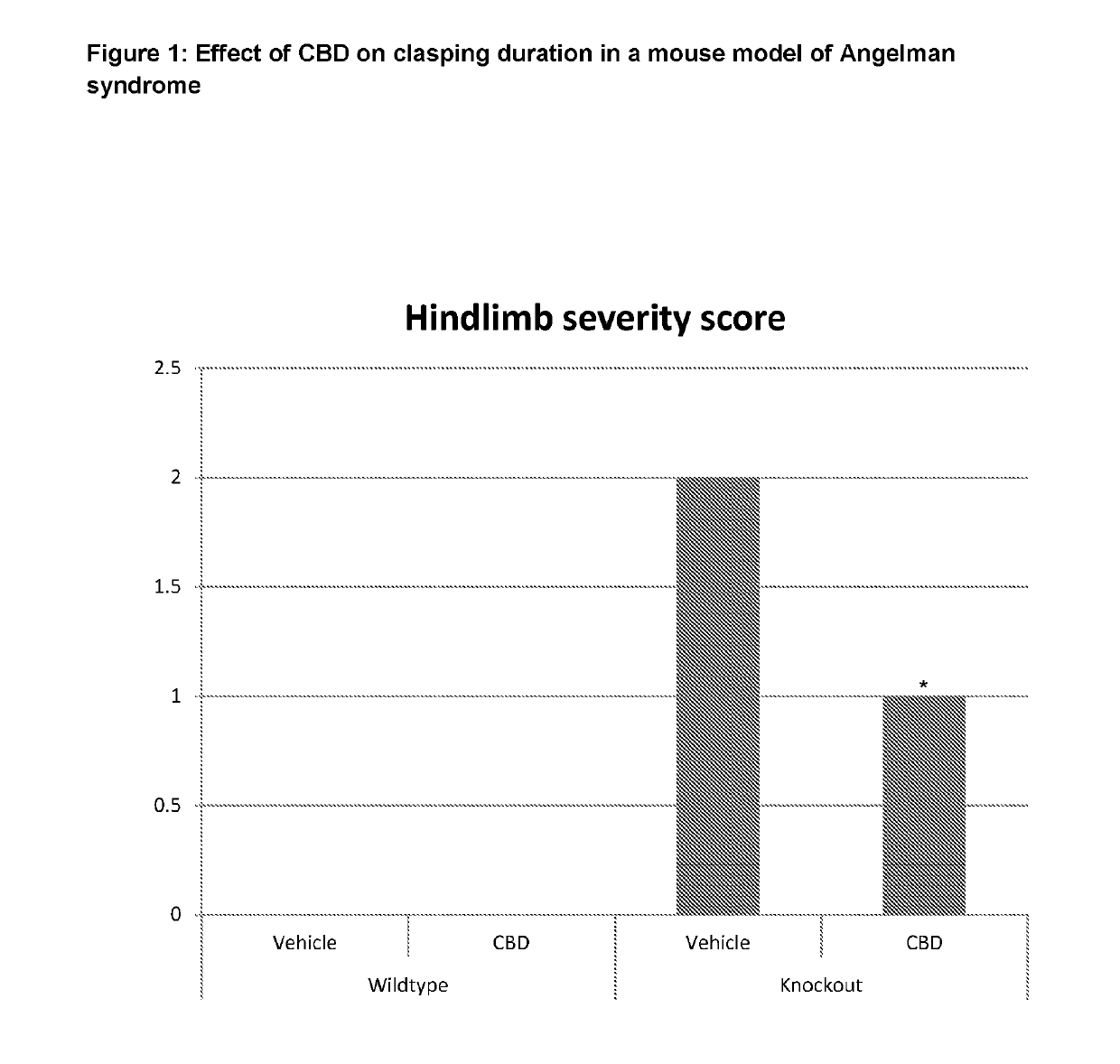
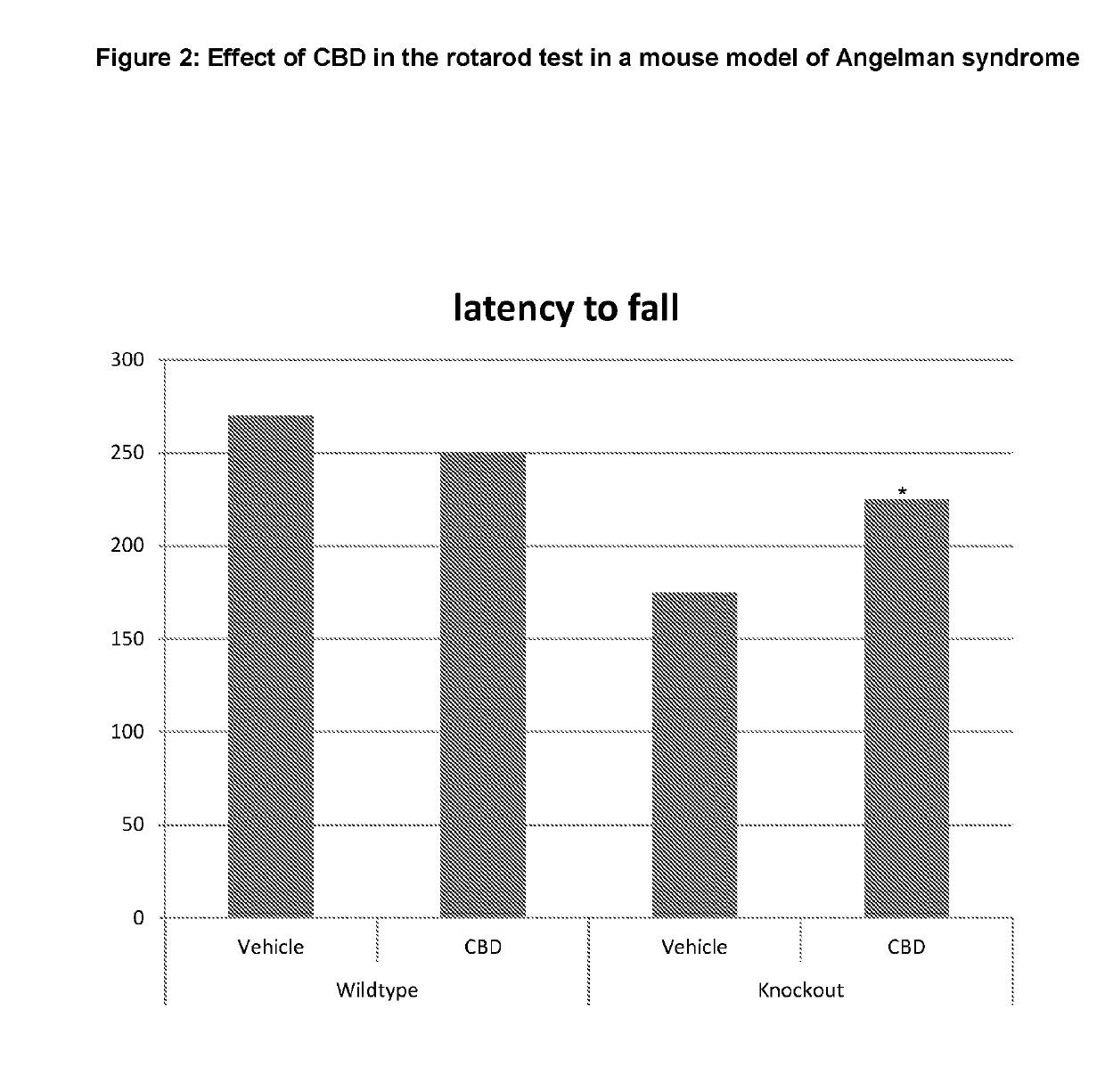
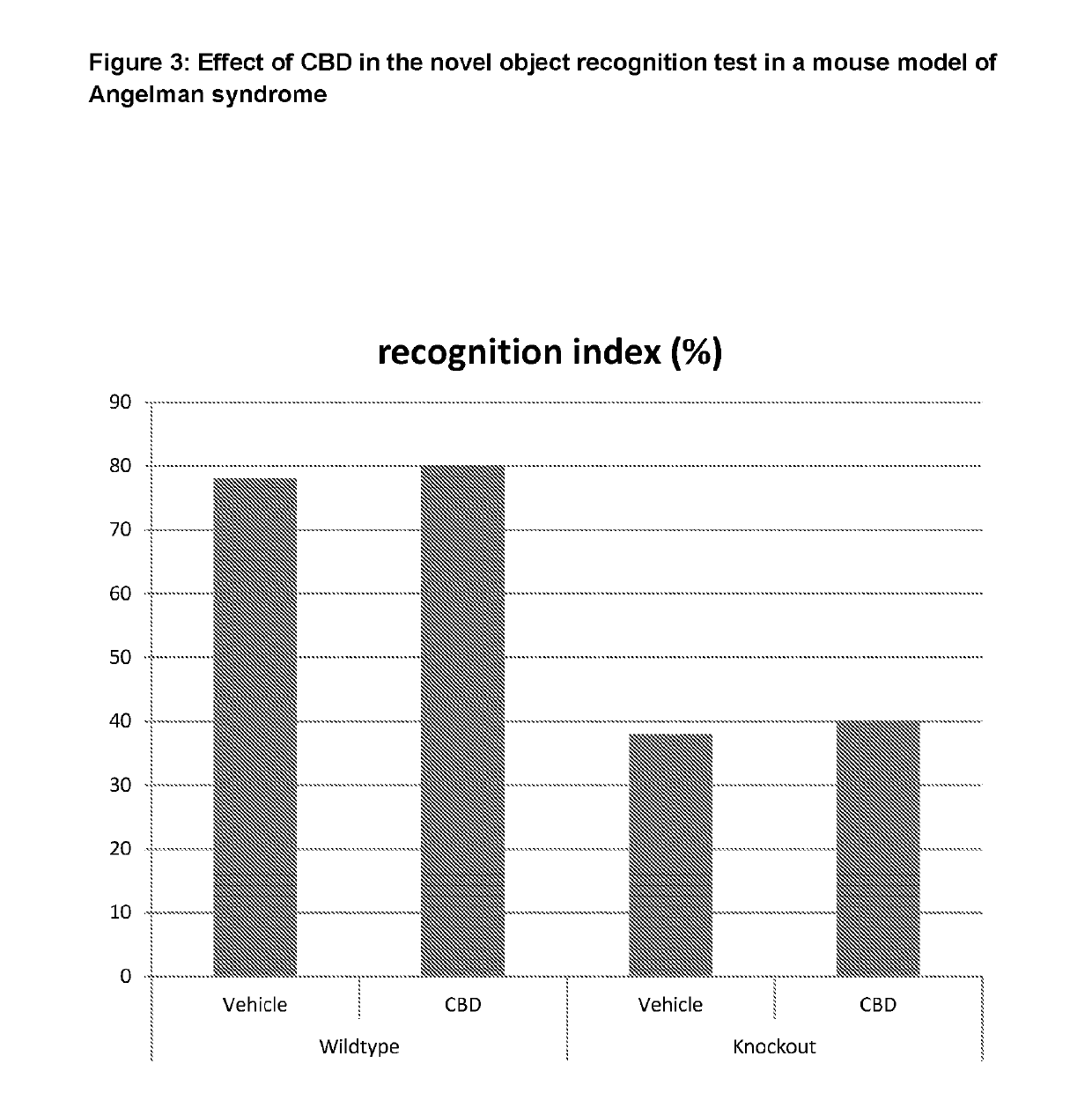
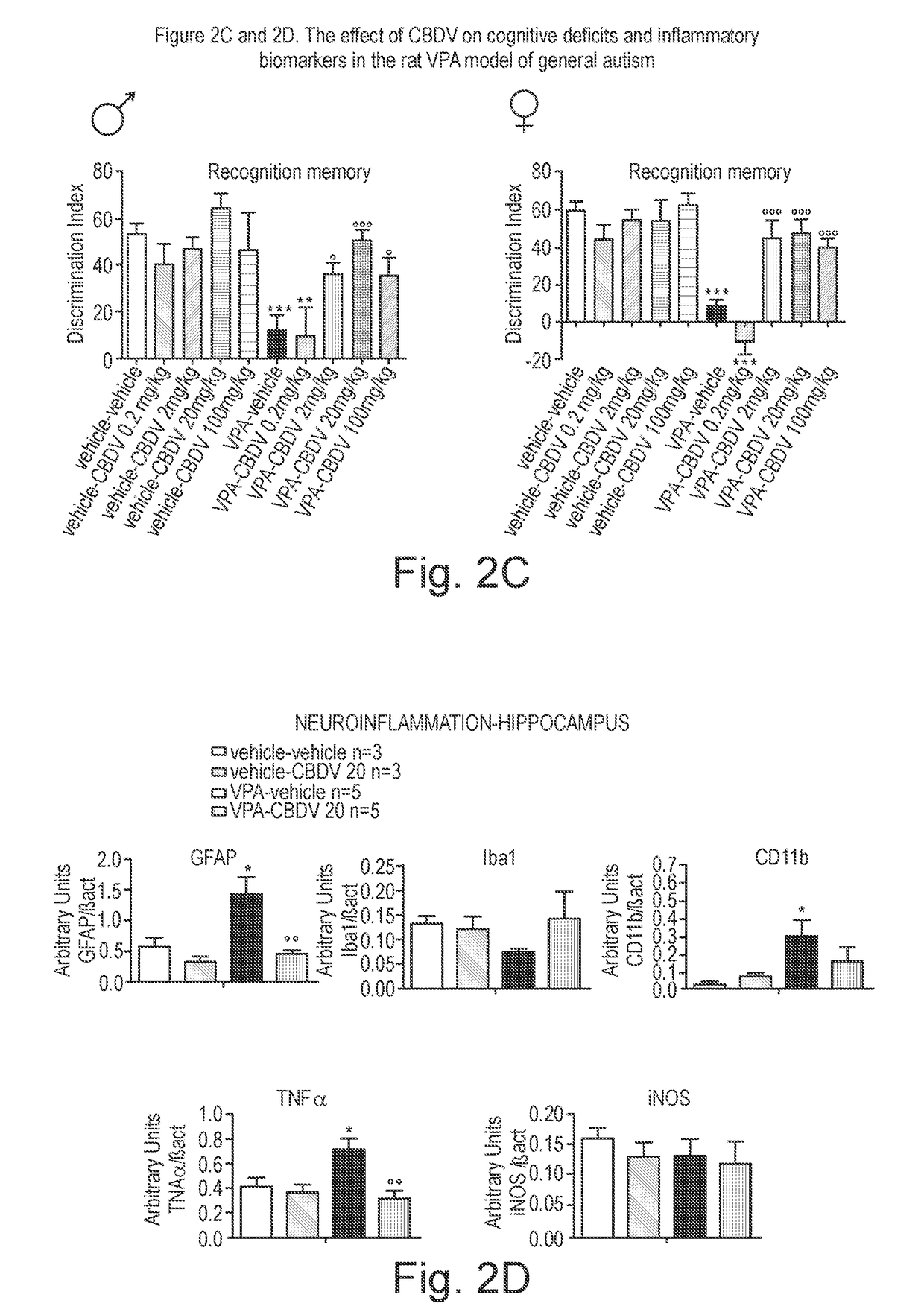
Use of cannabinoids in the treatment of angelman syndrome
ActiveUS20190321307A1Symptoms improvedNervous disorderHydroxy compound active ingredientsRodent modelAnxiety
The present invention relates to the use of cannabidiol (CBD) in the treatment of Angelman syndrome (AS). CBD has It been shown to be particularly effective in improving anxiety in rodent models of AS. The CBD is preferably substantially pure. It may take the form of a highly purified extract of Cannabis such that the CBD is present at greater than 98% of the total extract (w / w) and the other components of the extract are characterised. Alternatively, the CBD is synthetically produced. Alternatively, the CBD may be used as a botanical drug substance (BDS) from a Cannabis plant in which CBD is the predominant cannabinoid. The CBD may also be present in combination with other cannabinoids and non-cannabinoid components such as terpenes. In use the CBD may be used concomitantly with one or more other medicaments. Alternatively, the CBD may be formulated for administration separately, sequentially or simultaneously with one or more medicaments or the combination may be provided in a single dosage form. Where the CBD is formulated for administration separately, sequentially or simultaneously it may be provided as a kit or together with instructions to administer the one or more components in the manner indicated. It may also be used as the sole medication, i.e. as a monotherapy.
Owner:GW RES LTD
Methods of increasing tonic inhibition and treating secondary insomnia
Owner:OVID THERAPEUTICS
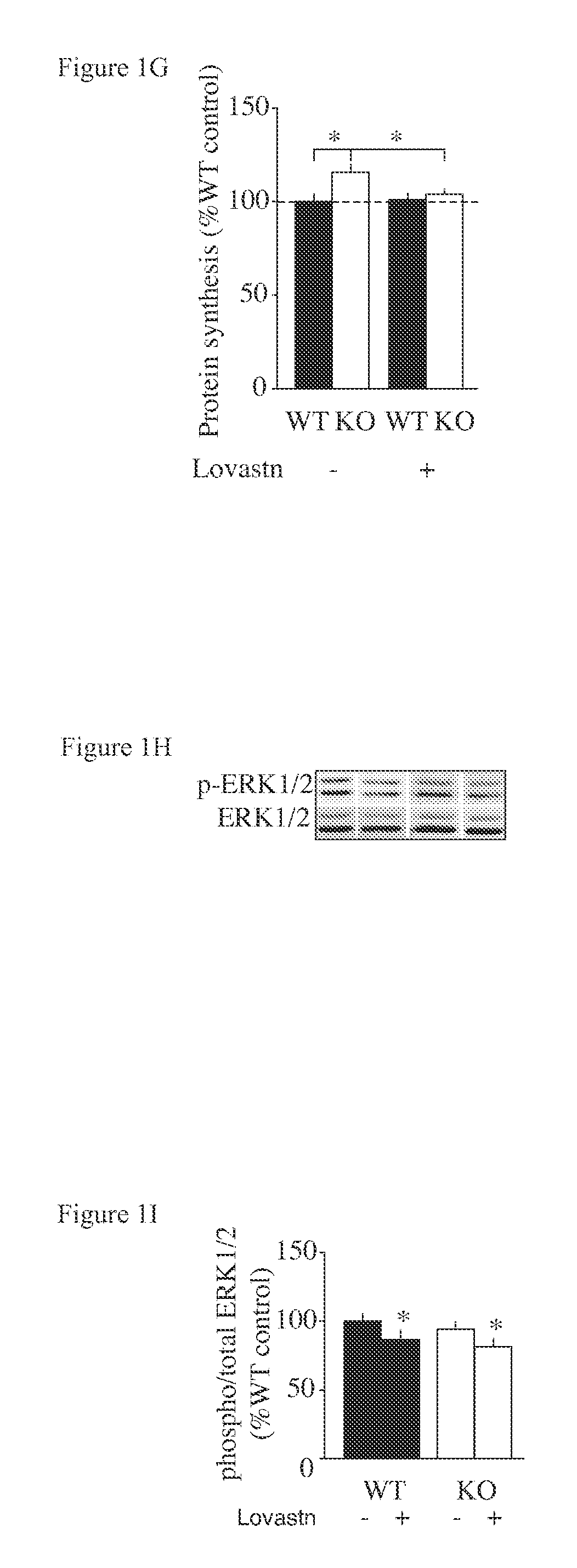
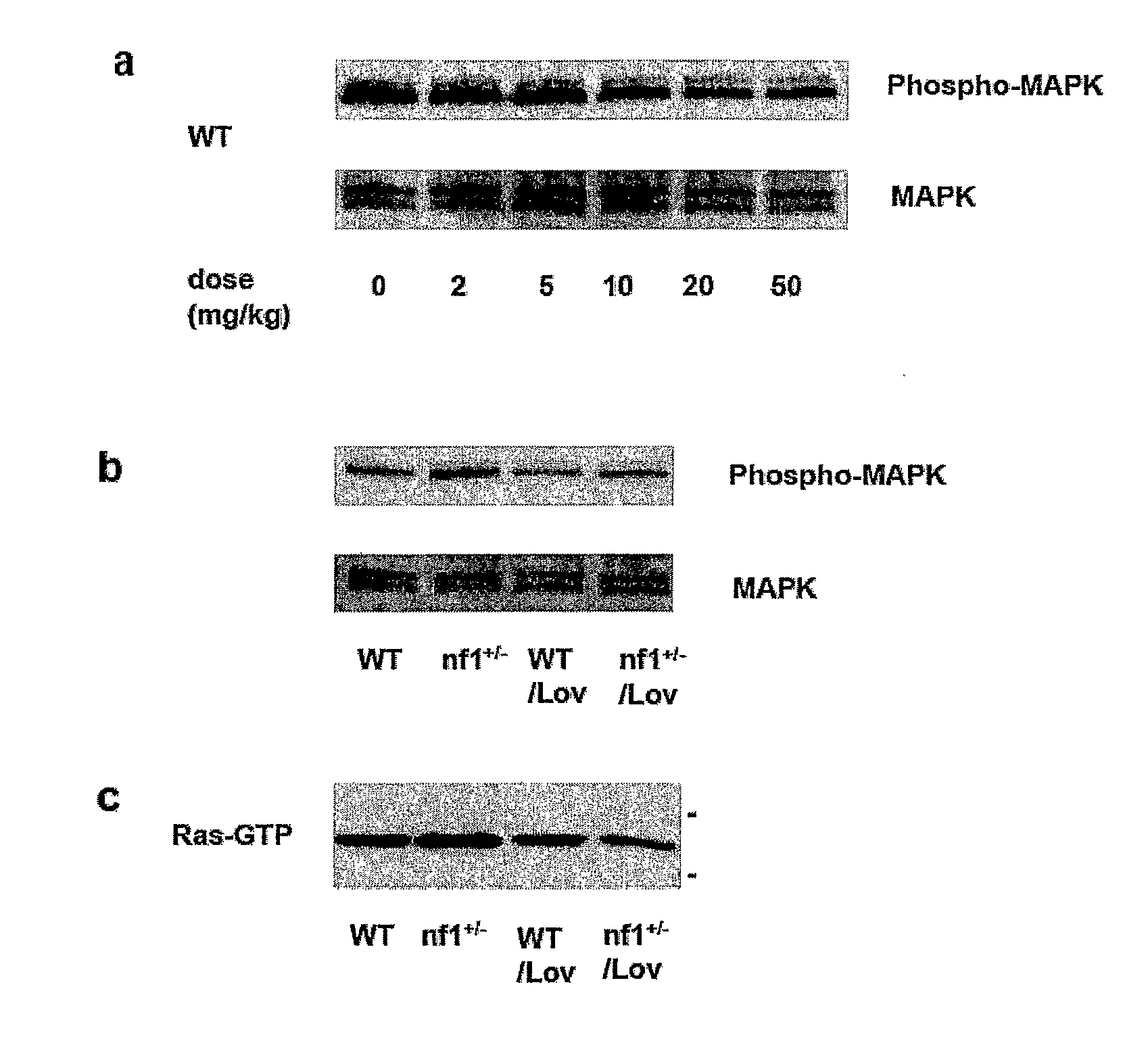
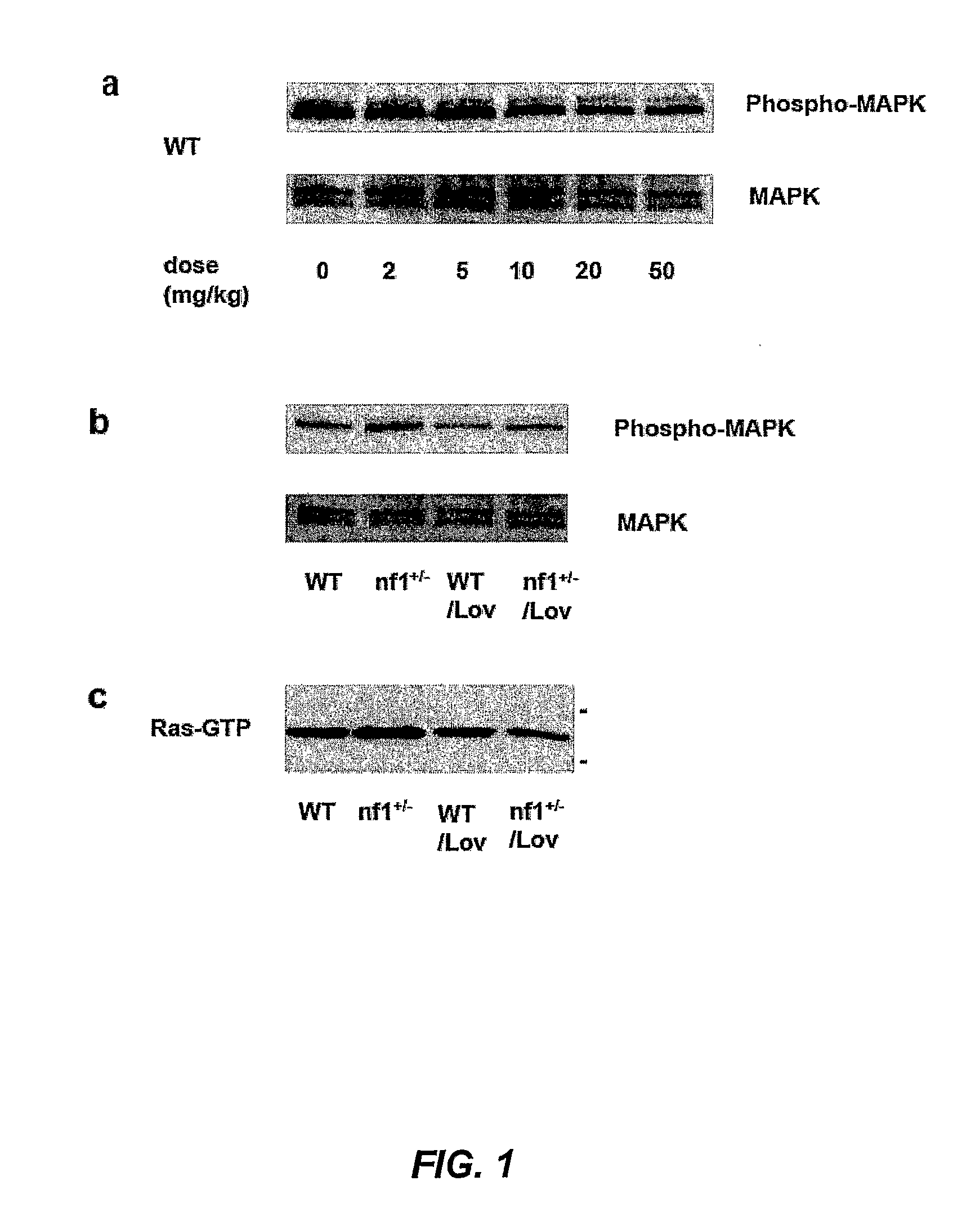
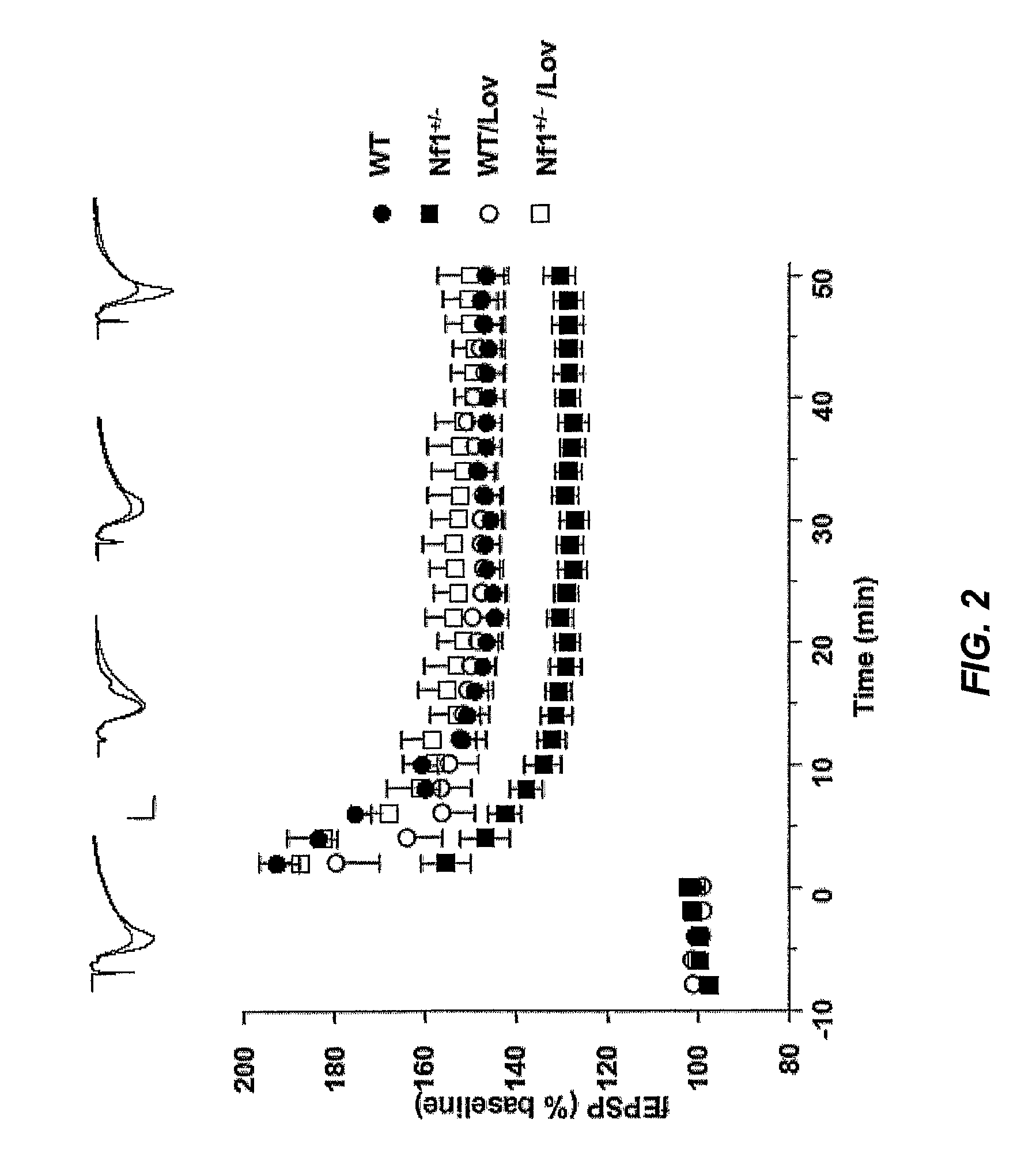
Treating Learning Deficits With Inhibitors of Hmg CoA Reductase
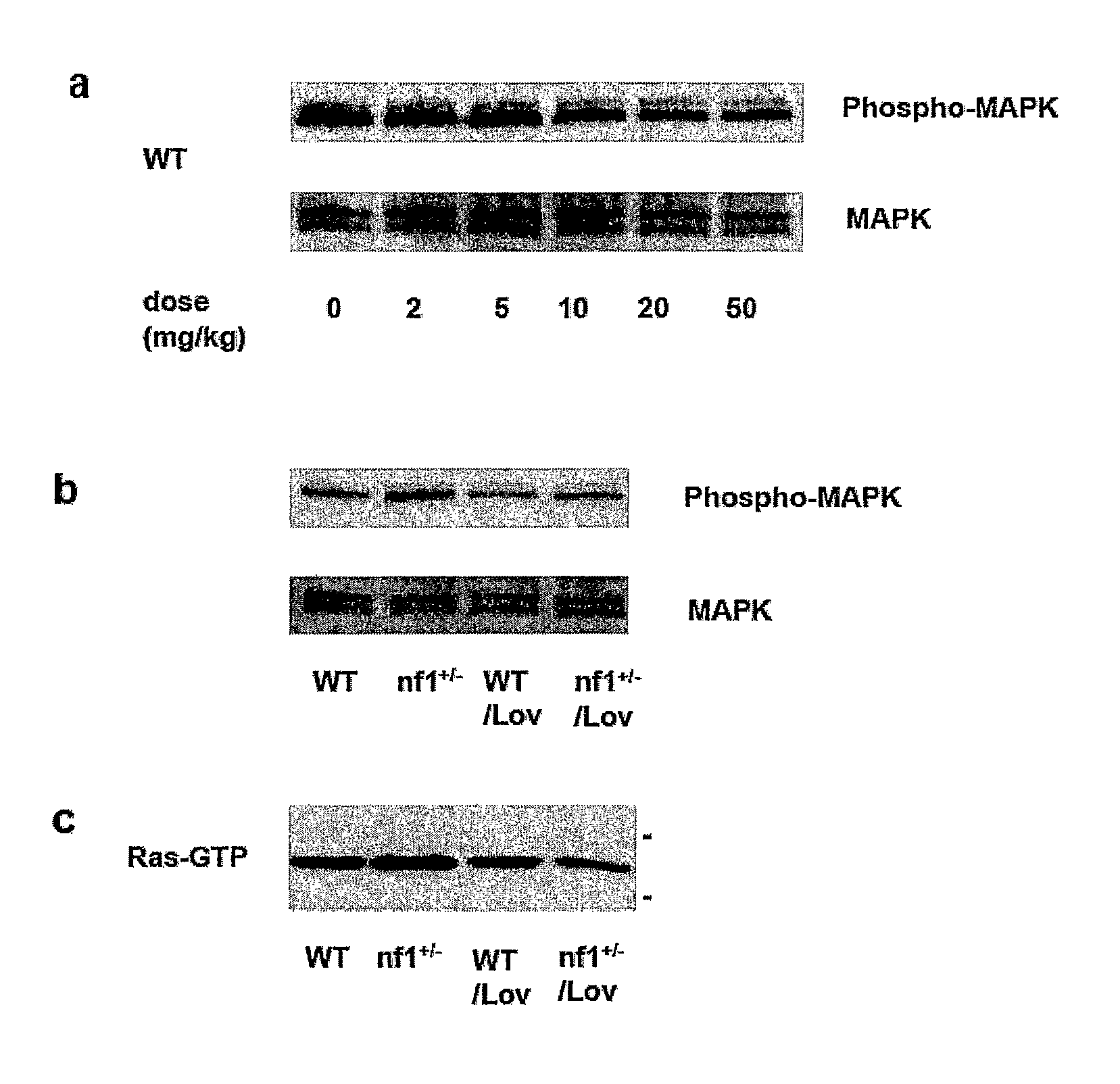
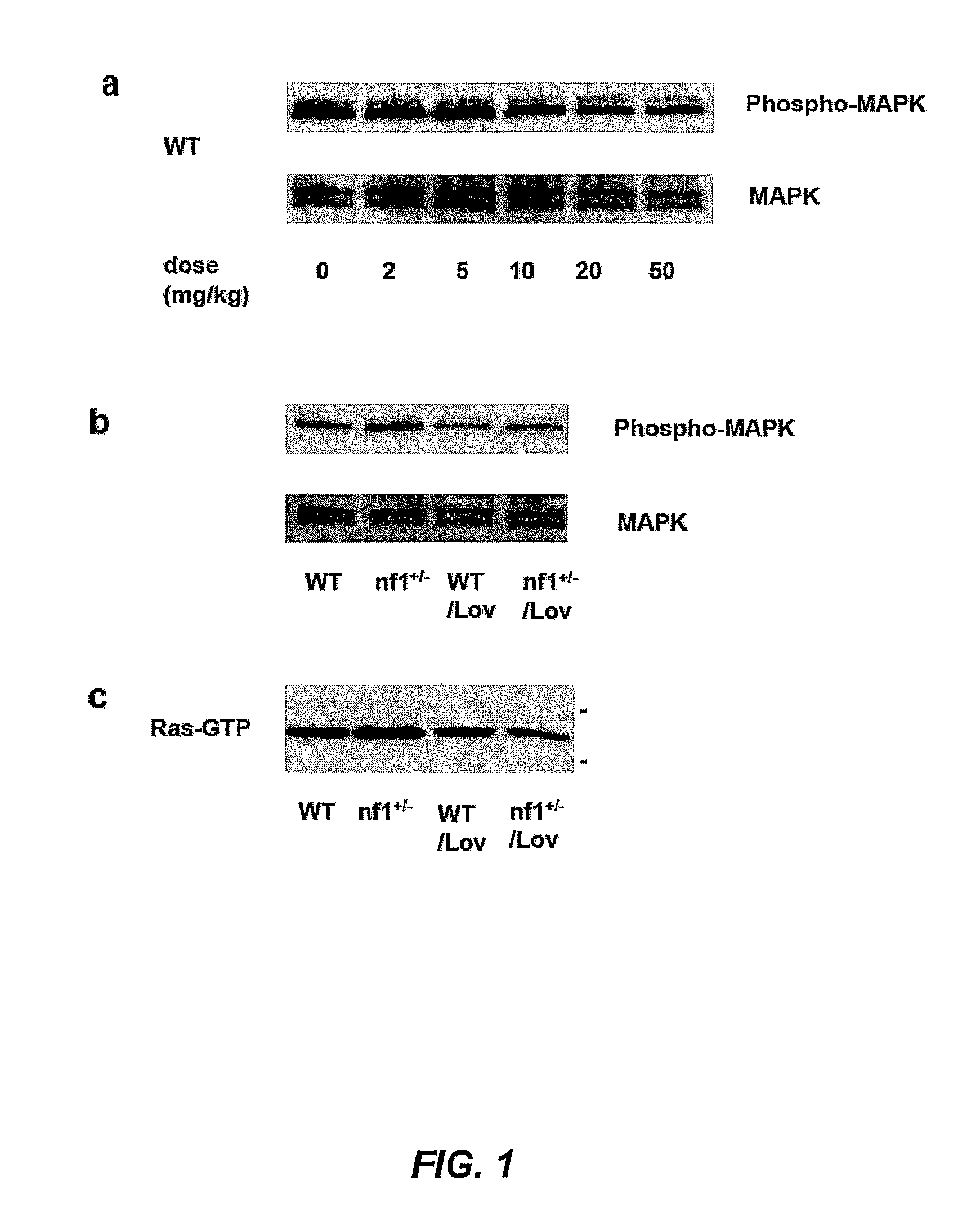
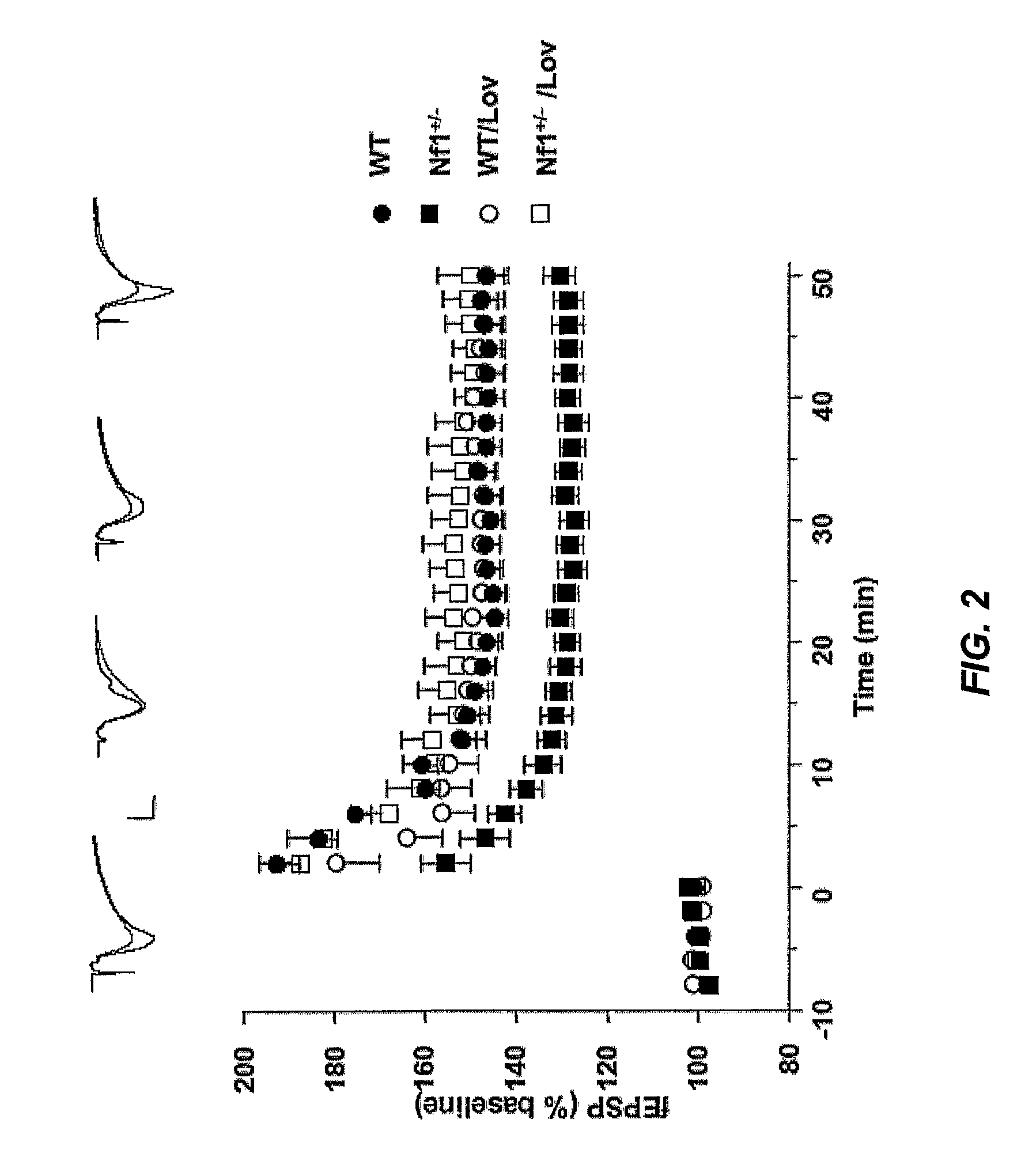
The disclosure provides methods of treating cognitive disorders by administering a HMG CoA reductase inhibitor. Cognitive deficits treatable with the inhibitor compound include those associated with Angelman Syndrome, Neurofibromatosis-1, certain forms of X-linked mental retardation, tuberous sclerosis, Down Syndrome, autism, and attention deficit / hyperactivity disorder.
Owner:RGT UNIV OF CALIFORNIA
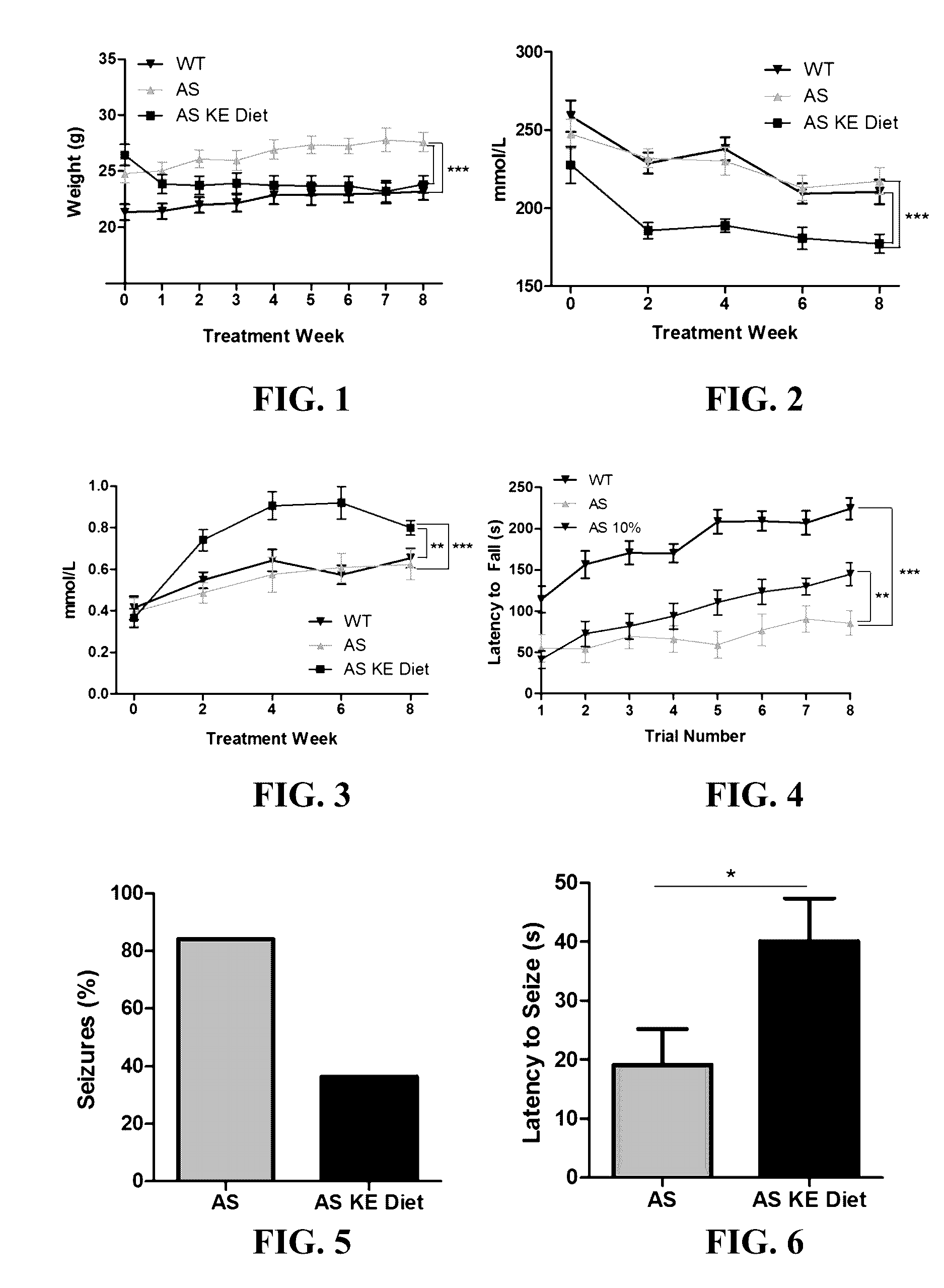
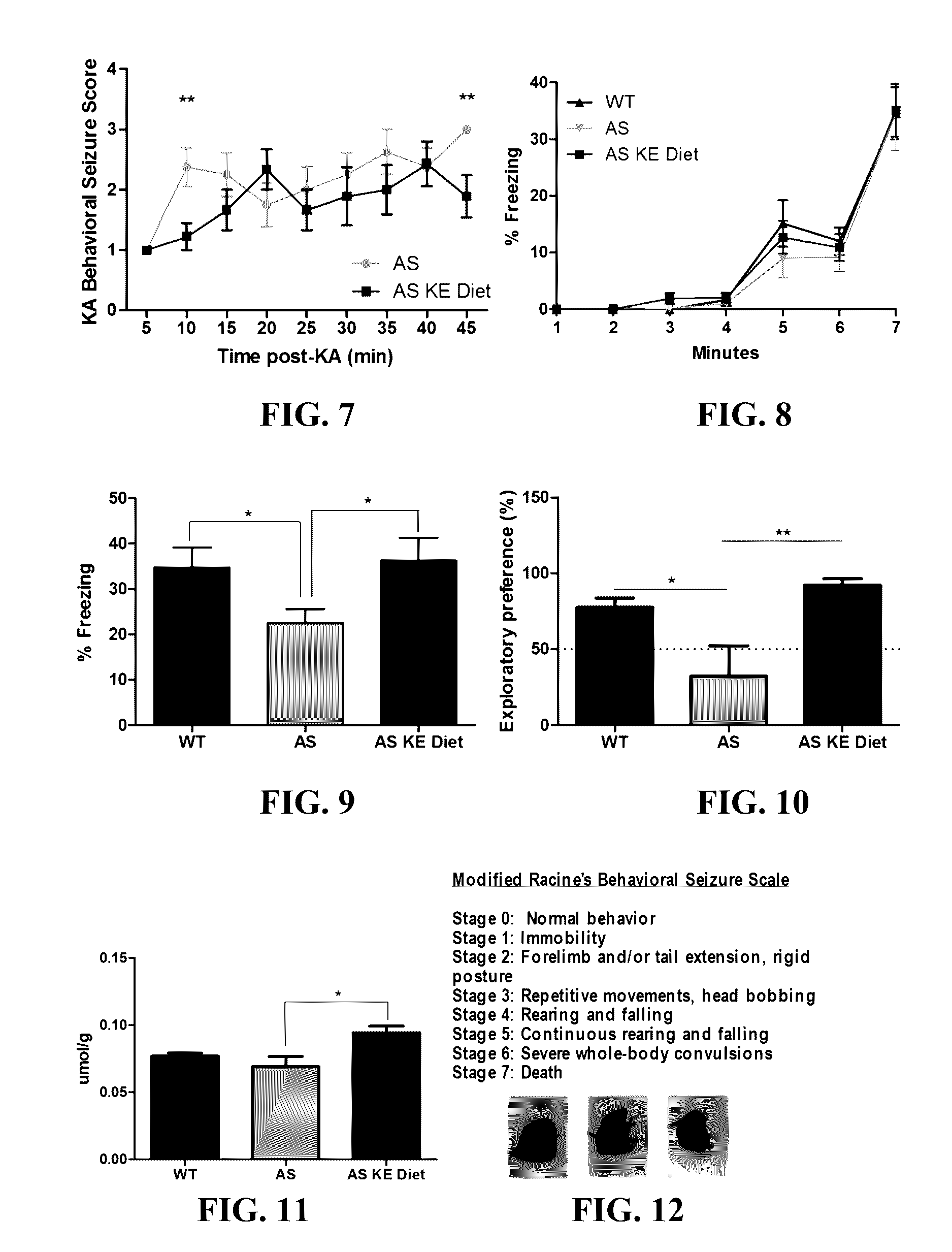
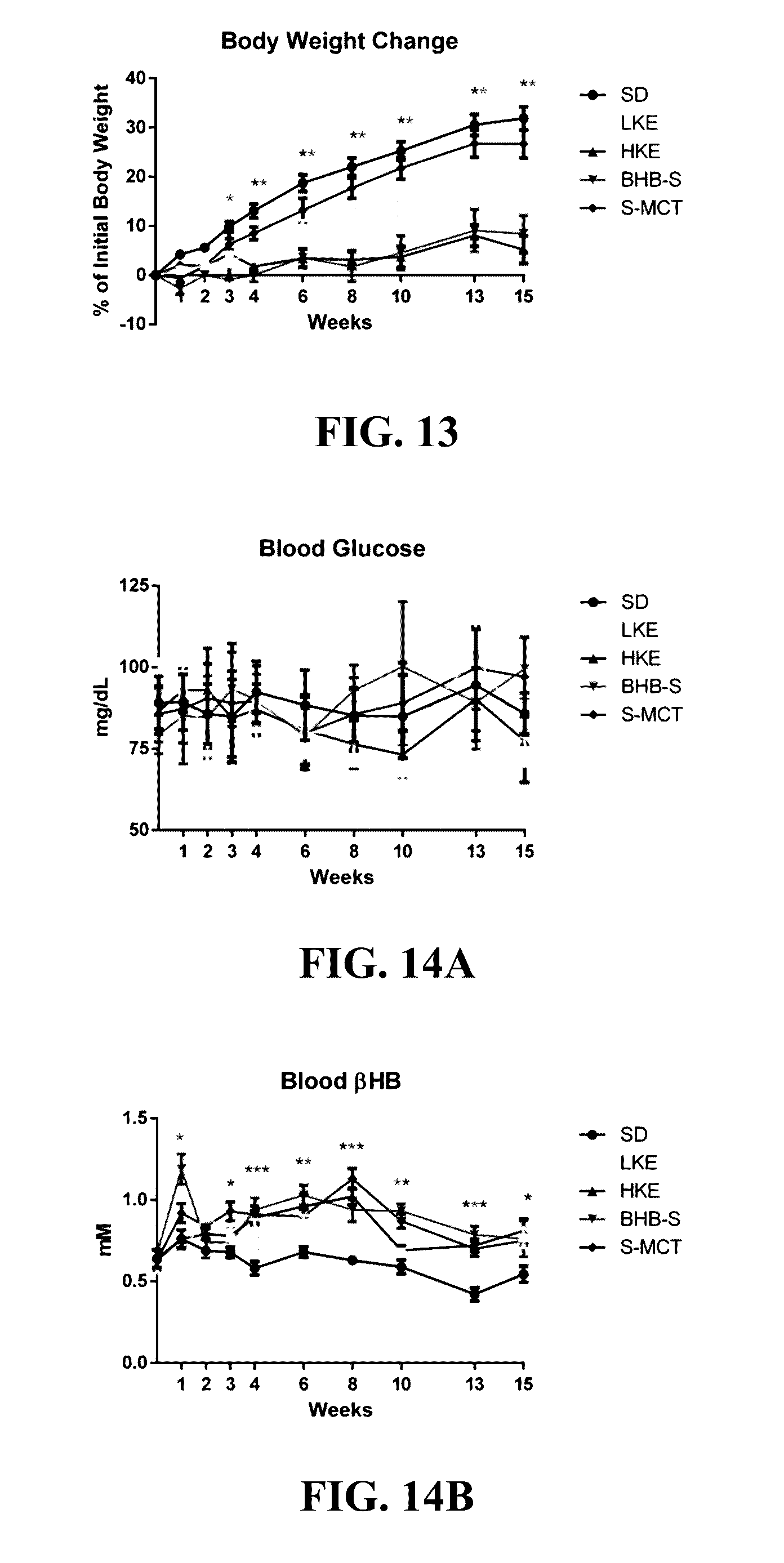
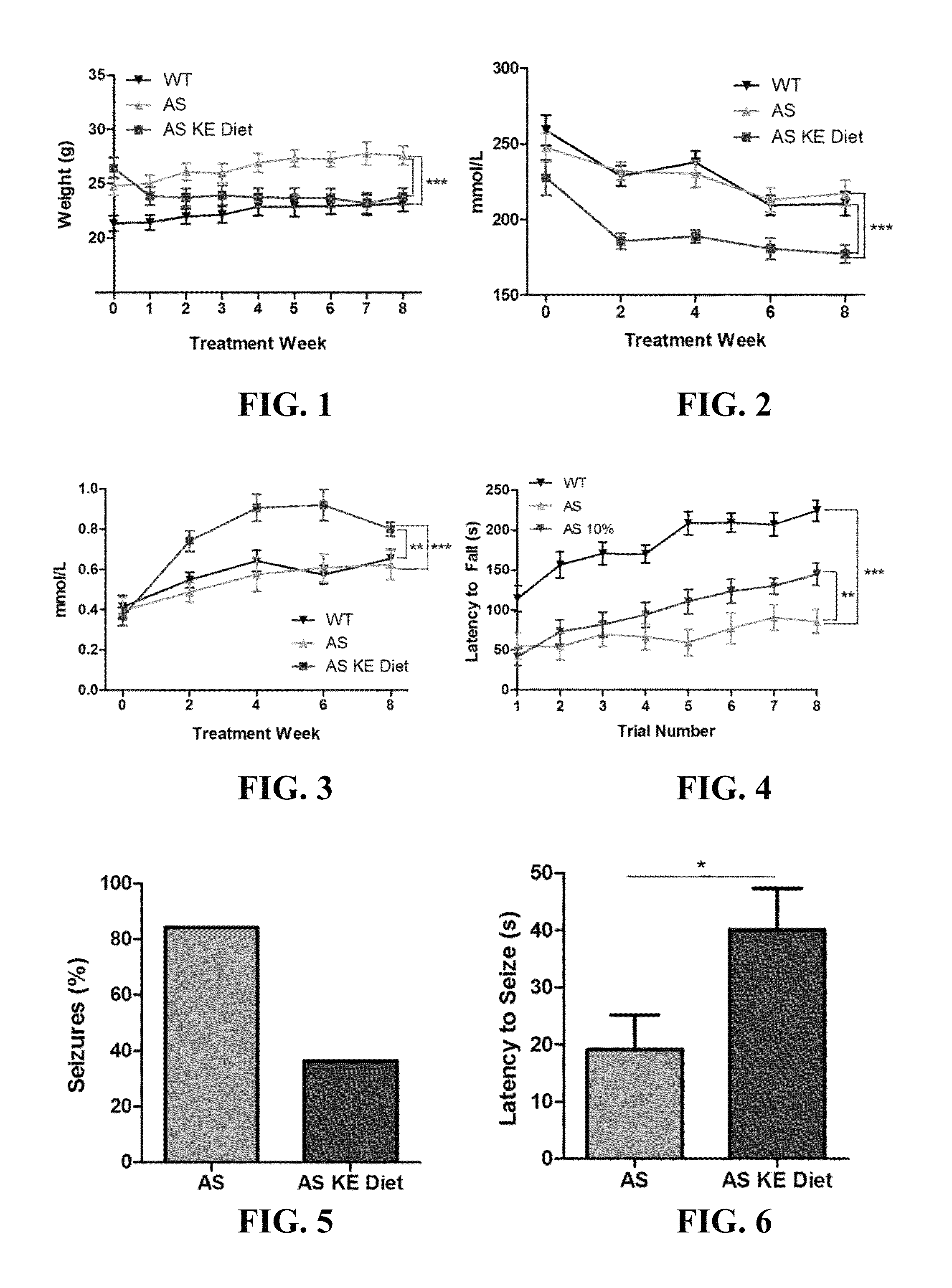
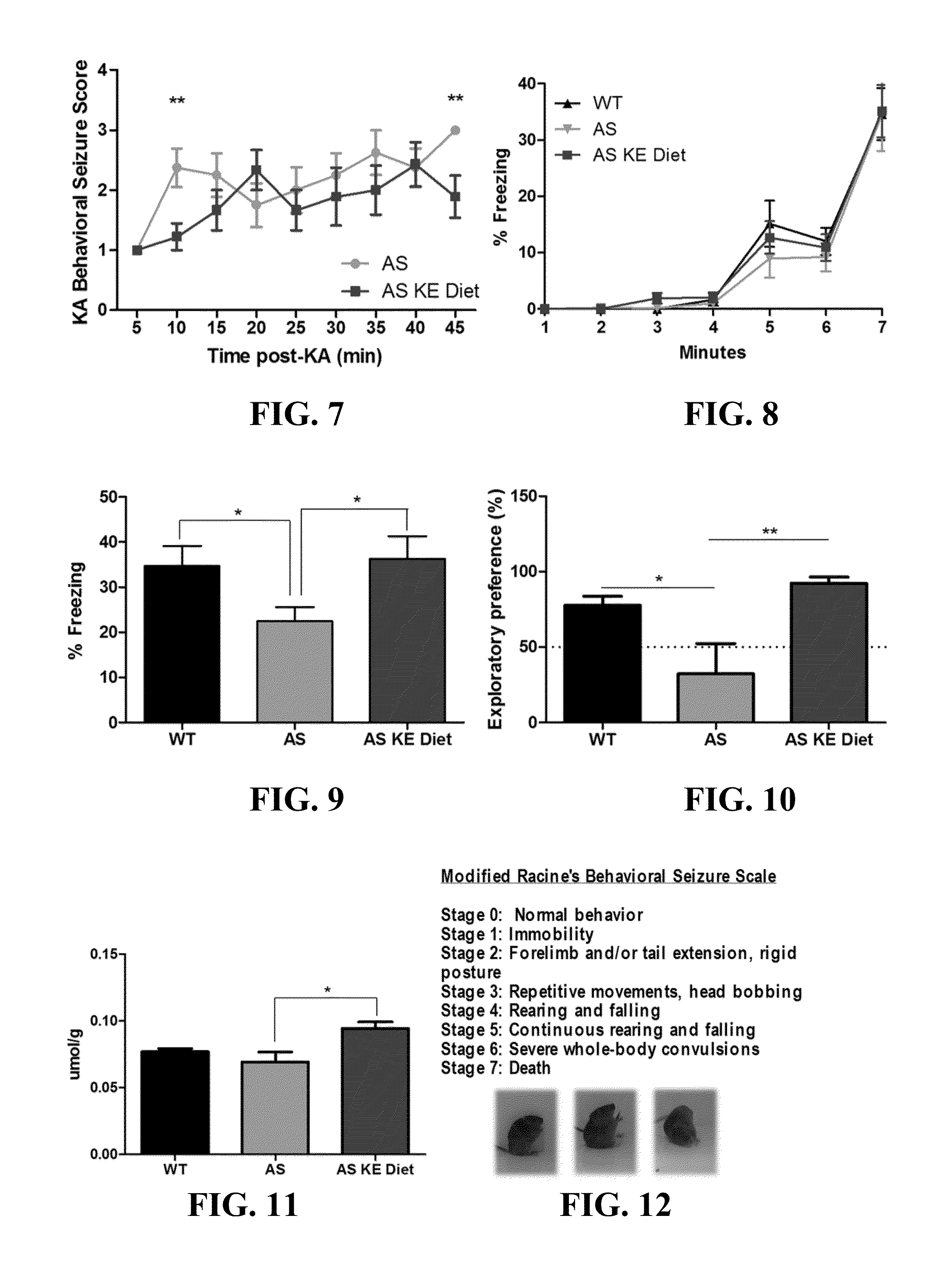
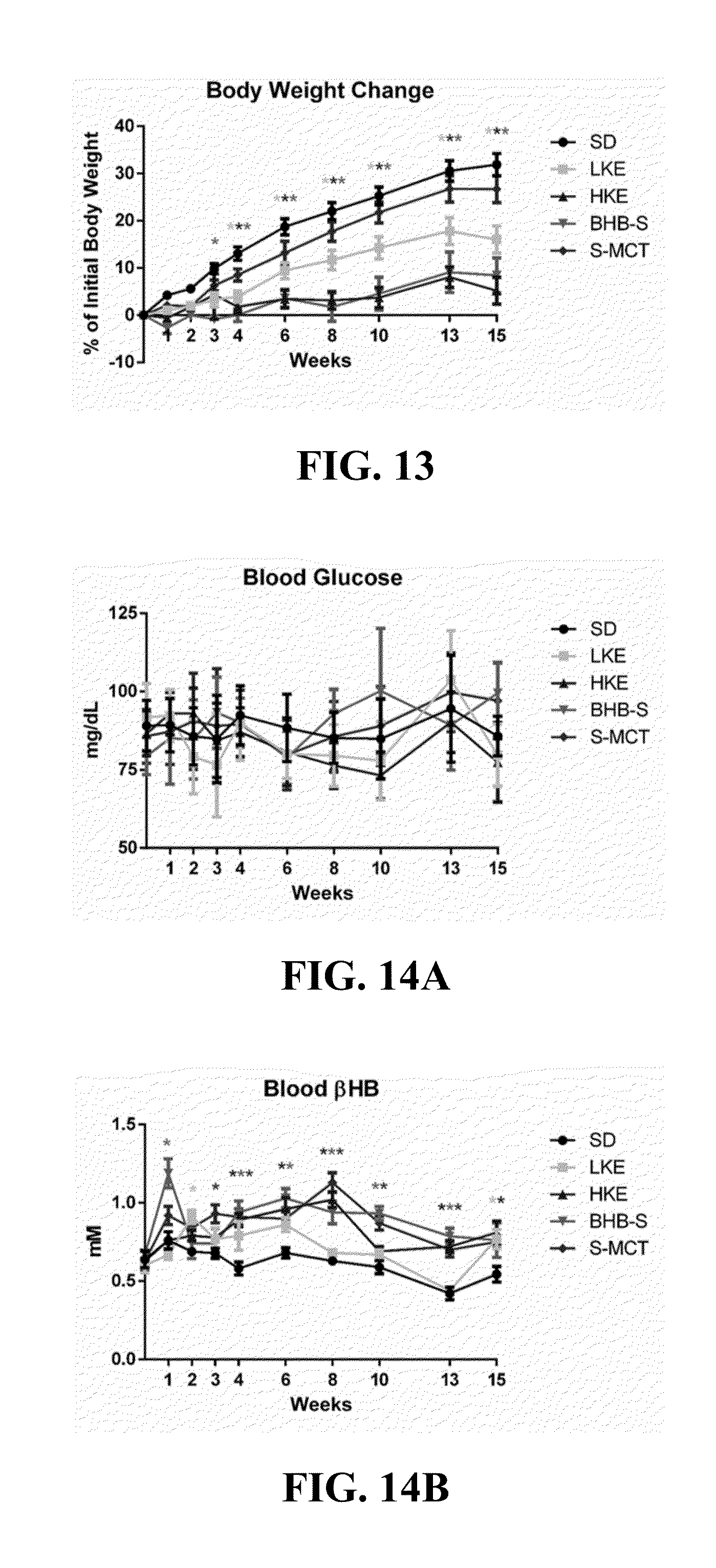
Ketone supplements for treatment of angelman syndrome
ActiveUS20170000754A1Lose weightReduced muscle toneEster active ingredientsAnhydride/acid/halide active ingredientsPhysiologyBlood ketone level
The invention concerns a method of treating Angelman Syndrome (AS) in a subject, comprising inducing ketosis in the subject by administering a therapeutically effective amount of a ketone ester, such as an R,S-1,3-butanediol acetoacetate ester, wherein administration of the ketone ester elevates the blood ketone level in the subject. Other aspects of the invention include a method of increasing cognitive function and / or motor function in a subject with AS; and a method of decreasing seizures and increasing the latency to seize in a subject with AS.
Owner:UNIV OF SOUTH FLORIDA
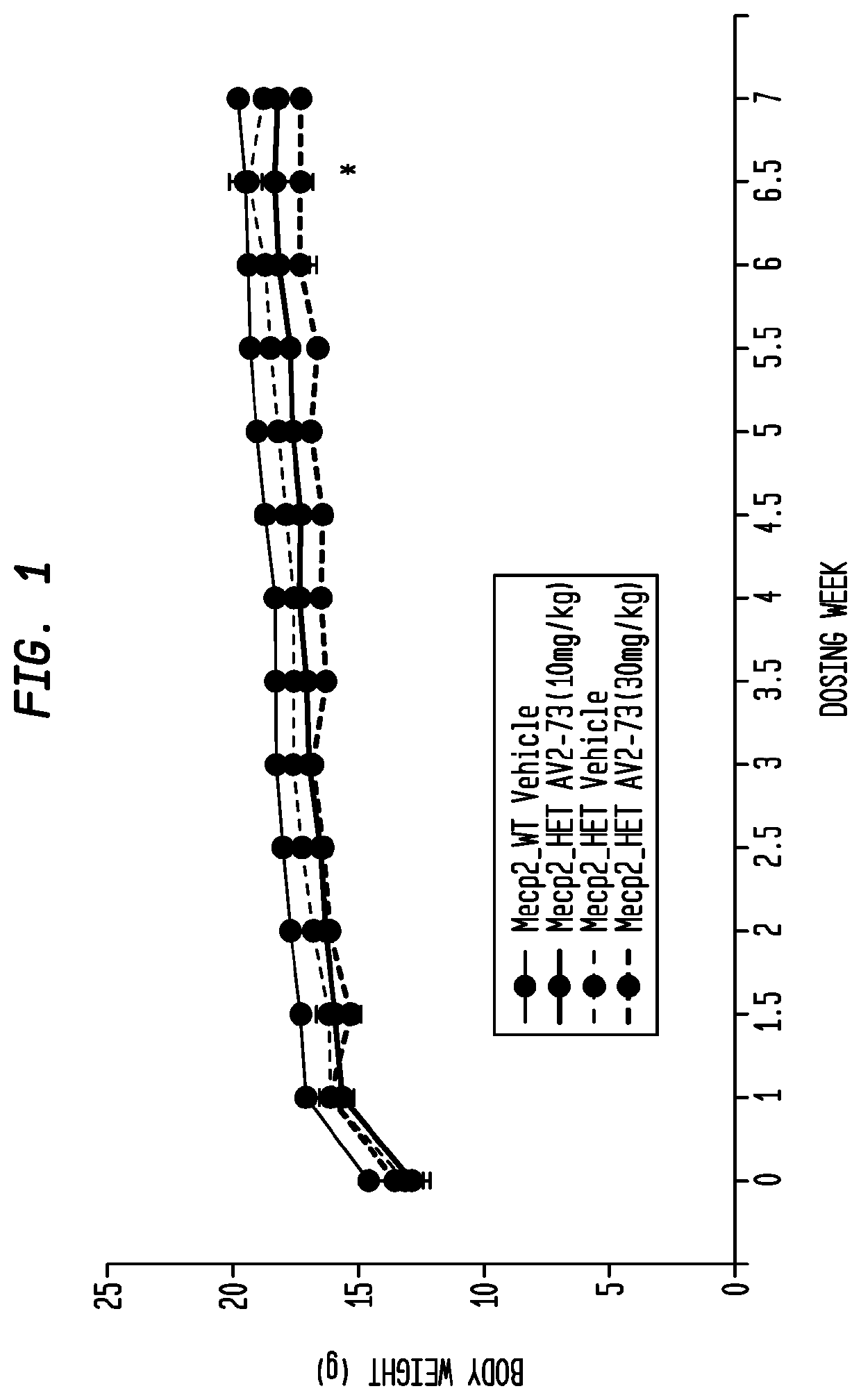
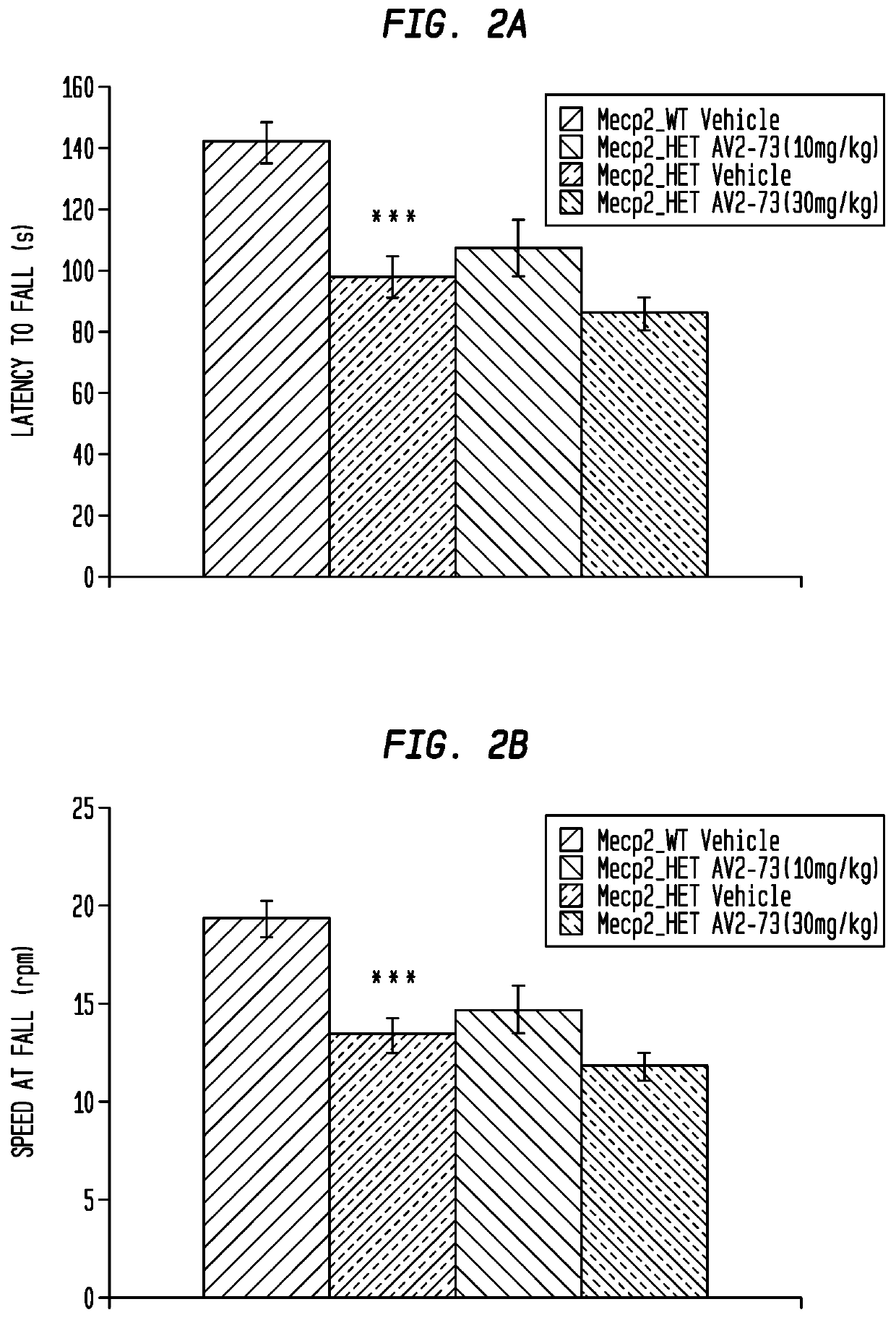
Neurodevelopmental disorder therapy
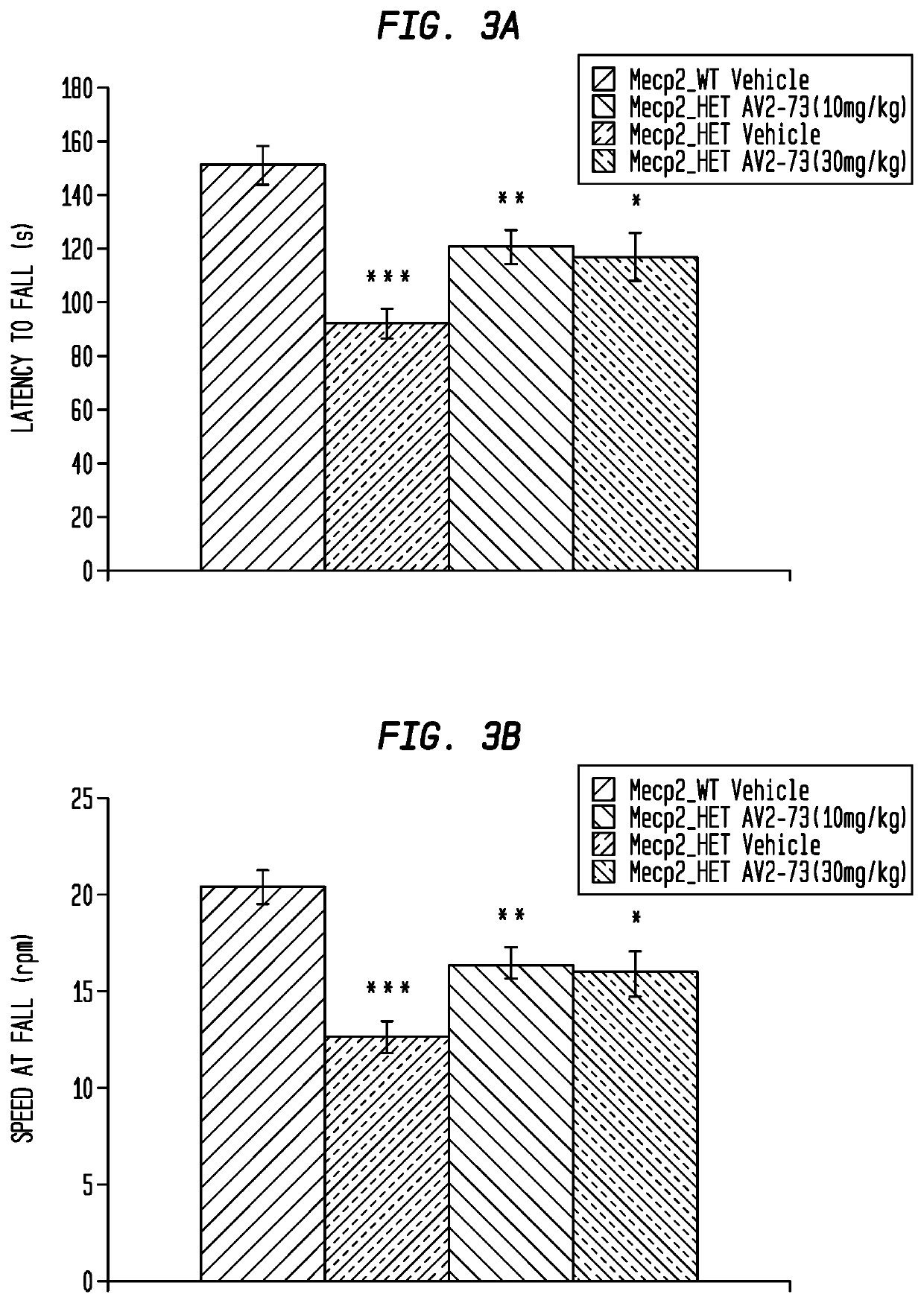
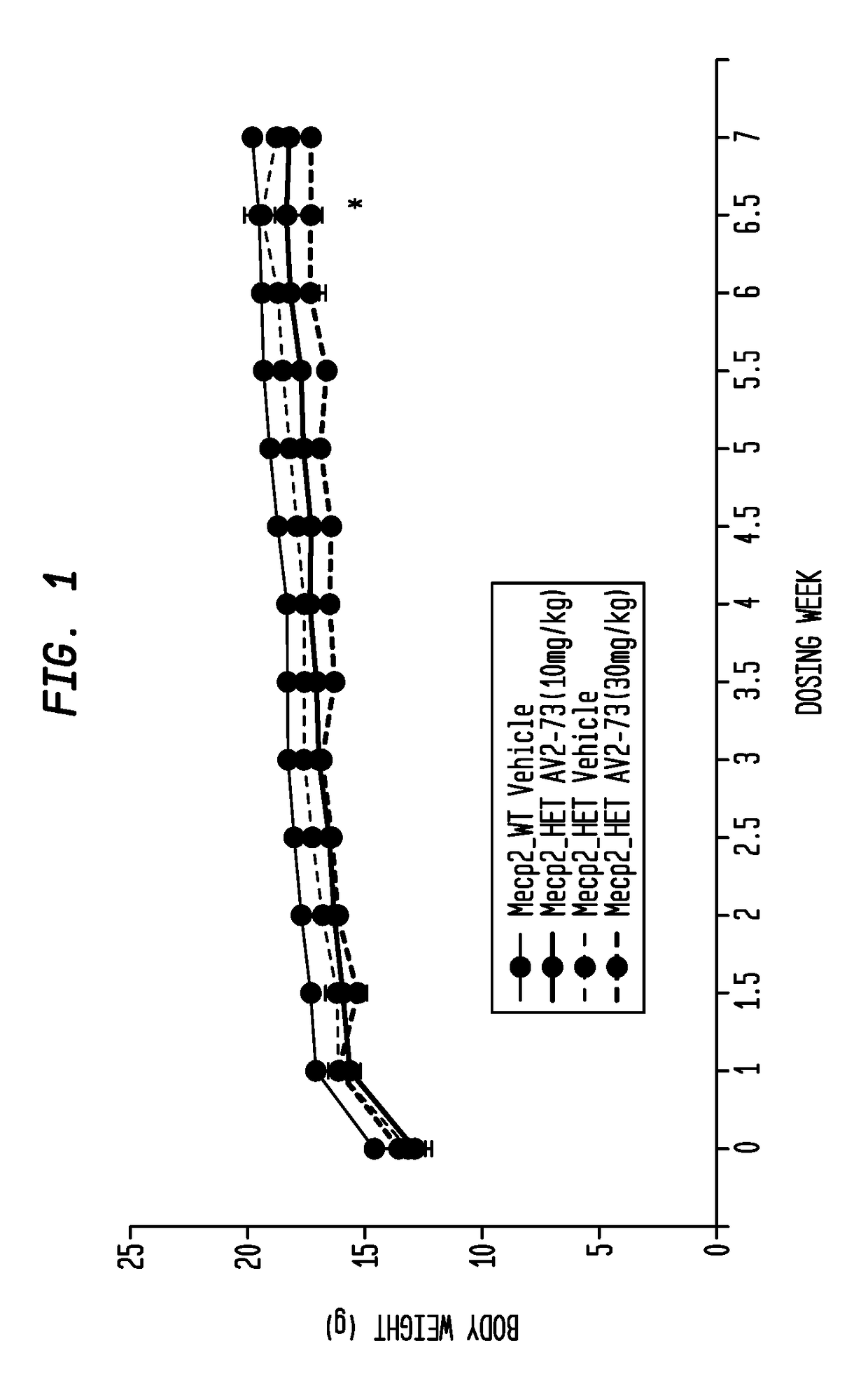
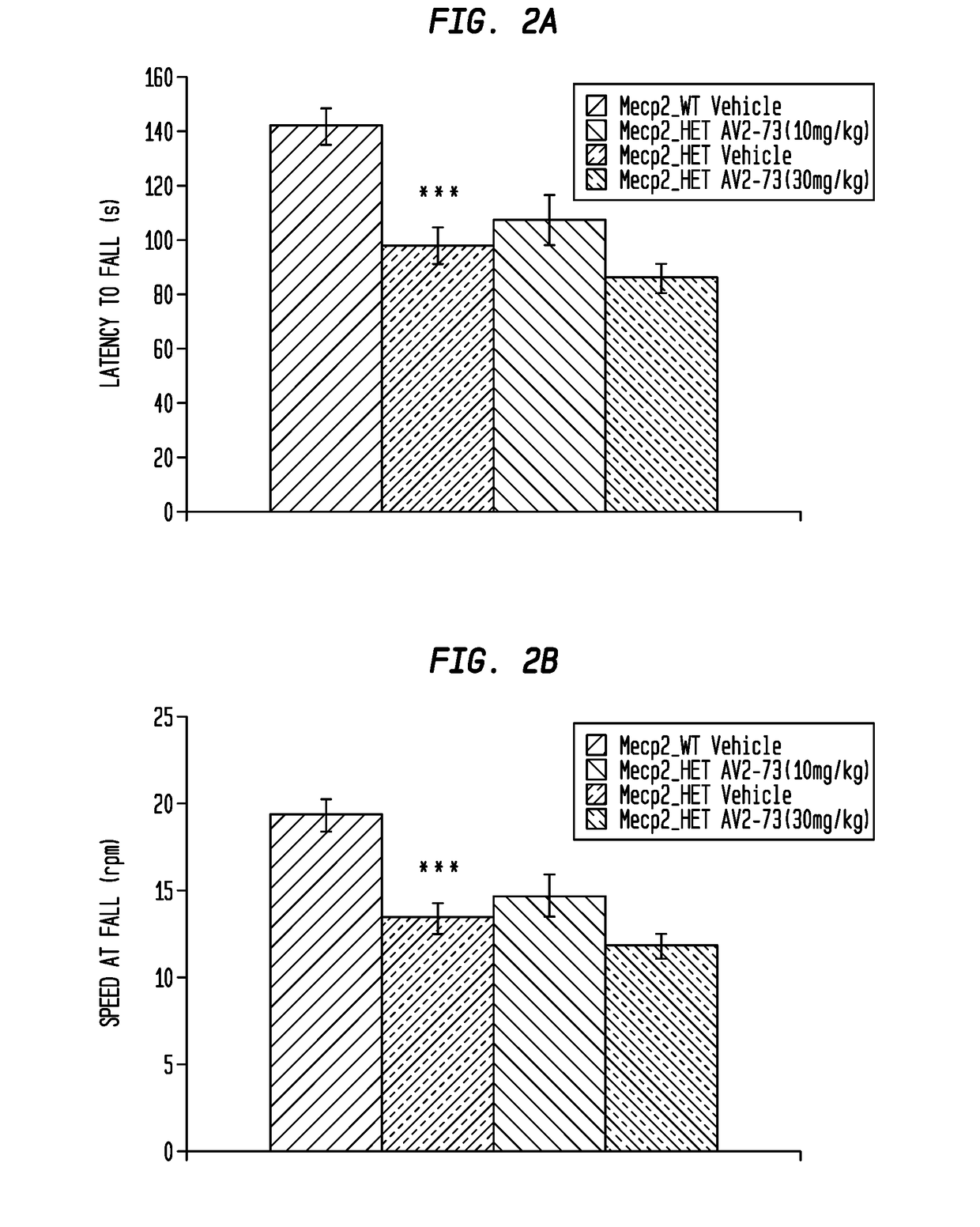
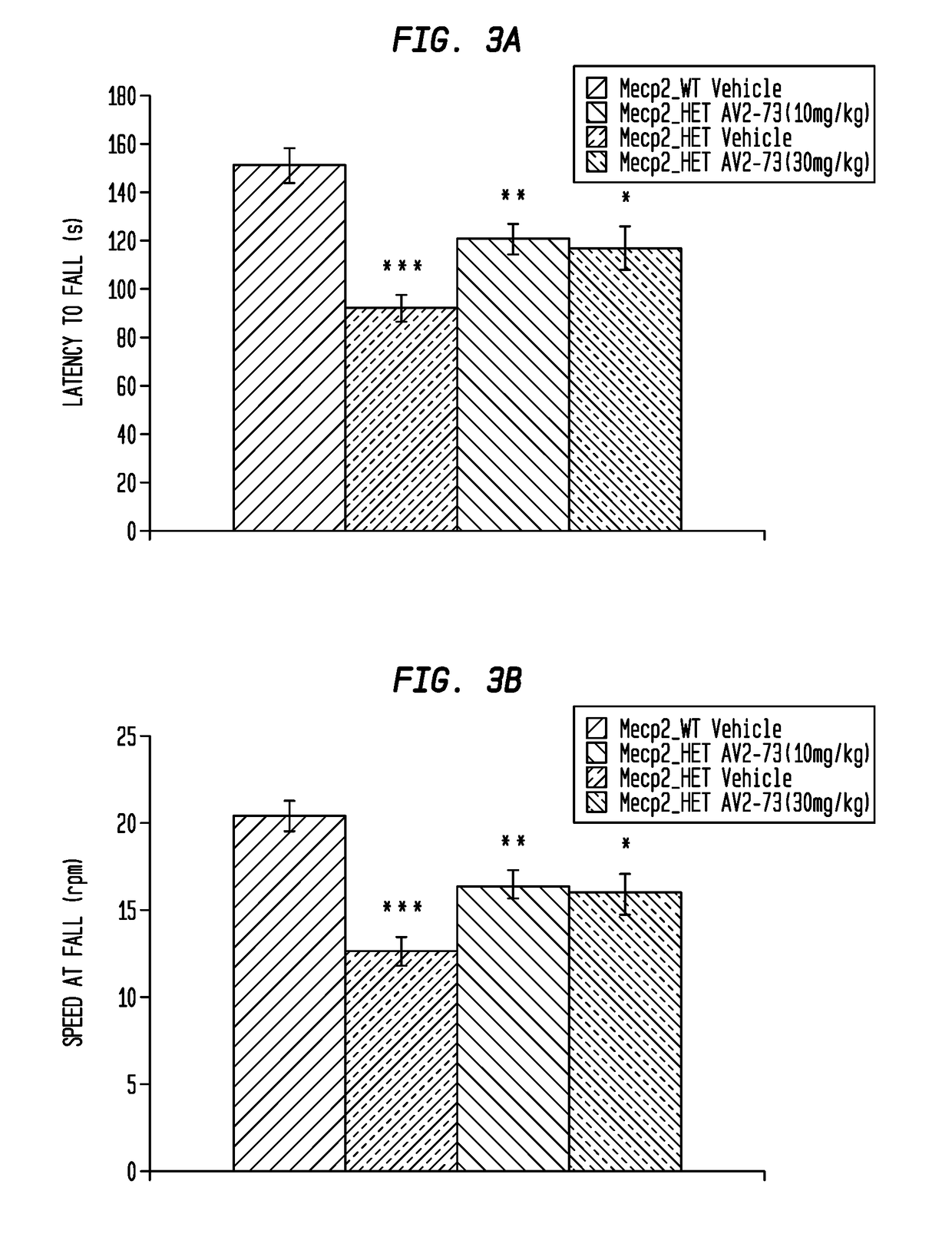
This invention addresses tetrahydro-N,N-dimethyl-2,2-diphenyl-3-furanmethanamine hydrochloride (ANAVEX2-73, AV2-73, or A2-73) in a method of treatment for neurodevelopmental disorders. Particular reference is made to the treatment of autism spectrum disorder, cerebral palsy, Rett syndrome, Angelman syndrome, Williams syndrome, pervasive developmental disorder not otherwise specified (PDD-NOS), childhood disintegrative disorder, and Smith-Magenis syndrome. Additional reference is made to multiple sclerosis.
Owner:ANAVEX LIFE SCI CORP
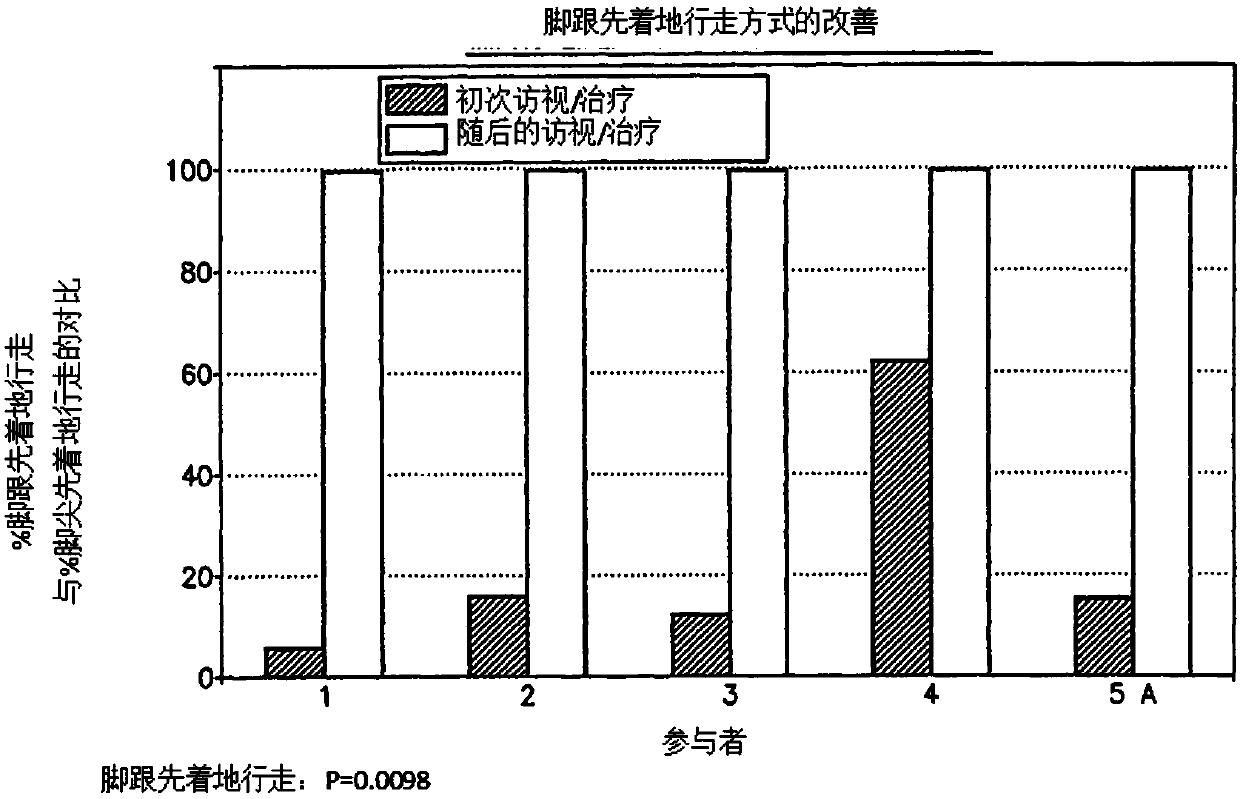
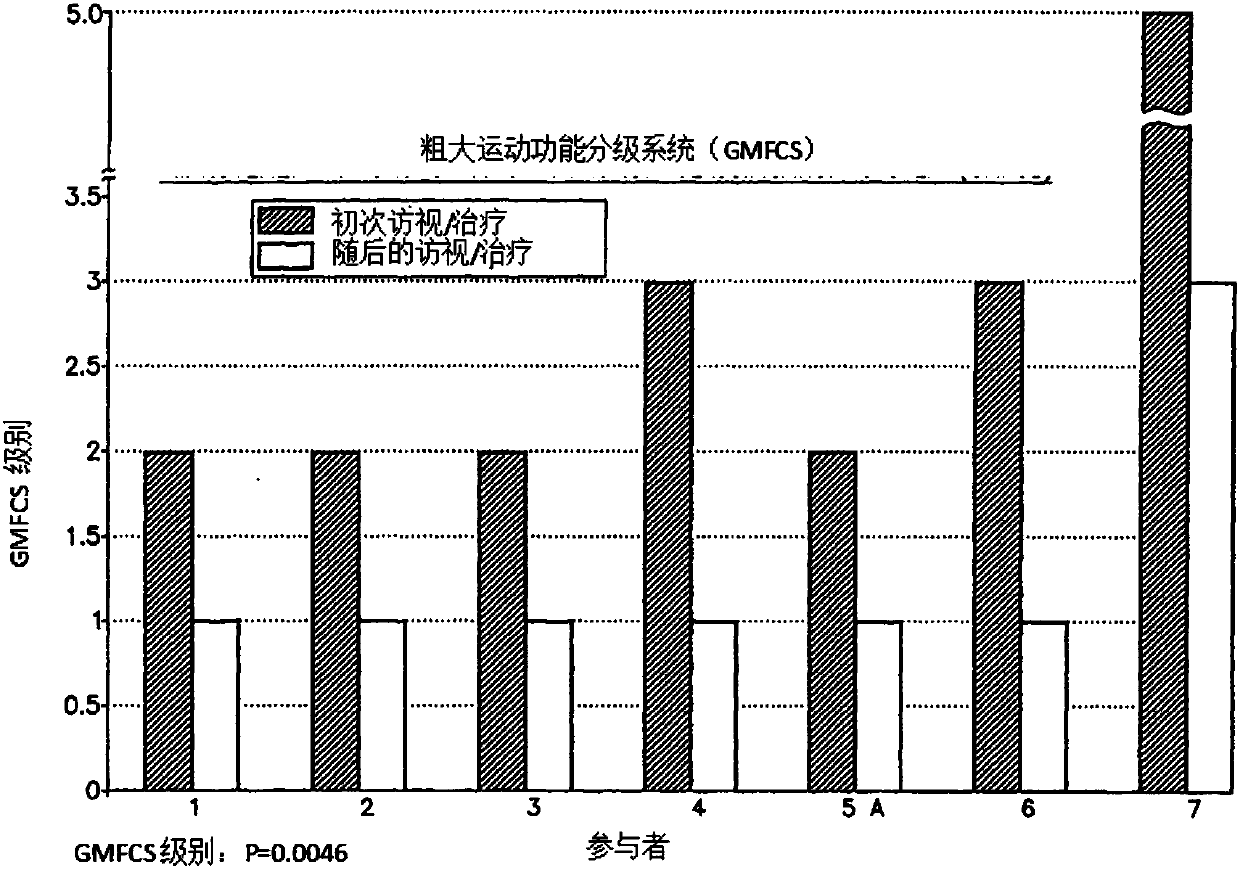
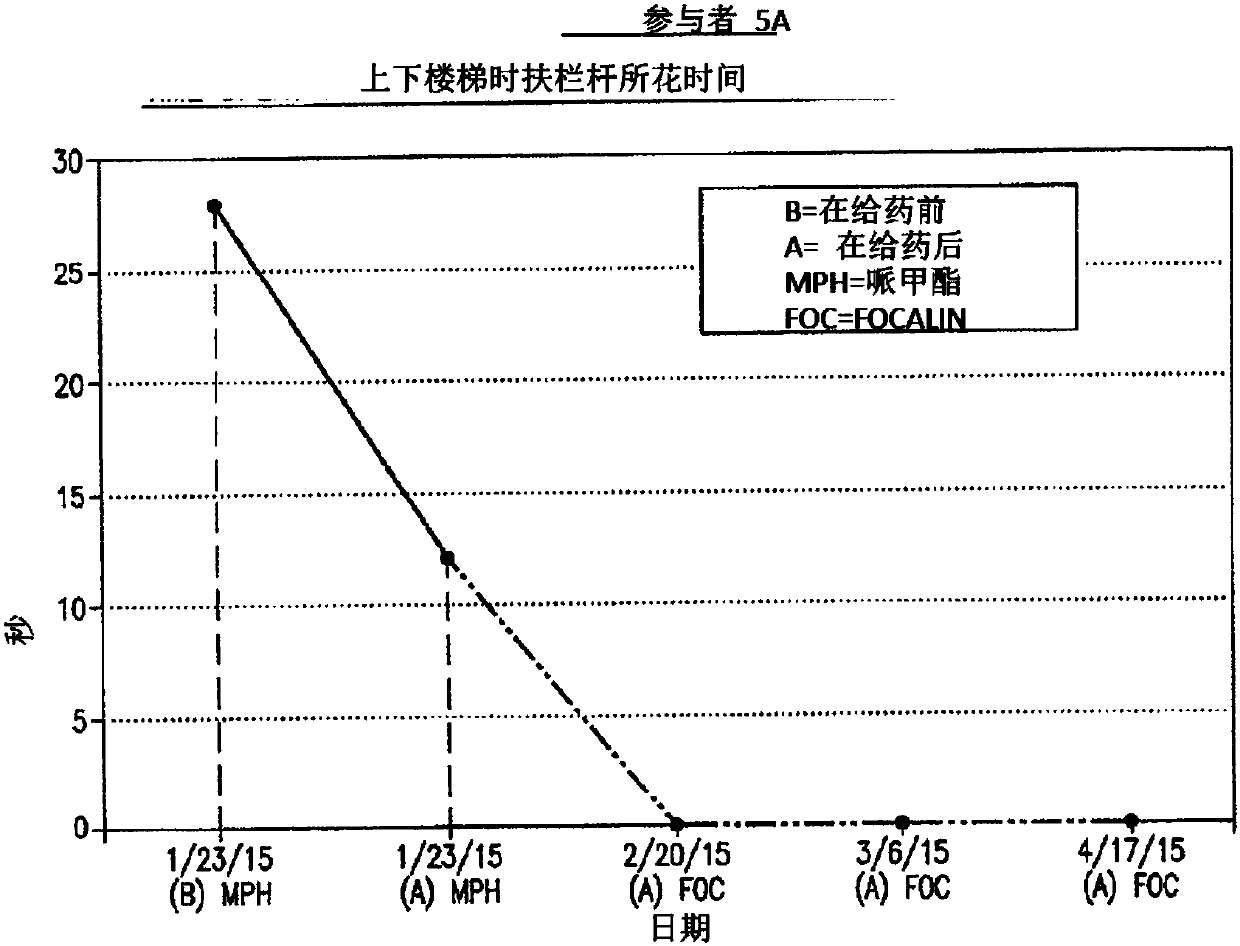
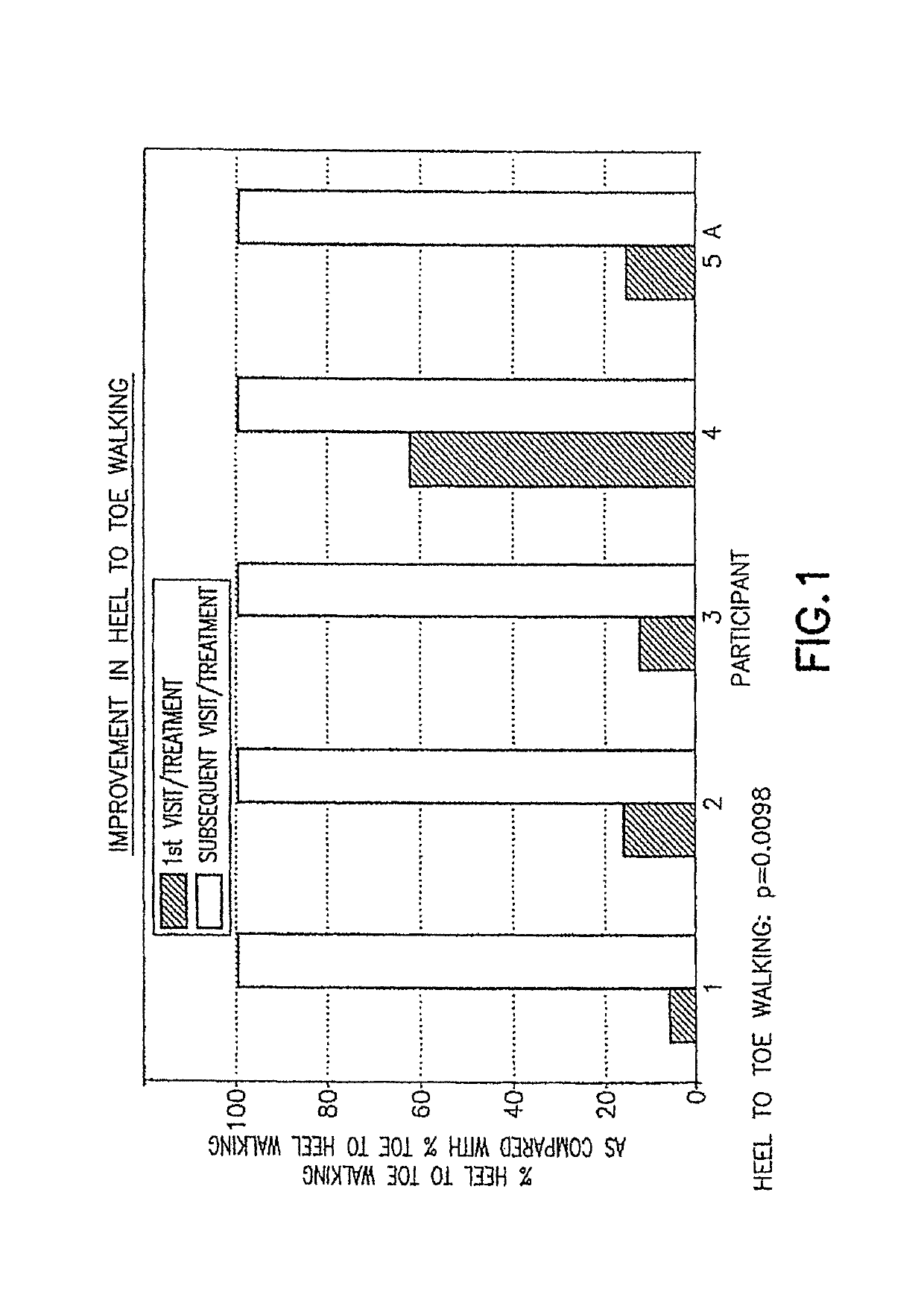
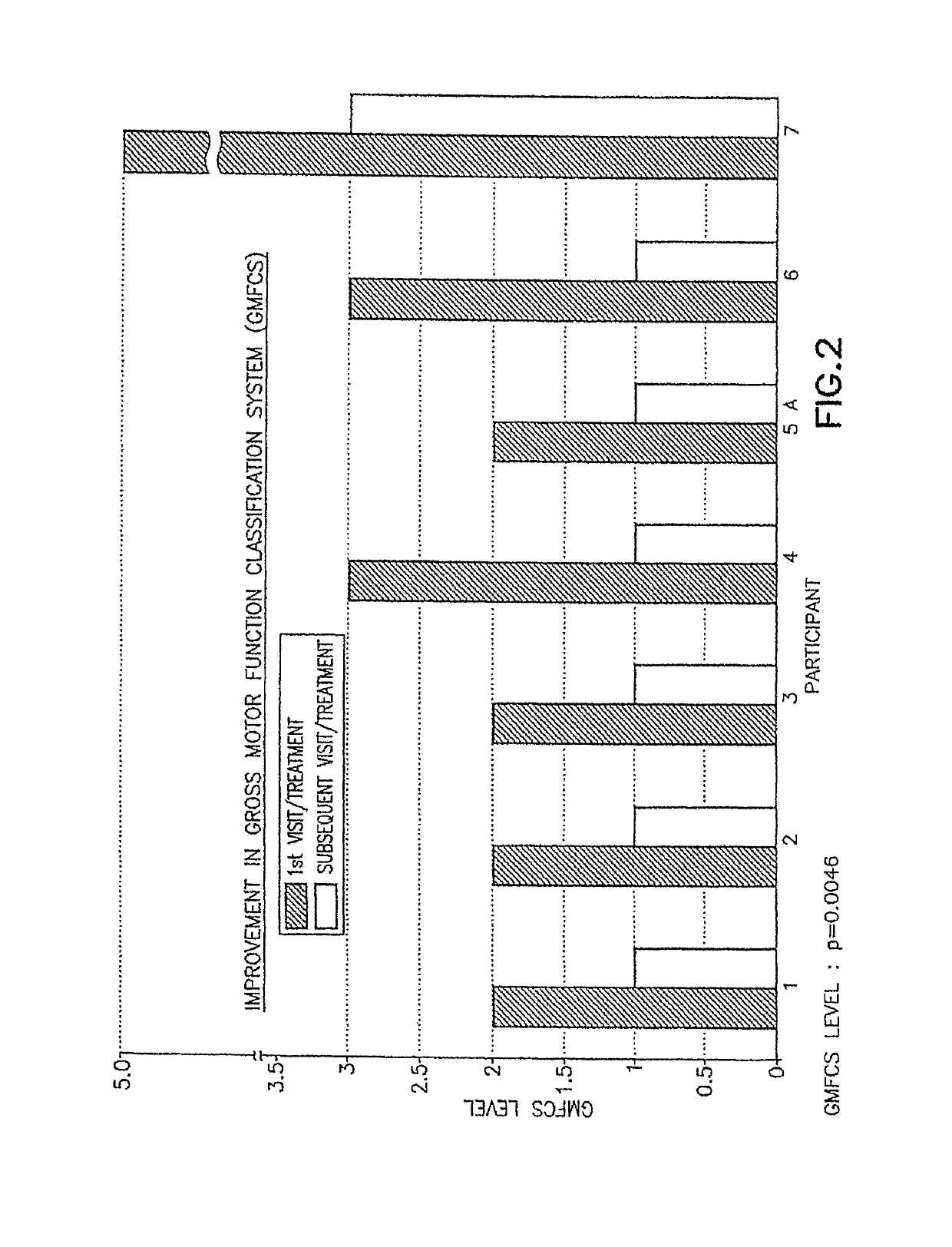
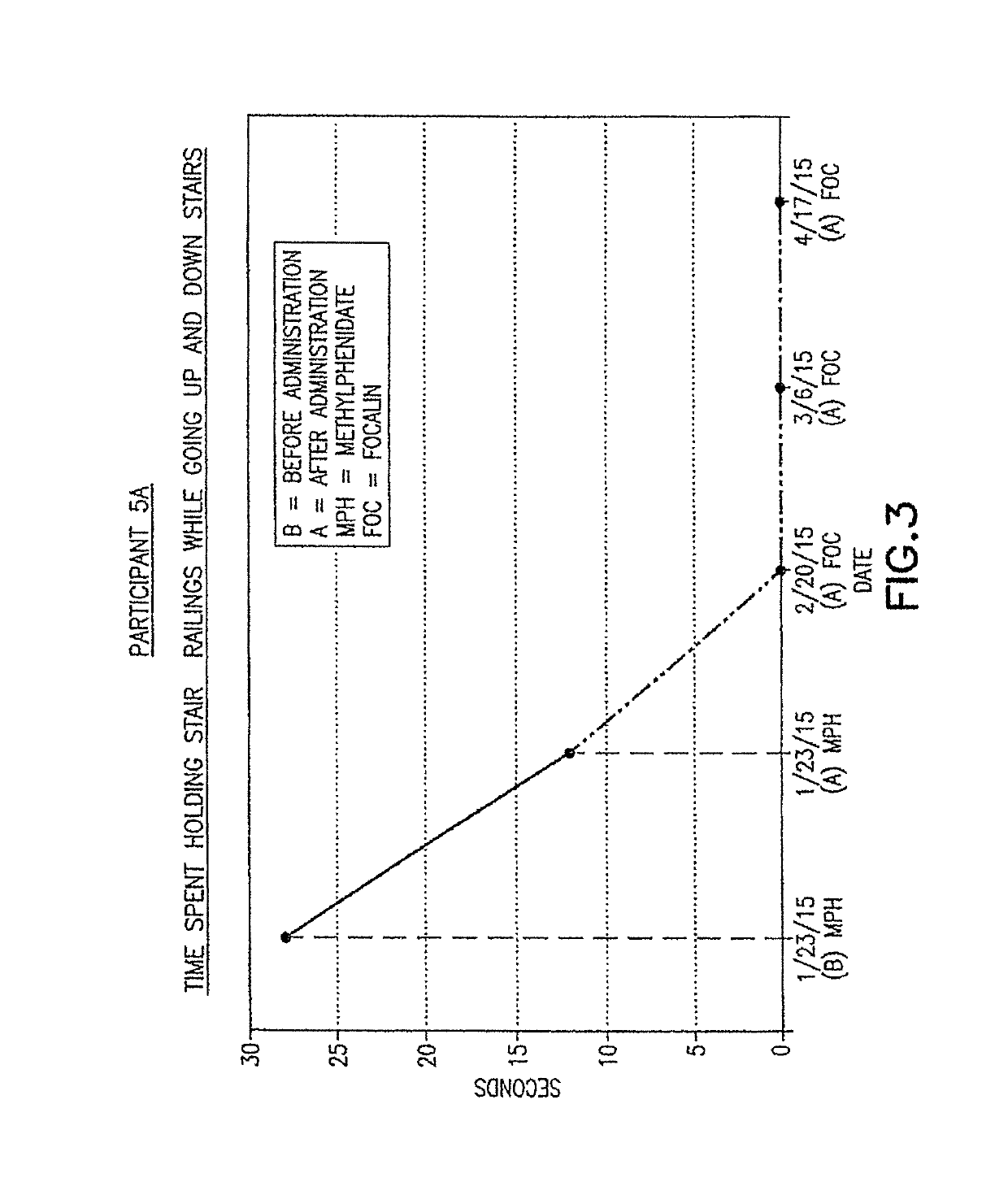
Pre-frontal cortex processing disorder gait and limb impairments treatment
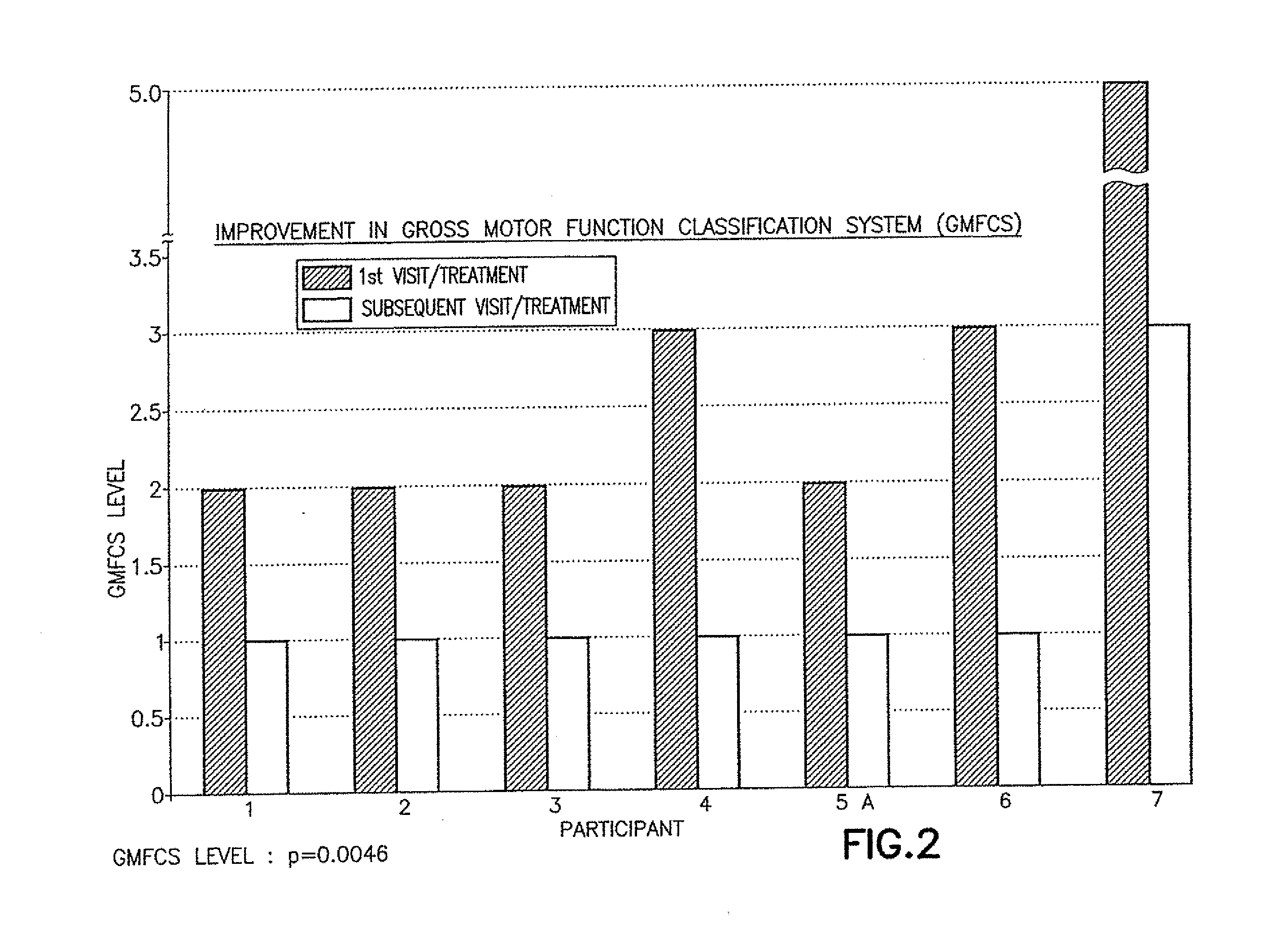
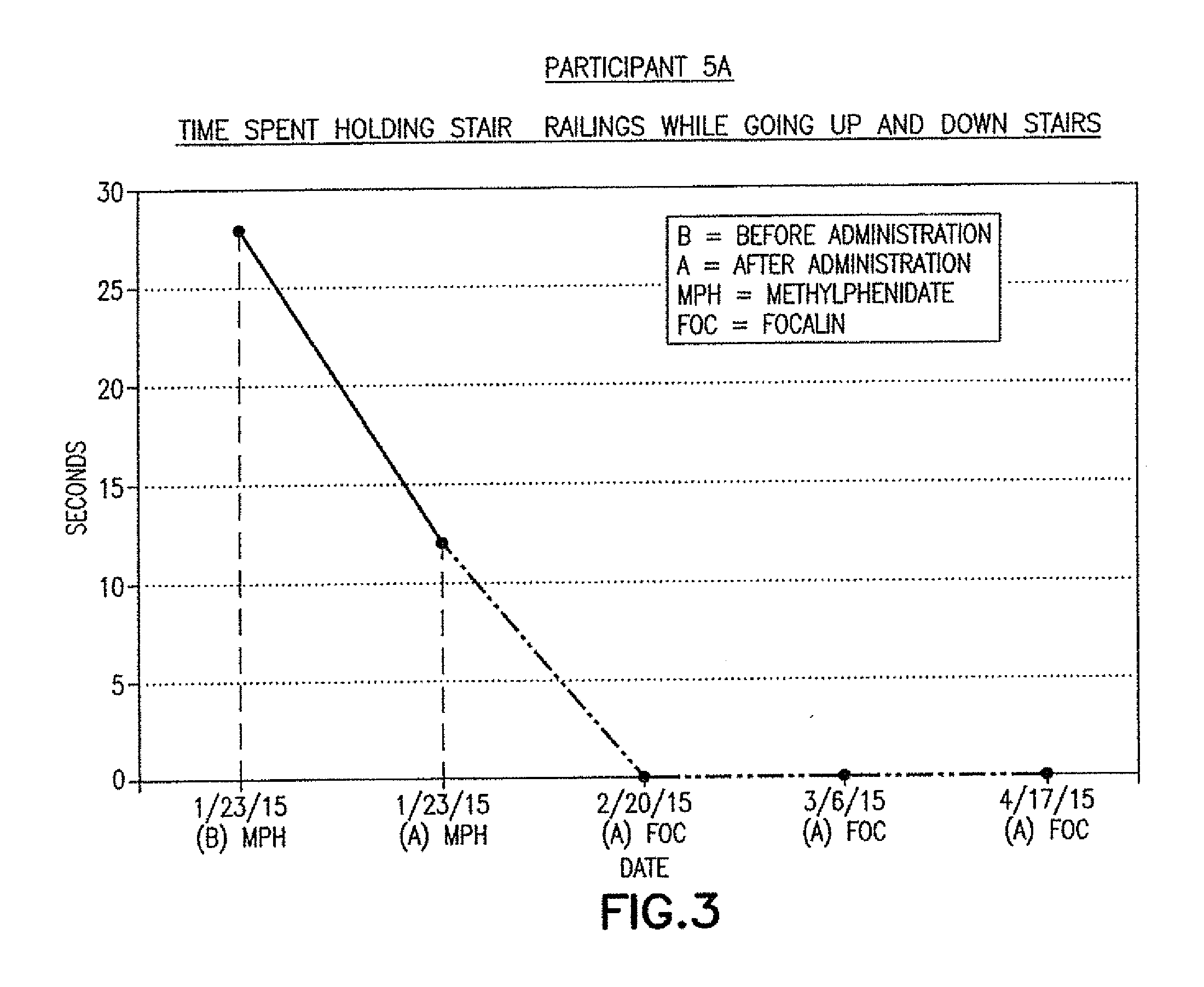
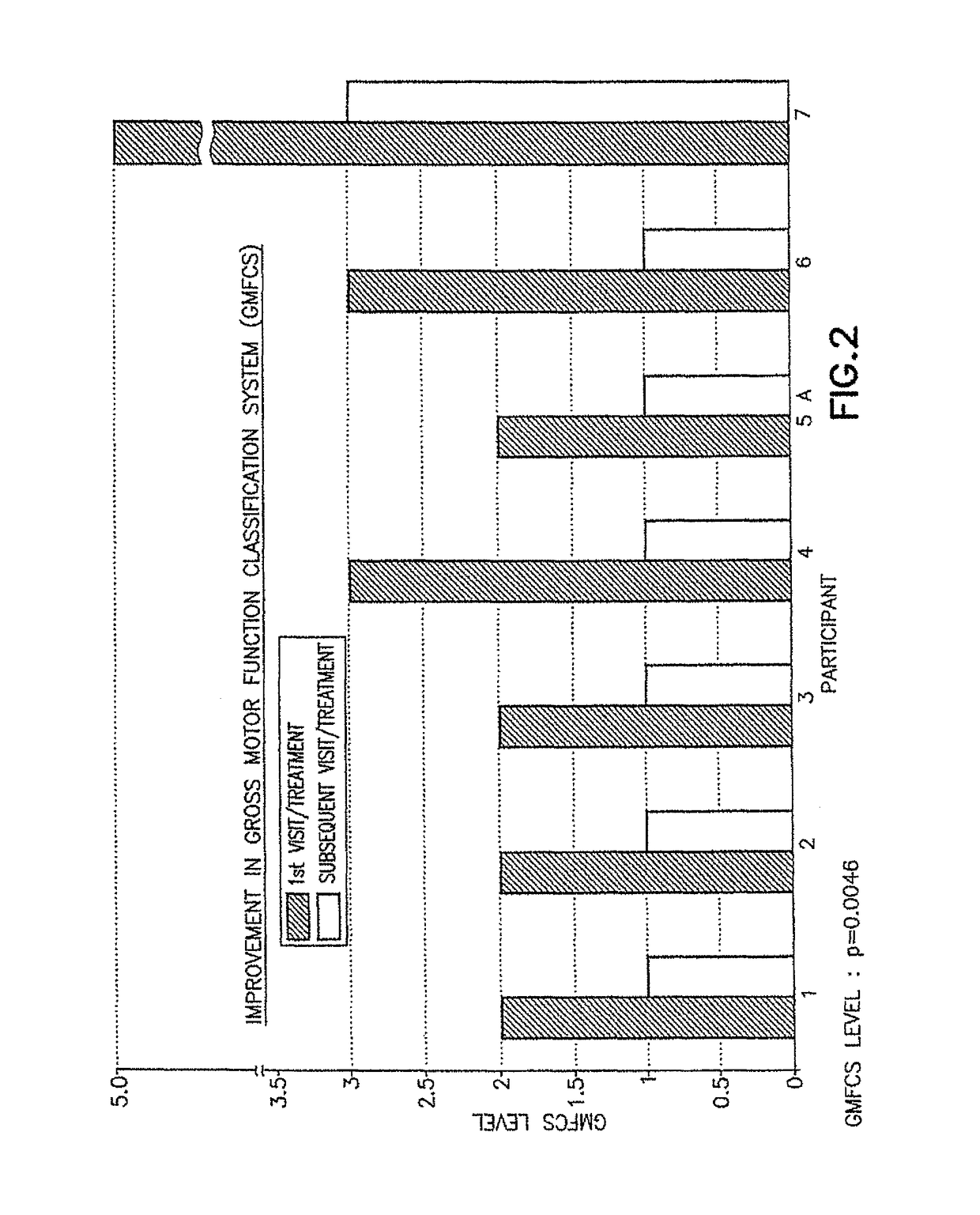
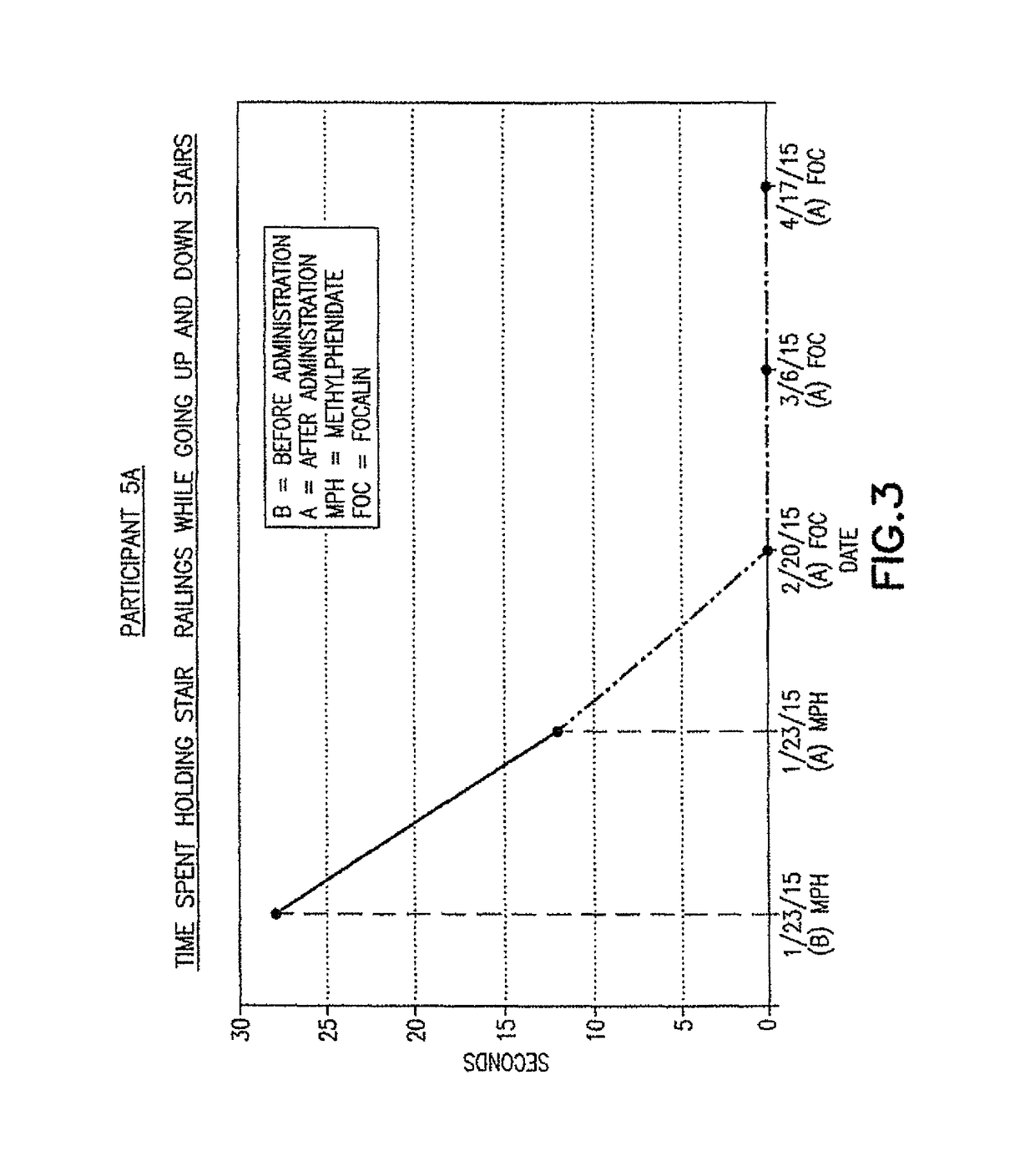
ActiveUS9682073B2Easy to controlConsequential diminishment in the speech, gait or limb impairmentOrganic active ingredientsDiagnostic recording/measuringDiseaseFragile X chromosome
A methylphenidate, particularly including dextro-threo-methylphenidate, is administered to a subject to treat a speech, gait or limb impairment secondary to a genetically acquired pre-frontal cortex processing disease or disorder, particularly including multiple sclerosis, cerebral palsy, Angelman syndrome, Rett syndrome and Fragile-X syndrome.
Owner:GILROSE PHARMA
Ketone esters for treatment of angelman syndrome
ActiveUS9364456B1Lose weightMore accessEster active ingredientsAnhydride/acid/halide active ingredientsKetoneBlood ketone level
The invention concerns a method of treating Angelman Syndrome (AS) in a subject, comprising inducing ketosis in the subject by administering a therapeutically effective amount of a ketone ester, such as an R,S-1,3-butanediol acetoacetate ester, wherein administration of the ketone ester elevates the blood ketone level in the subject. Other aspects of the invention include a method of increasing cognitive function and / or motor function in a subject with AS; and a method of decreasing seizures and increasing the latency to seize in a subject with AS.
Owner:UNIV OF SOUTH FLORIDA
Pre-frontal cortex processing disorder gait and limb impairments treatment
ActiveUS20160199366A1Easy to controlConsequential diminishment in the speech, gait or limb impairmentBiocideOrganic chemistryDiseaseFragile X chromosome
A methylphenidate, particularly including dextro-threo-methylphenidate, is administered to a subject to treat a speech, gait or limb impairment secondary to a genetically acquired pre-frontal cortex processing disease or disorder, particularly including multiple sclerosis, cerebral palsy, Angelman syndrome, Rett syndrome and Fragile-X syndrome.
Owner:GILROSE PHARMA
Neurodevelopmental disorder therapy
ActiveUS20190022052A1Organic active ingredientsNervous disorderSmith–Magenis syndromeCerebral palsied
This invention addresses tetrahydro-N,N-dimethyl-2,2-diphenyl-3-furanmethanamine hydrochloride (ANAVEX2-73, AV2-73, or A2-73) in a method of treatment for neurodevelopmental disorders. Particular reference is made to the treatment of autism spectrum disorder, cerebral palsy, Rett syndrome, Angelman syndrome, Williams syndrome, pervasive developmental disorder not otherwise specified (PDD-NOS), childhood disintegrative disorder, and Smith-Magenis syndrome. Additional reference is made to multiple sclerosis.
Owner:ANAVEX LIFE SCI CORP
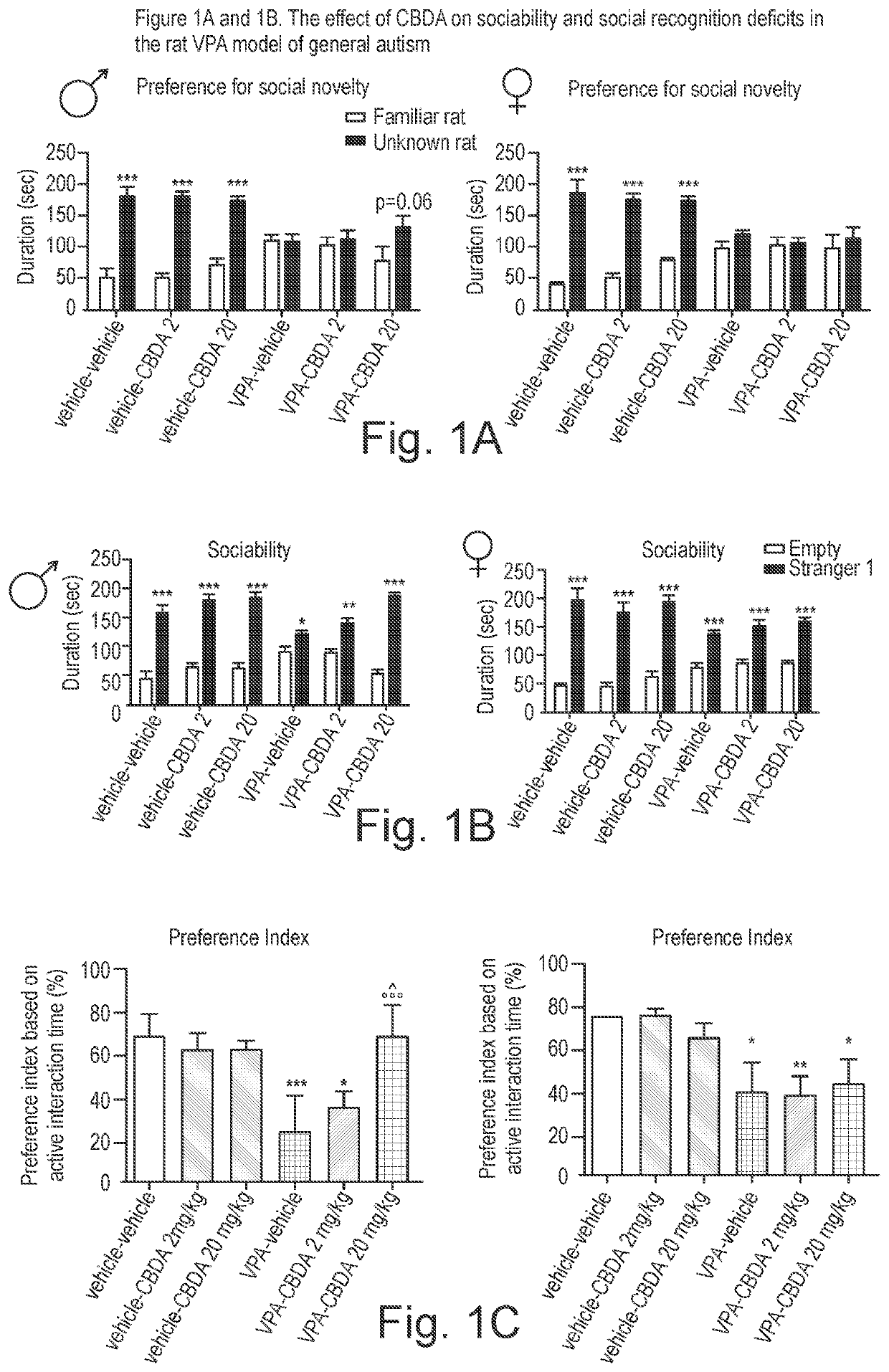
Use of cannabidiolic acid in the treatment of autism spectrum disorder and associated disorders
ActiveUS20190117619A1Symptoms improvedNervous disorderHydroxy compound active ingredientsDiseaseRodent model
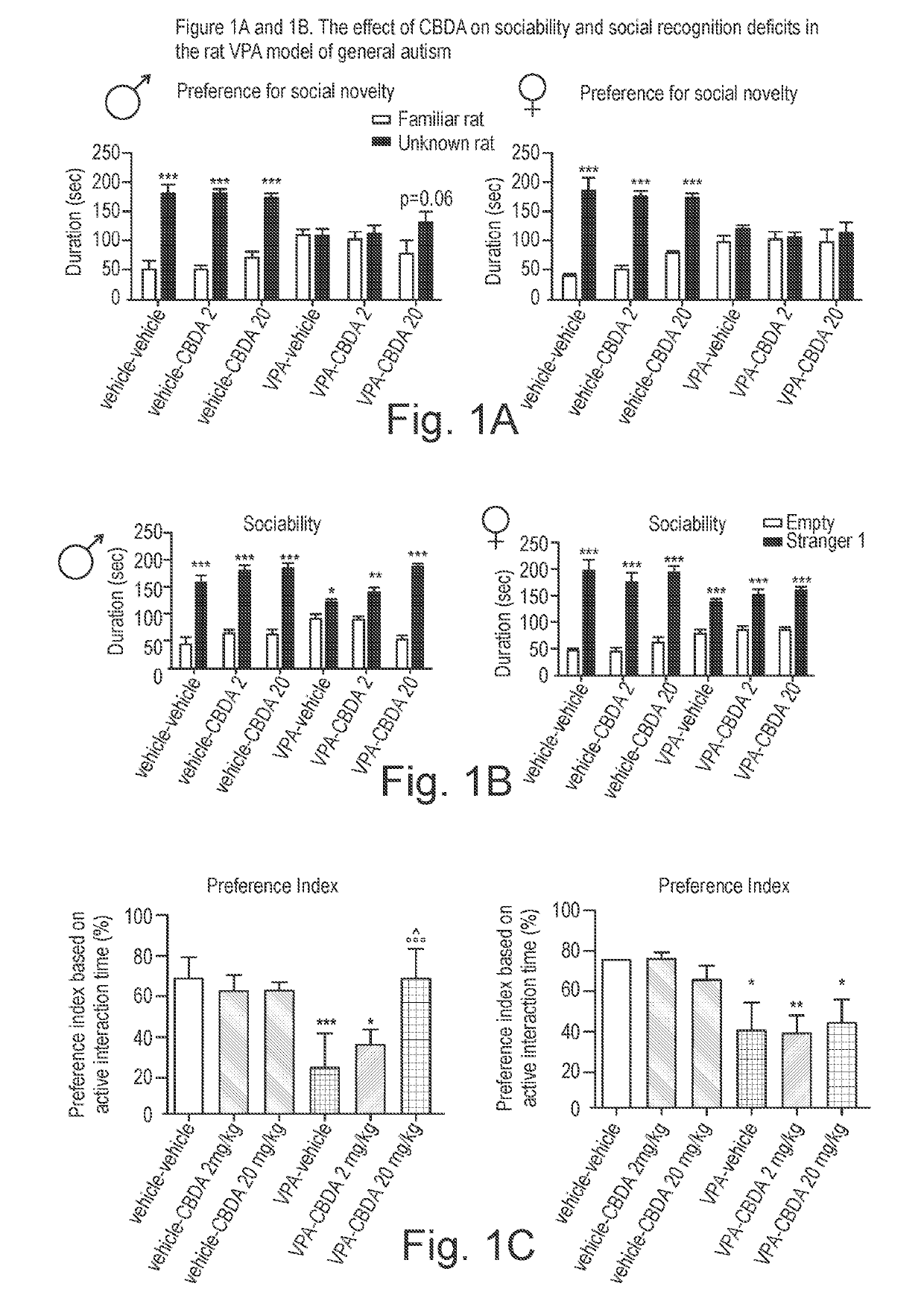
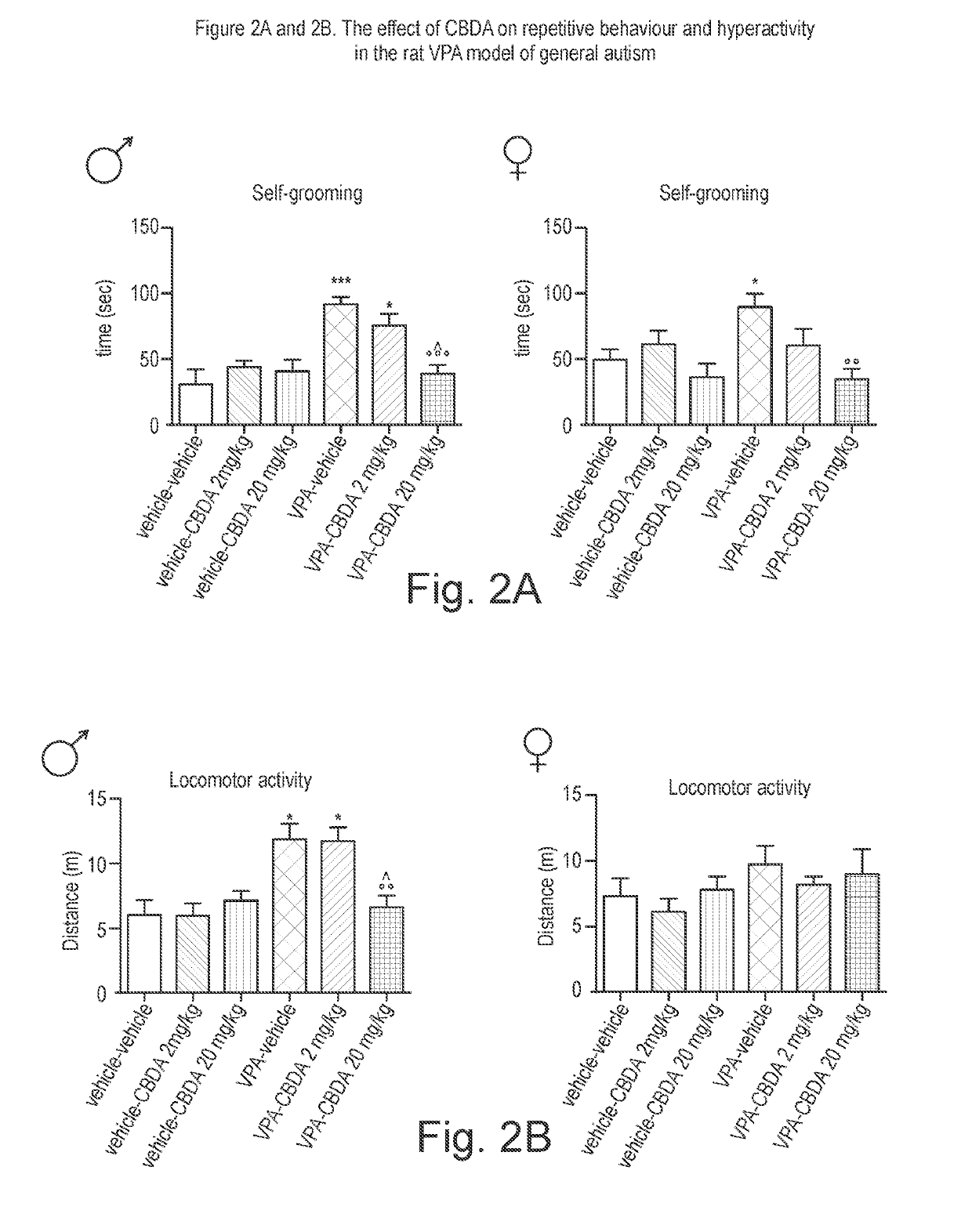
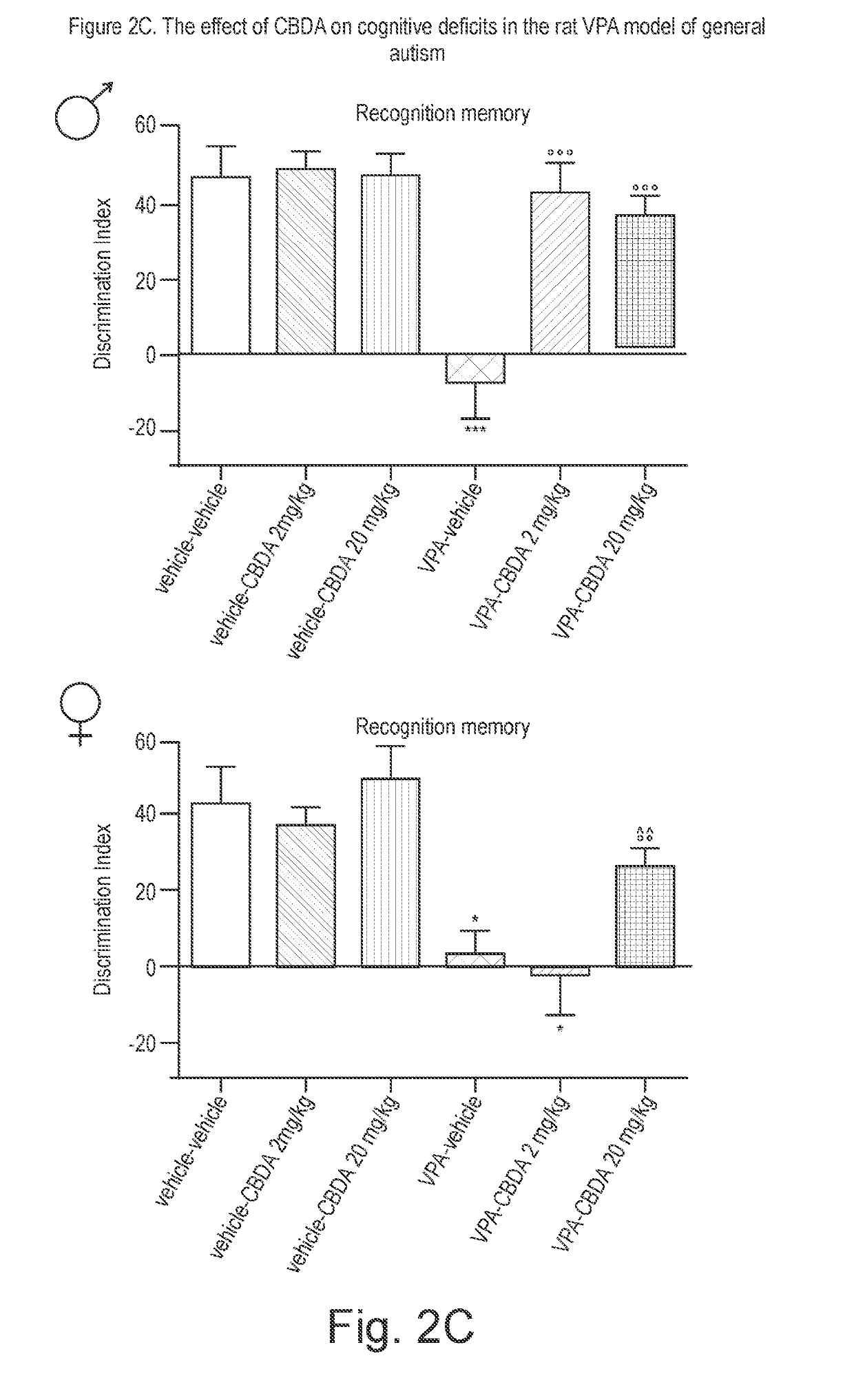
The present invention relates to the use of cannabidiolic acid (CBDA) in the treatment of autism spectrum disorder (ASD) and ASD-associated disorders, such as Fragile X syndrome (FXS); Rett syndrome (RS); or Angelman syndrome (AS). CBDA has been shown to be particularly effective in improving cognitive dysfunction in rodent models of ASD, FXS, RS and AS. The CBDA is preferably substantially pure. It may take the form of a highly purified extract of cannabis such that the CBDA is present at greater than 95% of the total extract (w / w) and the other components of the extract are characterised. Alternatively, the CBDA is synthetically produced.
Owner:GW RES LTD
Treating learning deficits with inhibitors of Hmg CoA reductase
The disclosure provides methods of treating cognitive disorders by administering a HMG CoA reductase inhibitor. Cognitive deficits treatable with the inhibitor compound include those associated with Angelman Syndrome, Neurofibromatosis-1, certain forms of X-linked mental retardation, tuberous sclerosis, Down Syndrome, autism, and attention deficit / hyperactivity disorder.
Owner:RGT UNIV OF CALIFORNIA
Pre-frontal cortex processing disorder speech, gait and limb impairments treatment
ActiveUS10085414B2Easy to controlConsequential diminishment in the speech, gait or limb impairmentAnimal housingHeterocyclic compound active ingredientsExtremity injuryFragile X chromosome
A methylphenidate, particularly including dextro-threo-methylphenidate, is administered to a subject to treat a speech, gait or limb impairment secondary to a genetically acquired pre-frontal cortex processing disease or disorder, particularly including multiple sclerosis, cerebral palsy, Angelman syndrome, Rett syndrome and Fragile-X syndrome.
Owner:GILROSE PHARMA
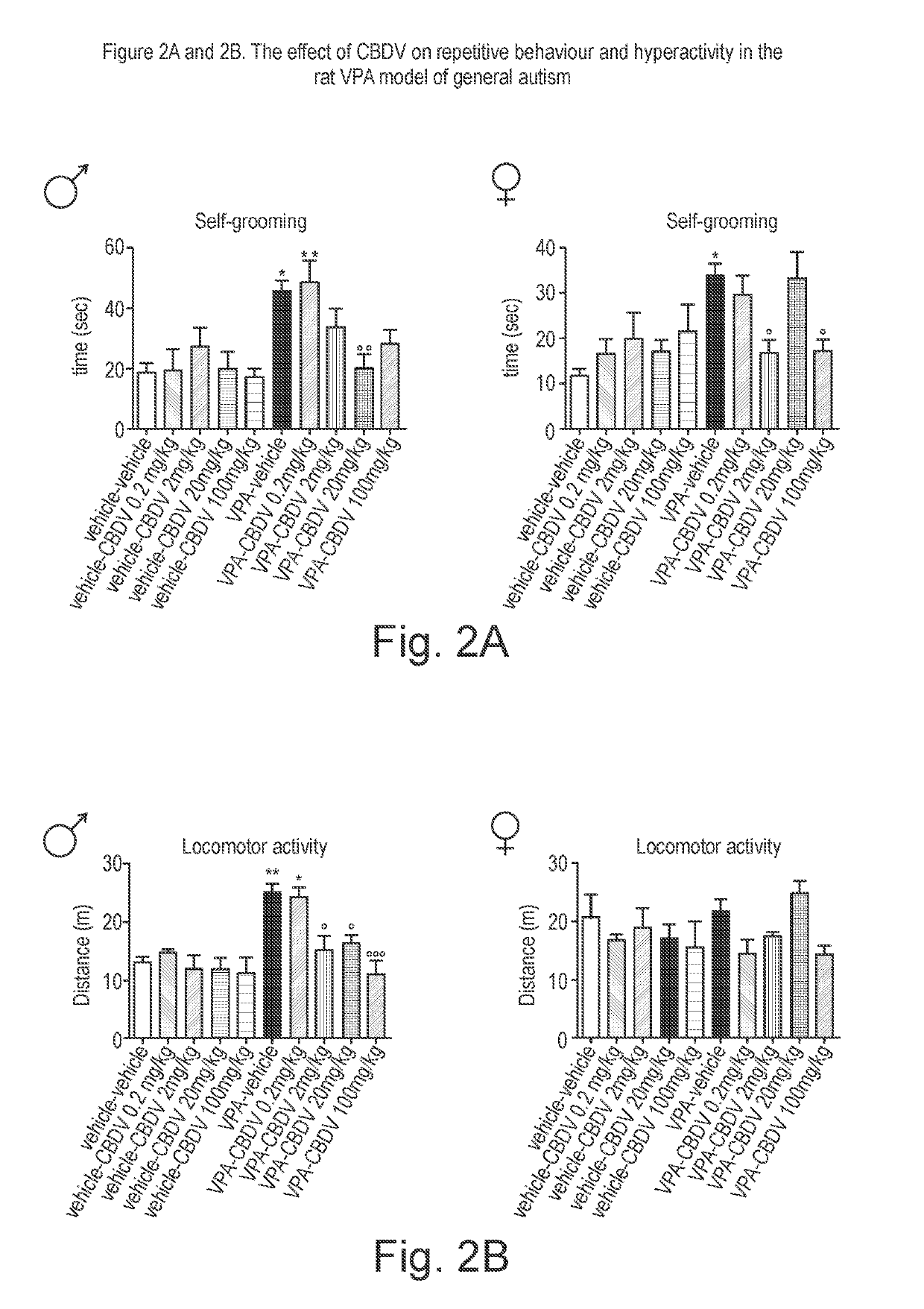
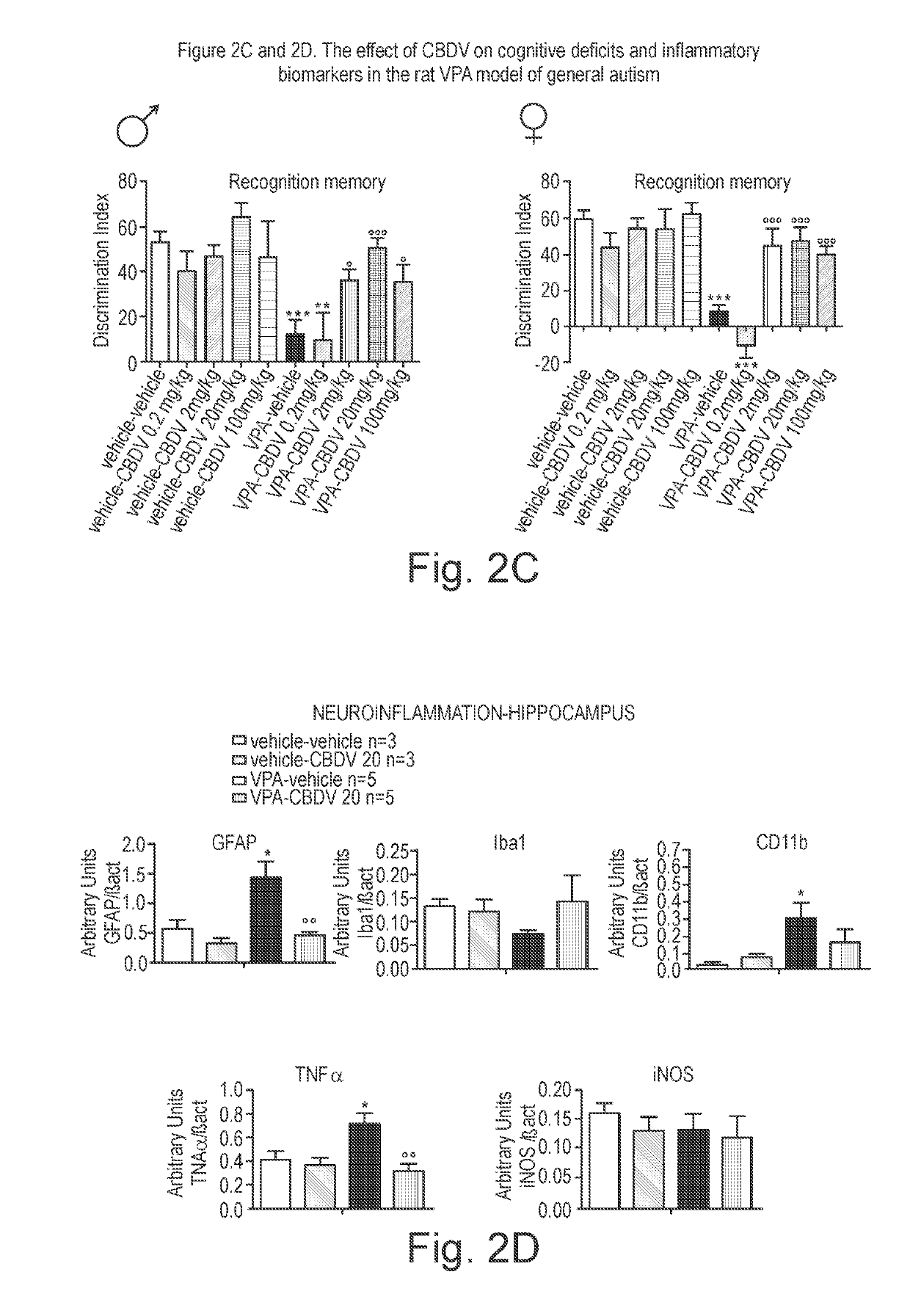
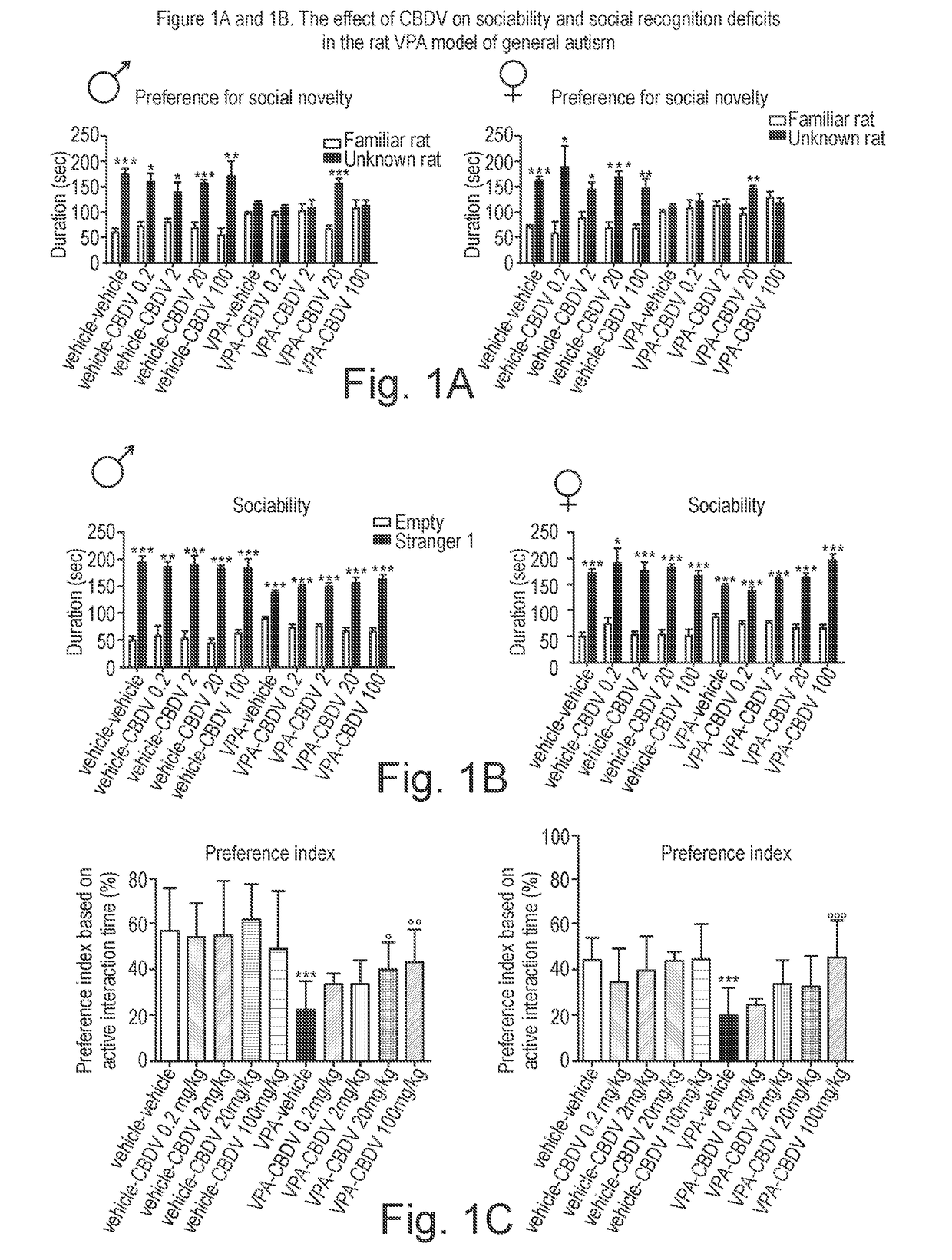
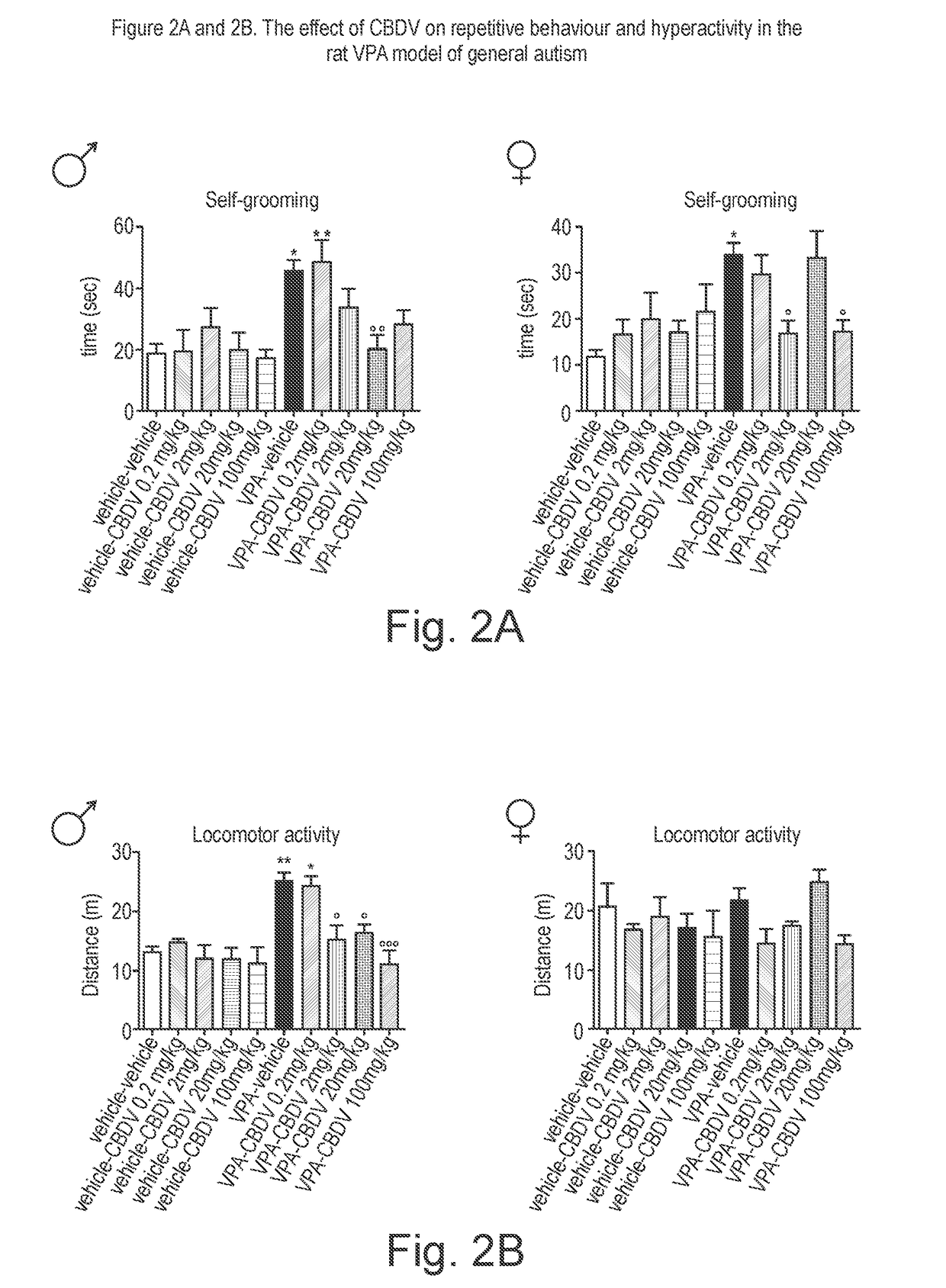
Use of cannabidivarin in the treatment of autism spectrum disorder, associated disorders and schizophrenia
InactiveUS20190070128A1Symptoms improvedNervous disorderHydroxy compound active ingredientsCannabisDisease
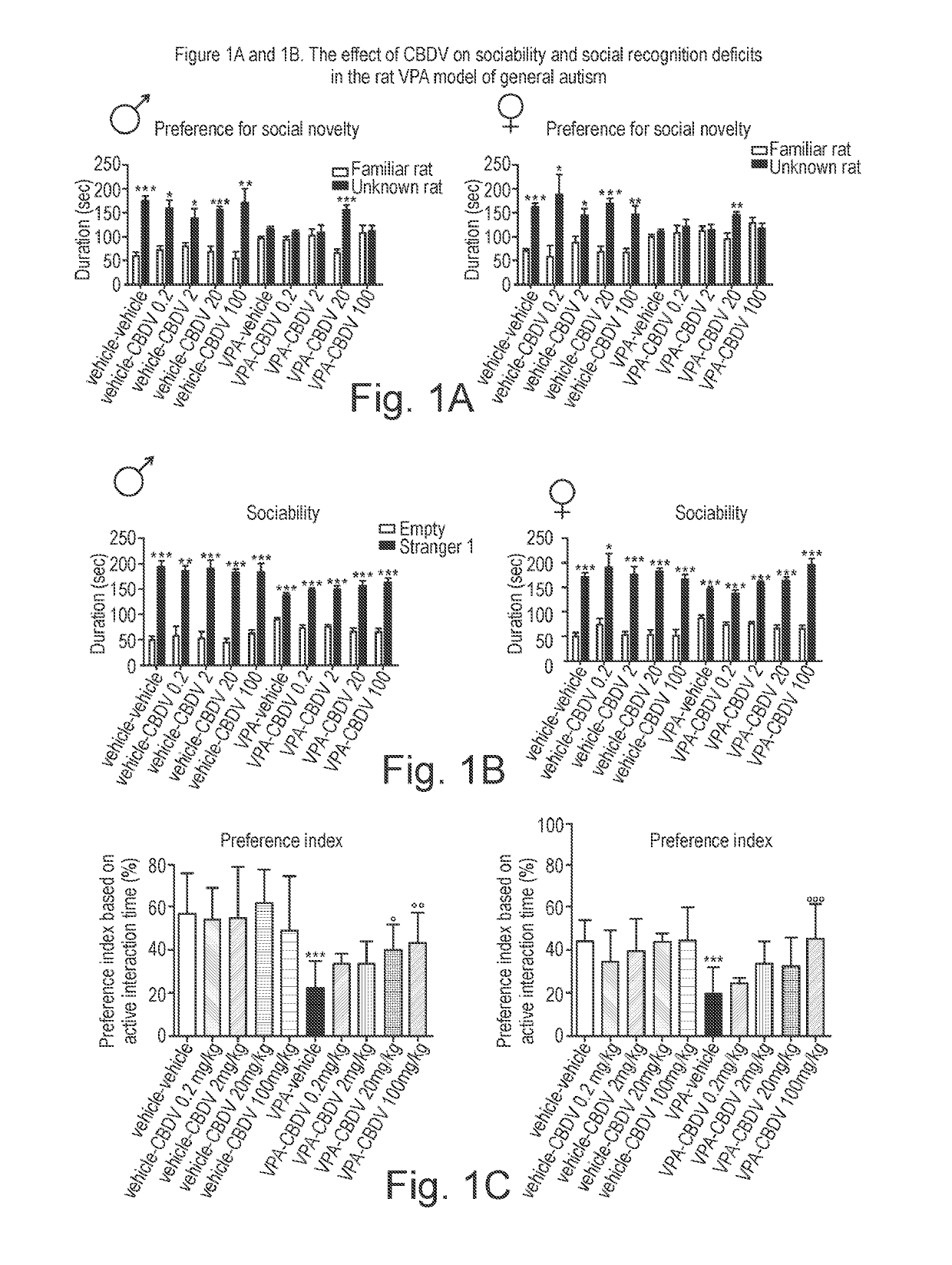
The present invention relates to the use of cannabidivarin (CBDV) in the treatment of autism spectrum disorder (ASD) and ASD-associated disorders such as Fragile X syndrome (FXS); Rett syndrome (RS); or Angelman syndrome (AS). In a further embodiment the invention relates to the use of CBDV in the treatment of schizophrenia. CBDV has been shown to be particularly effective in improving cognitive dysfunction in rodent models of ASD, FXS, RS, AS and schizophrenia. The CBDV is preferably substantially pure. It may take the form of a highly purified extract of cannabis such that the CBDV is present at greater than 95% of the total extract (w / w) and the other components of the extract are characterised. Alternatively, the CBDV is synthetically produced.
Owner:GW RES LTD
Pre-frontal cortex processing disorder speech, gait and limb impairments treatment
ActiveUS10420318B2Easy to controlConsequential diminishment in the speech, gait or limb impairmentAnimal housingHeterocyclic compound active ingredientsCerebral palsiedRett syndrome
Owner:GILROSE PHARMA
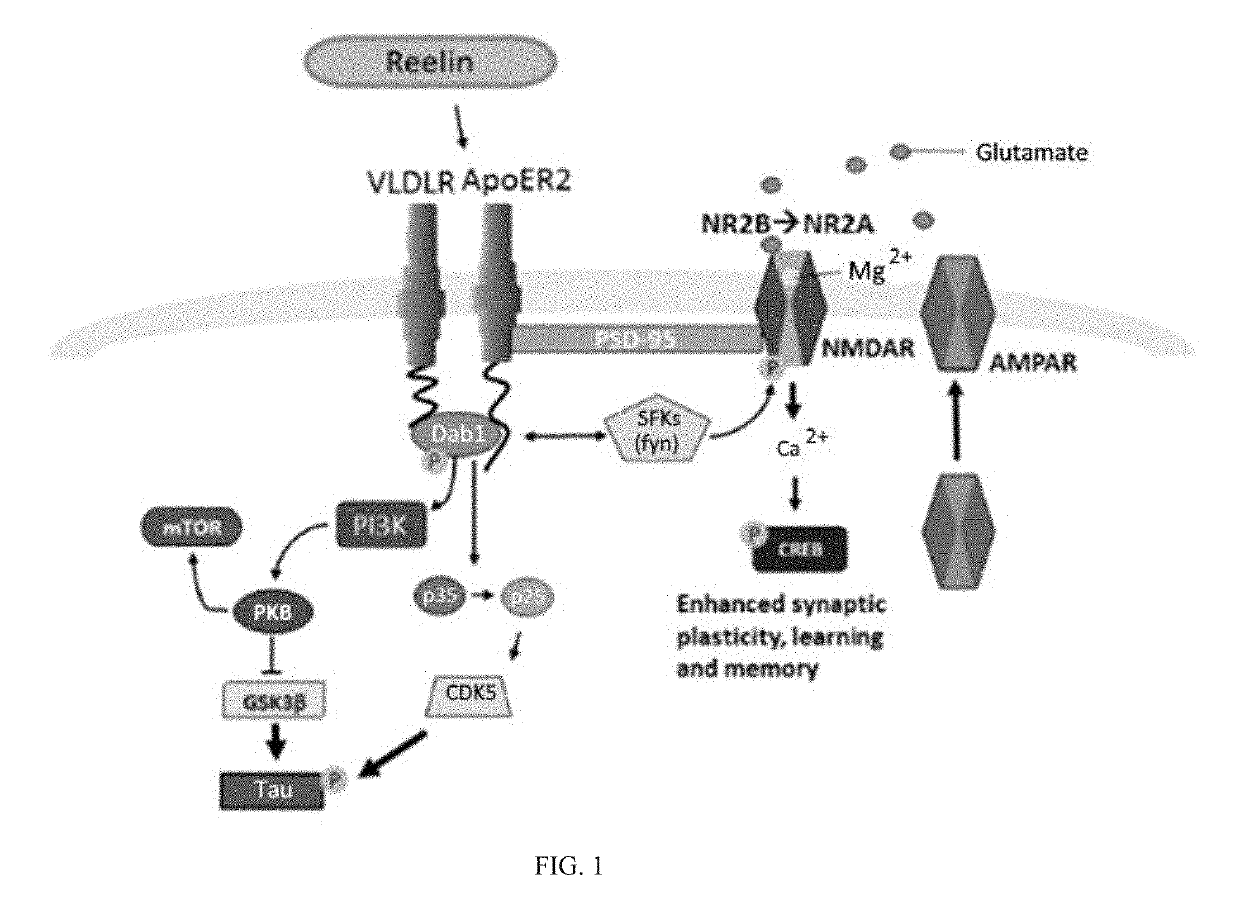
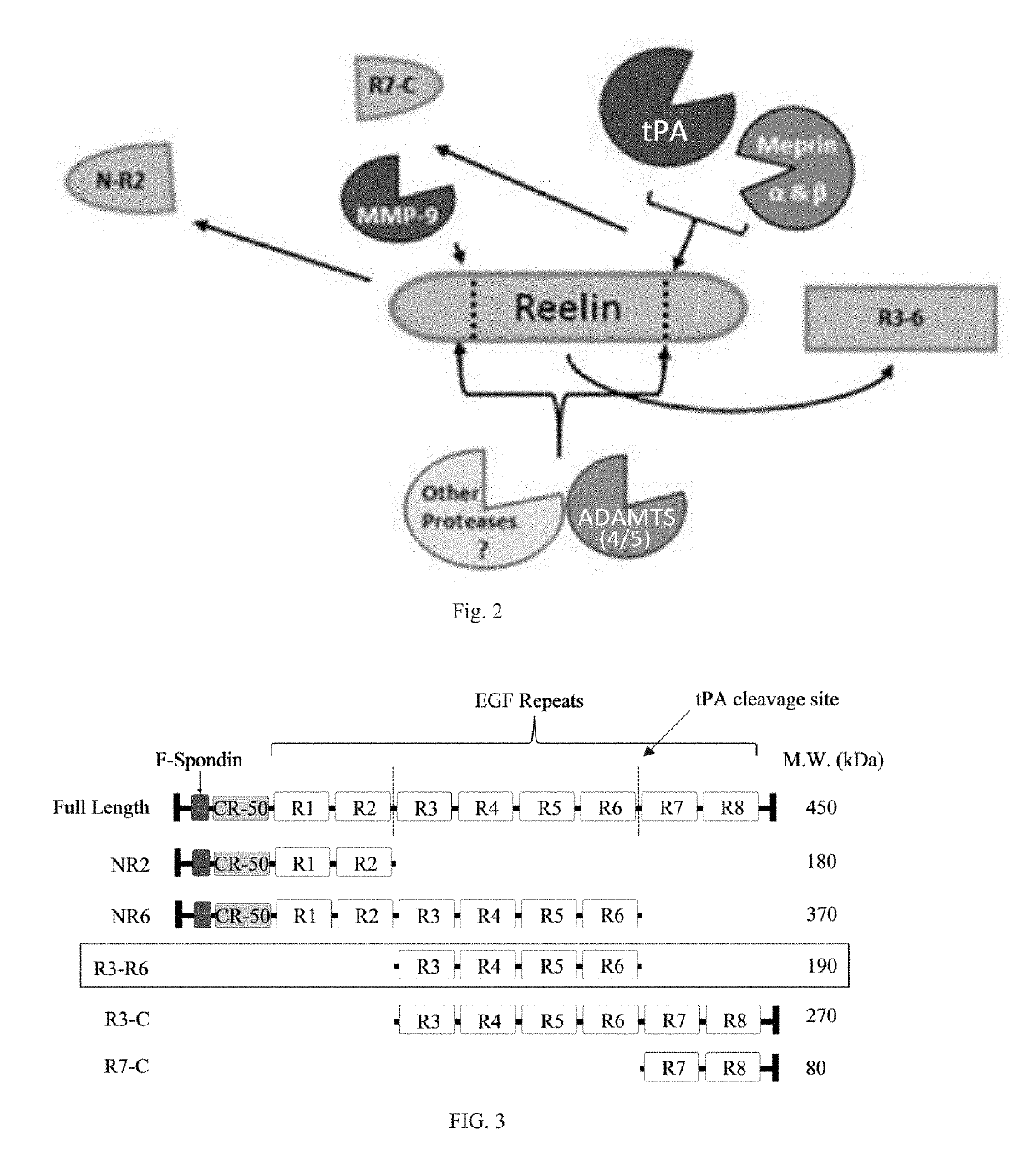
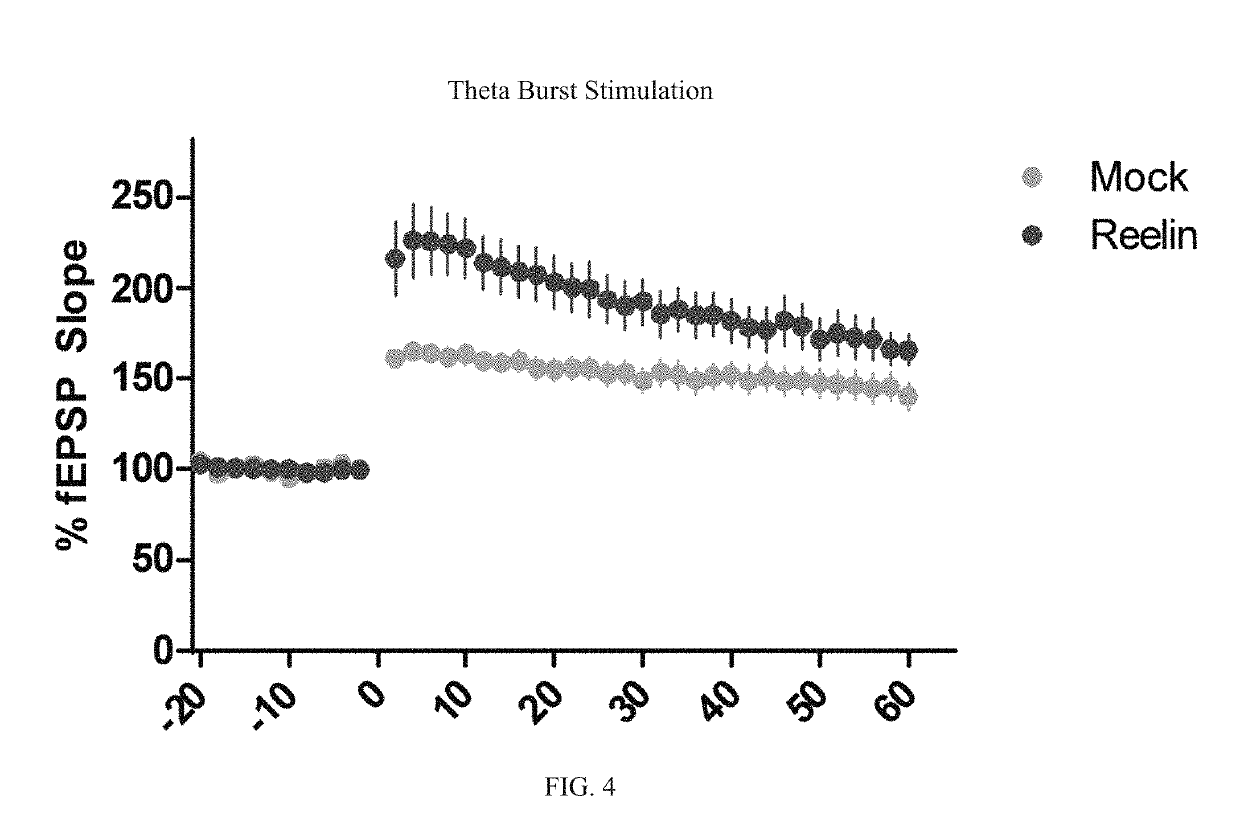
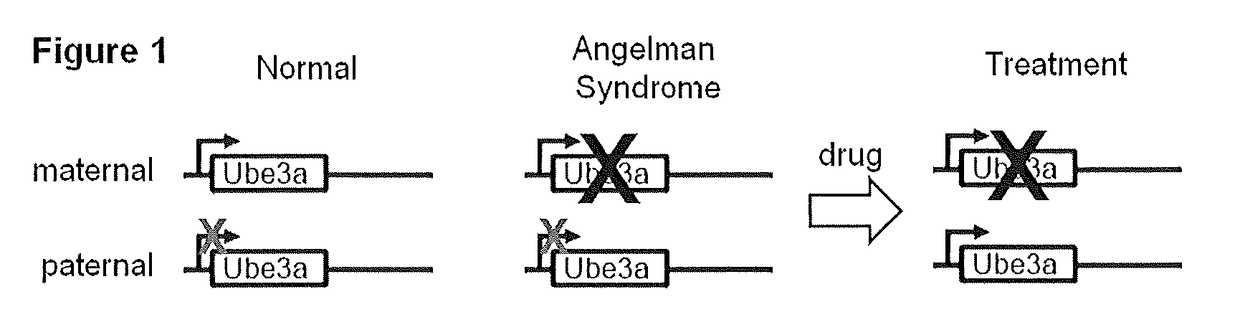
Reelin compositions for treatment of neurological disorders
PendingUS20190169246A1Improve plasticityImprove cognitive functionNervous disorderPeptide/protein ingredientsFragile X chromosomeReelin
Changes in Reelin levels as well as Reelin signaling alter cognitive function. This can be accomplished by administering a therapeutically effective amount of a repeat fragment of Reelin, or a construct formed from fragment repeats of Reelin to a patient or subject. Changes to Reelin levels can be used to treat various neurodegenerative diseases, neuronal insults, or stroke, such as fragile X syndrome, William's syndrome, Rett syndrome, Down's syndrome, Angelman syndrome, autism, ischemia, hypoxia, Alzheimer's disease, and schizophrenia. Reelin can also be used to alter dendritic spine density, diminished long-term potentiation, and diminished synaptic plasticity and associative learning deficits. Constructs formed from repeat region 3 of full length Reelin and repeat region 5 of full length Reelin, or repeat region 3 of full length Reelin and repeat region 6 of full length Reelin have been found particularly useful.
Owner:UNIV OF SOUTH FLORIDA
Methods and compositions for unsilencing imprinted genes
The present invention provides methods and compositions for inducing expression of Ube3a in a cell by contacting the cell with a topoisomerase inhibitor. Particular embodiments include a method of treating a genomic imprinting disorder, such as Angelman syndrome, in a subject by administering to the subject an effective amount of a topoisomerase inhibitor.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
2-[bis(4-fluorophenyl)methyl]-2,7-diazaspiro[4.5]decan-10-one derivatives and related compounds as inhibitors of the human dopamine-active-transporter (DAT) protein for the treatment of e.g. attention deficit disorder (ADD)
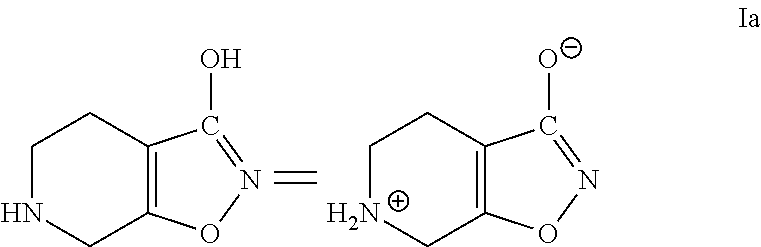
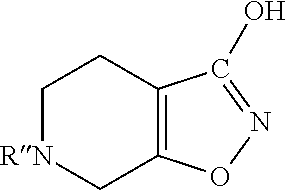
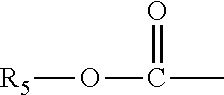
InactiveUS20170260185A1Good choiceUseful in treatmentNervous disorderOrganic chemistrySubstance abuserImpulse control disorder
The present invention provides compounds of formula (I) and in particular 2-[bis(4-fluorophenyl)methyl]-2,7-diazaspiro[4.5]decan-10-one derivatives and related compounds as inhibitors of human dopamine-active-transporter (DAT) protein for the treatment of sexual dysfunction, affective disorders, anxiety, depression, chronic fatigue, Tourette syndrome, Angelman syndrome, attention deficit disorder (ADD), attention deficit hyperactivity disorder (ADHD), obesity, pain, obsessive-compulsive disorder, movement disorders, CNS disorders, sleep disorders, narcolepsy, conduct disorder, substance abuse (including smoking cessation), eating disorders, and impulse control disorders.
Owner:CHRONOS THERAPEUTICS
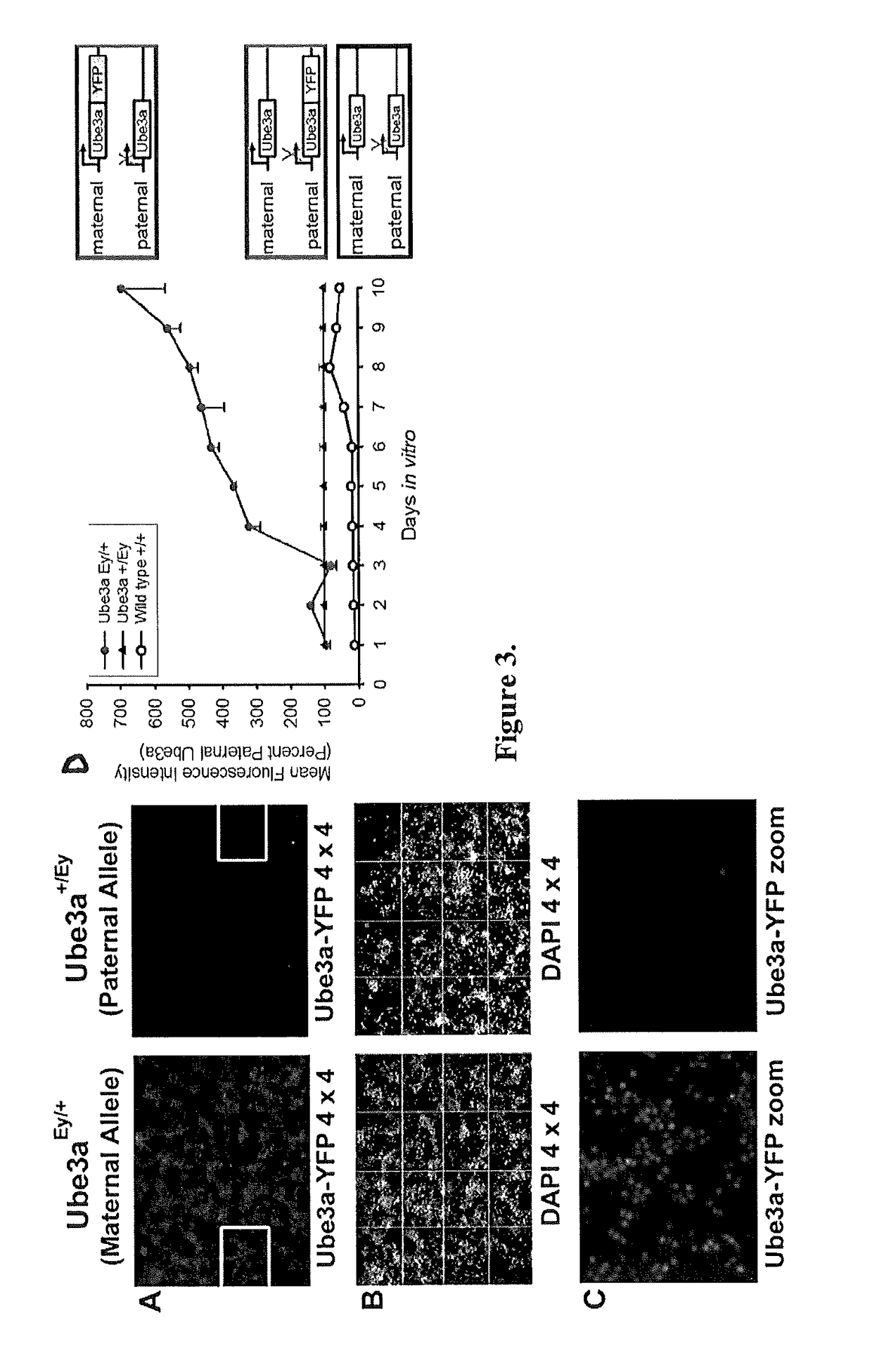
Animal model of angelman syndrome
The present invention concerns non-human animals with cells having a genome that is lacking the entire E3 ubiquitin ligase (Ube3a) gene (including all isoforms and alternative promoters). These animals are useful for modeling Angelman Syndrome. The invention also includes methods for assessing the effect of an agent, such as potential therapeutics, on an animal model by exposing the animal or cells, tissues, or organs isolated therefrom, to an agent of interest.
Owner:UNIV OF SOUTH FLORIDA +2
Methods for treatment of autism spectrum disorders
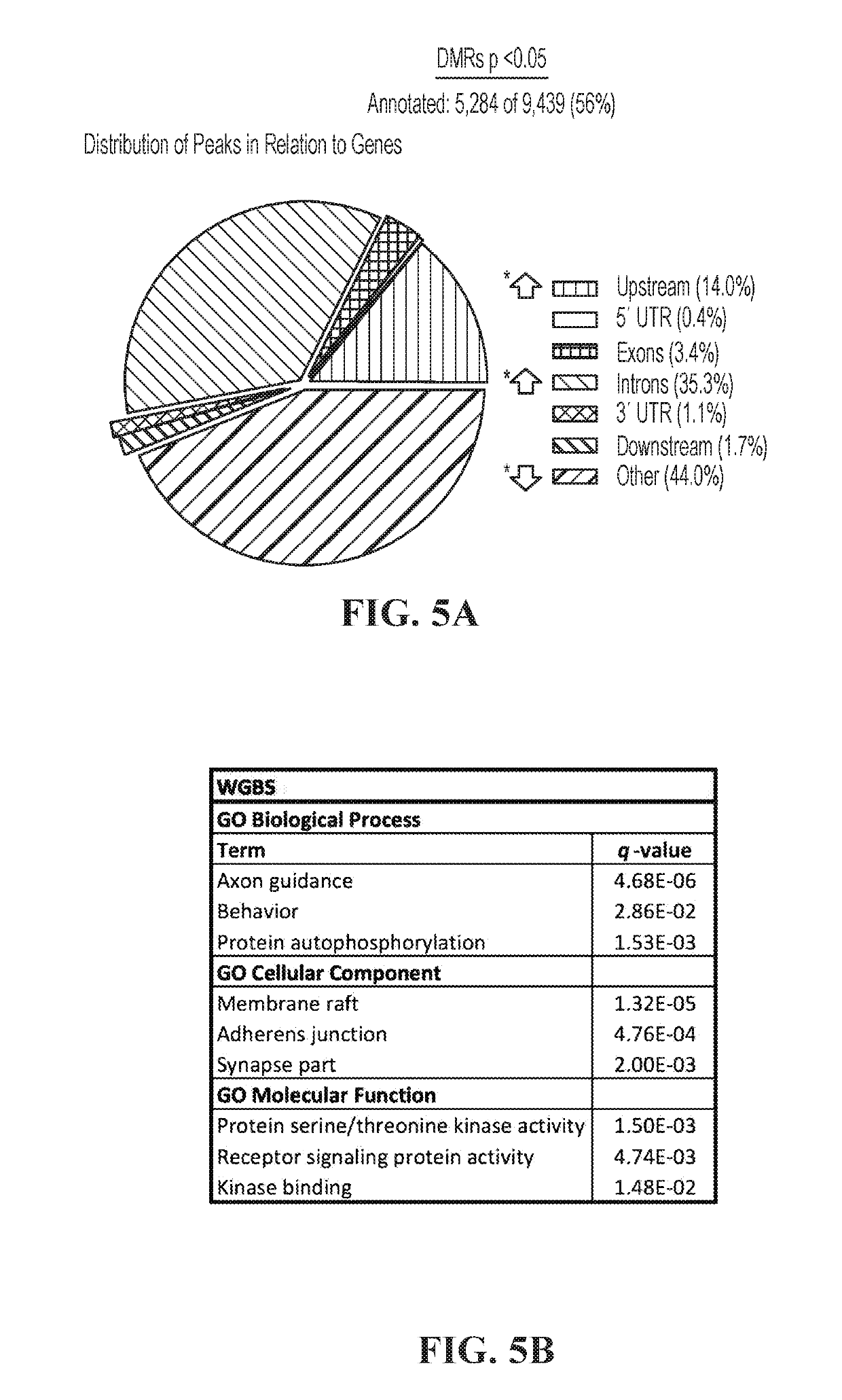
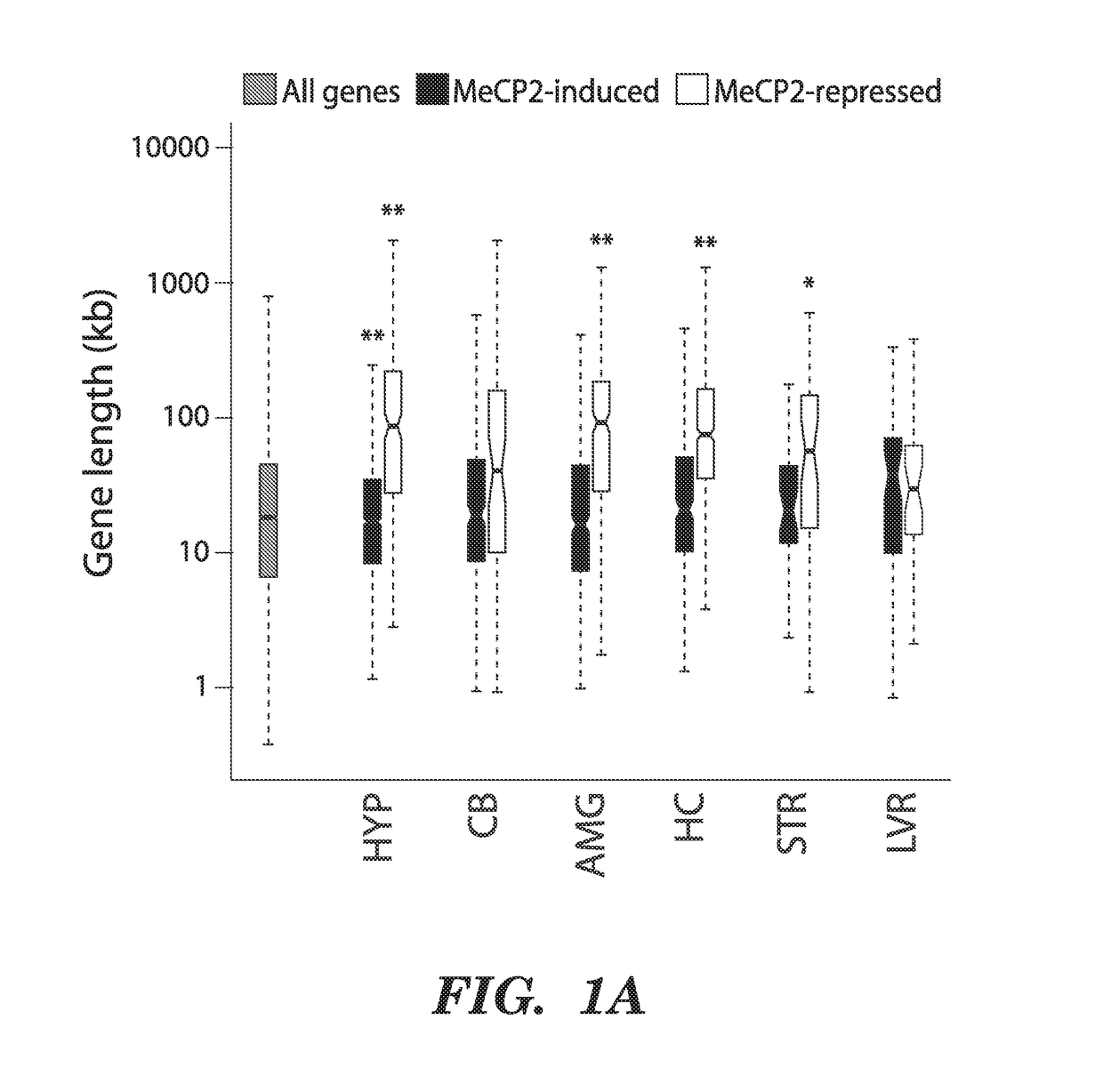
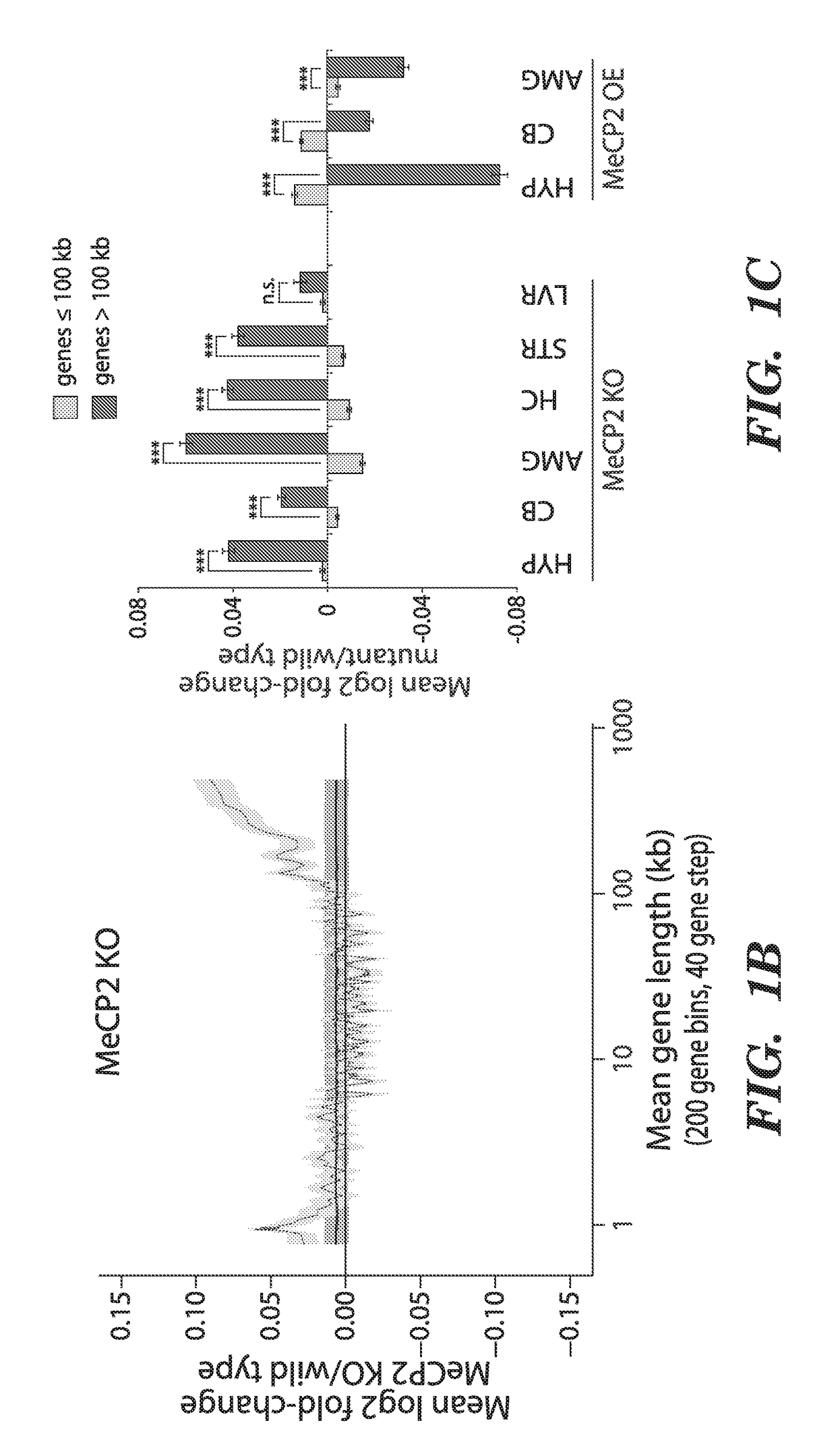
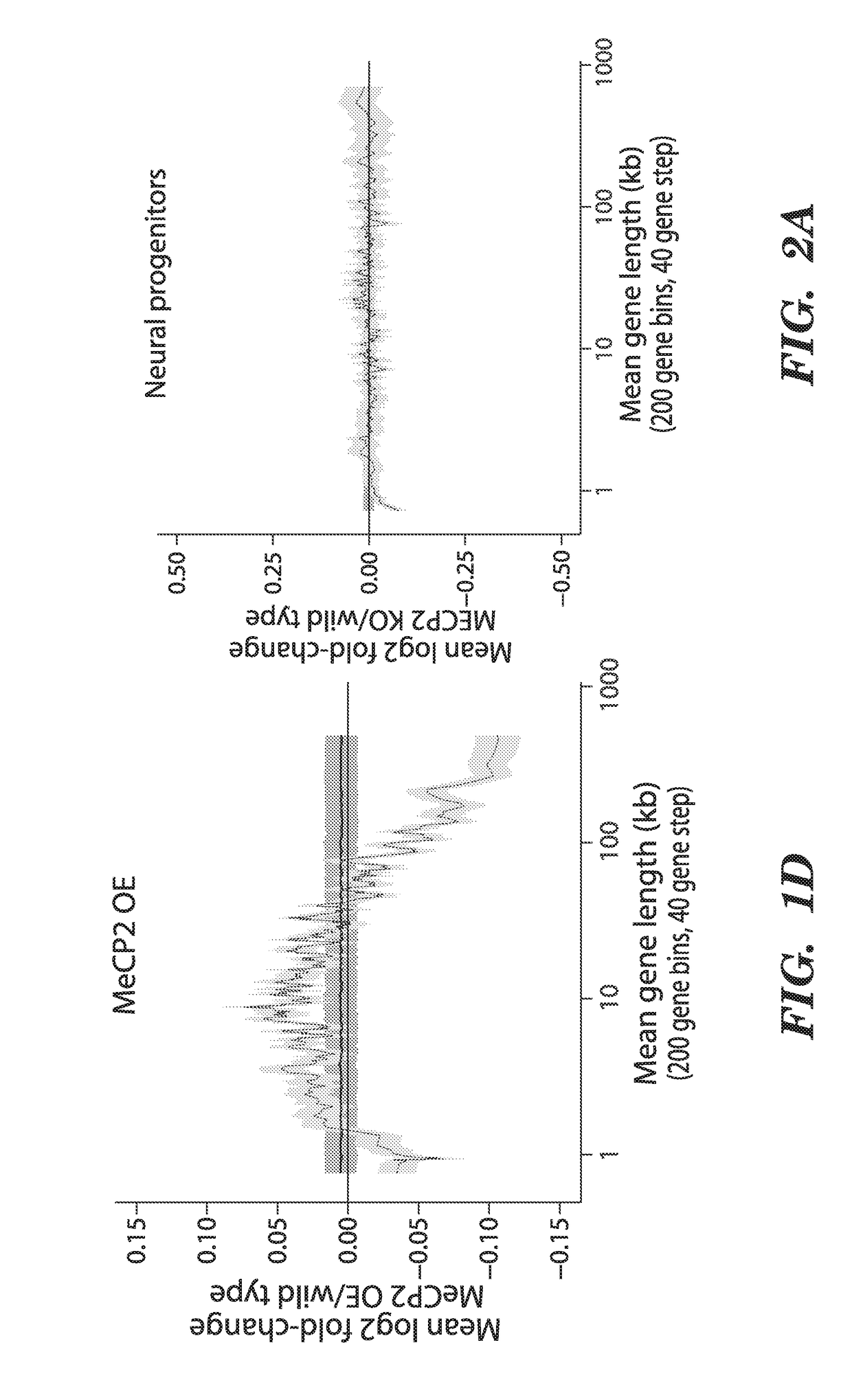
We have determined that MeCP2 protein mediates modulation of long-gene expression in the brain and results in neurological dysfunction associated with autism spectrum disorders, including but not limited to, Fragile X Syndrome, Rett syndrome, and Angelman syndrome (AS). In particular, a lack of MeCP2 protein causes up-regulation of long gene expression in the brain which corresponds with the pathology of Rett syndrome and Fragile X Syndrome, while too much MeCP2 protein results in excessive repression of long gene expression in the brain and pathology related to MeCP2 duplication syndrome. Accordingly, embodiments of the invention are directed to methods for treatment of autism spectrum disorders. The methods involve administration, to a subject, agents that modulate the expression of long genes in the brain.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
2-[bis(4-fluorophenyl)methyl]-2,7-diazaspiro[4.5]decan-10-one derivatives and related compounds as inhibitors of the human dopamine-active-transporter (dat) protein for the treatment of e.g. attention deficit disorder (add)
InactiveCN107001367AOrganic active ingredientsNervous disorderSubstance abuserImpulse control disorder
The present invention provides compounds of formula (I) and in particular 2-[bis(4-fluorophenyl)methyl]-2,7- diazaspiro[4.5]decan-10-one derivatives and related compounds as inhibitors of human dopamine-active-transporter (DAT) protein for the treatment of sexual dysfunction, affective disorders, anxiety, depression, chronic fatigue, Tourette syndrome, Angelman syndrome, attention deficit disorder (ADD), attention deficit hyperactivity disorder (ADHD), obesity, pain, obsessive-compulsive disorder, movement disorders, CNS disorders, sleep disorders, narcolepsy, conduct disorder, substance abuse (including smoking cessation), eating disorders, and impulse control disorders.
Owner:CHRONOS THERAPEUTICS
Methods and compositions for unsilencing imprinted genes
ActiveUS20180016584A1Organic active ingredientsVector-based foreign material introductionTopoisomerase inhibitorCancer research
The present invention provides methods and compositions for inducing expression of Ube3a in a cell by contacting the cell with a topoisomerase inhibitor. Particular embodiments include a method of treating a genomic imprinting disorder, such as Angelman syndrome, in a subject by administering to the subject an effective amount of a topoisomerase inhibitor.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
Use of cannabidivarin in the treatment of autism spectrum disorder, associated disorders and schizophrenia
The present invention relates to the use of cannabidivarin (CBDV) in the treatment of autism spectrum disorder (ASD) and ASD-associated disorders such as Fragile X syndrome (FXS); Rett syndrome (RS); or Angelman syndrome (AS). In a further embodiment the invention relates to the use of CBDV in the treatment of schizophrenia. CBDV has been shown to be particularly effective in improving cognitive dysfunction in rodent models of ASD, FXS, RS, AS and schizophrenia. The CBDV is preferably substantially pure. It may take the form of a highly purified extract of cannabis such that the CBDV is present at greater than 95% of the total extract (w / w) and the other components of the extract are characterised. Alternatively, the CBDV is synthetically produced.
Owner:GW RES LTD
Methods of treatment and/or prevention of disorders and symptoms related to bkca and/or sk channelophathies
PendingUS20220313664A1Nervous disorderTetracycline active ingredientsNeuro-degenerative diseaseSpectrum disorder
The present invention relates to compounds and compositions for use in a method of treating and / or preventing neurological or non-neurological channelopathies, i.e., pathologies which are caused specifically by defects in some of the calcium-activated potassium channels. Specific neurological channelopathies and / or synaptopathies targeted by the present invention include specific neuropsychiatric pathologies, such as particularly Fragile X Syndrome (FXS), Angelman syndrome, Williams-Beuren syndrome, autism, Autism spectrum disorders (ASDs), hyperacusis, Smith-Magenis syndrome, Prader-Willi syndrome, 7q11.23 duplication syndrome, and cri-du-chat syndrome, as well as other neuropathic disorders such as trembling, epilepsy and neuropathic pains associated and neurodegenerative diseases such as Parkinson's and Alzheimer diseases. Compounds, compositions and methods of the present invention are also particularly useful and efficient in alleviating neurological symptoms common to these neuropsychiatric pathologies.
Owner:UNIV DE BORDEAUX +2
Gene chip for detecting Angelman syndromes, using method and kit for gene chip
ActiveCN102559872AEasy to operateHigh detection specificityMicrobiological testing/measurementPrenatal diagnosisNucleotide
The invention relates to a gene chip for detecting Angelman syndromes, and a using method and a kit for the gene chip. The gene chip comprises a solid-phase carrier and a probe fixed to the solid-phase carrier, wherein the oligonucleotide probe is a nucleotide sequence shown as SEQ ID NO. 1-50. The using method comprises the following steps of: (a) preparing a deoxyribonucleic acid (DNA) fragment of a sample; (b) marking the DNA fragment by using fluorescence; (c) eluting the marked product; (d) hybridizing the marked product and the gene chip; and (e) scanning and detecting a hybridization signal of the gene chip to obtain a result. The invention also relates to the kit for the gene chip. The gene chip has simple operating steps and is high in detection specificity and stability and low in cost, and the scanned result extracted from the sample can be completed within one working day, so the gene chip is low in cost and suitable for the genetic mutation detection of clinical patients, prenatal diagnosis and the detection of normal human heterozygote carriers.
Owner:SHANGHAI BIOGERM MEDICAL TECH CO LTD
Use of cannabidiolic acid in the treatment of autism spectrum disorder and associated disorders
The present invention relates to the use of cannabidiolic acid (CBDA) in the treatment of autism spectrum disorder (ASD) and ASD-associated disorders, such as Fragile X syndrome (FXS); Rett syndrome (RS); or Angelman syndrome (AS). CBDA has been shown to be particularly effective in improving cognitive dysfunction in rodent models of ASD, FXS, RS and AS. The CBDA is preferably substantially pure. It may take the form of a highly purified extract of cannabis such that the CBDA is present at greater than 95% of the total extract (w / w) and the other components of the extract are characterised. Alternatively, the CBDA is synthetically produced.
Owner:GW RES LTD
Traditional Chinese medicine for treating Angelman syndrome
InactiveCN107823461AAccurate analysisCorrect matchHeavy metal active ingredientsAnthropod material medical ingredientsCurative effectOfficinalis
The invention discloses a traditional Chinese medicine for treating an Angelman syndrome, and belongs to the technical field of the traditional Chinese medicine. The clinicopathologic analysis and therapeutic problems of the Angelman syndrome are solved. Analyzed by the traditional Chinese medicine viewpoint, the Angelman syndrome belongs to the inherent shortage, deficiency of the kidney and vital energy, pathogenic influence and heart spirit confused by phlegm. The traditional Chinese medicines of tonifying kidney and Qi, invigorating the circulation of blood and dispelling wind, and dissipating phlegm for resuscitation are combined as a therapeutic drug. The traditional Chinese medicine for treating the Angelman syndrome is mainly prepared by the following medicines by weight: 15 partsof fructus trichosanthis, 12 parts of bombyx batryticatus, 15 parts of the root of red-rooted salvia, 12 parts of rhizoma gastrodiae, 15 parts of radix polygonati officinalis, 15 parts of rehmannia glutinosa libosch, 13 parts of radix achyranthis bidentatae, 20 parts of crude dragon bone, 20 parts of crude ruddle, 25 parts of concha ostreae, 10 parts of uncaria, and 8 parts of rhizoma acori graminei. The traditional Chinese medicine for treating the Angelman syndrome is capable of creating a positive solution theory and a therapeutic solution of the traditional Chinese medicine for correctly analyzing the Angelman syndrome, and getting the remarkable curative effect.
Owner:防城港市蓝瀚达科技有限公司
Methods of Treating Seizure Disorders
InactiveUS20130225671A1Reduces AGS severityReduce incidenceBiocideNervous disorderNeurofibromatosis type IFragile X chromosome
Seizure disorders are treated by Ras inhibitors. In an embodiment, the Ras inhibitor includes a farnesyl transferase inhibitor. In another embodiment, the farnesyl transferase inhibitor includes a 3-hydroxy-3-methylglutaryl-Coenzyme A reductase inhibitor. In another embodiment, the 3-hydroxy-3-methylglutaryl-Coenzyme A reductase inhibitor is not a Ras inhibitor. Subjects having fragile X syndrome, autism, Angelman syndrome, Costello syndrome, cardio facio cutaneous syndrome, neurofibromatosis type I, Noonan syndrome and Coffin-Lowry syndrome that have seizure disorders can be treated.
Owner:MASSACHUSETTS INST OF TECH
Pre-frontal cortex processing disorder, gait and limb impairment treatment
A methylphenidate, particularly including dextro-threo-methylphenidate, is administered to a subject to treat a gait or limb impairment secondary to a genetically acquired pre-frontal cortex processing disease or disorder, particularly including multiple sclerosis, cerebral palsy, Angelman syndrome, Rett syndrome and Fragile-X syndrome.
Owner:GILROSE PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![2-[bis(4-fluorophenyl)methyl]-2,7-diazaspiro[4.5]decan-10-one derivatives and related compounds as inhibitors of the human dopamine-active-transporter (DAT) protein for the treatment of e.g. attention deficit disorder (ADD) 2-[bis(4-fluorophenyl)methyl]-2,7-diazaspiro[4.5]decan-10-one derivatives and related compounds as inhibitors of the human dopamine-active-transporter (DAT) protein for the treatment of e.g. attention deficit disorder (ADD)](https://images-eureka.patsnap.com/patent_img/68a634a7-23d1-4986-b3f4-d8b07a0017a5/US20170260185A1-20170914-C00001.png)
![2-[bis(4-fluorophenyl)methyl]-2,7-diazaspiro[4.5]decan-10-one derivatives and related compounds as inhibitors of the human dopamine-active-transporter (DAT) protein for the treatment of e.g. attention deficit disorder (ADD) 2-[bis(4-fluorophenyl)methyl]-2,7-diazaspiro[4.5]decan-10-one derivatives and related compounds as inhibitors of the human dopamine-active-transporter (DAT) protein for the treatment of e.g. attention deficit disorder (ADD)](https://images-eureka.patsnap.com/patent_img/68a634a7-23d1-4986-b3f4-d8b07a0017a5/US20170260185A1-20170914-C00002.png)
![2-[bis(4-fluorophenyl)methyl]-2,7-diazaspiro[4.5]decan-10-one derivatives and related compounds as inhibitors of the human dopamine-active-transporter (DAT) protein for the treatment of e.g. attention deficit disorder (ADD) 2-[bis(4-fluorophenyl)methyl]-2,7-diazaspiro[4.5]decan-10-one derivatives and related compounds as inhibitors of the human dopamine-active-transporter (DAT) protein for the treatment of e.g. attention deficit disorder (ADD)](https://images-eureka.patsnap.com/patent_img/68a634a7-23d1-4986-b3f4-d8b07a0017a5/US20170260185A1-20170914-C00003.png)
![2-[bis(4-fluorophenyl)methyl]-2,7-diazaspiro[4.5]decan-10-one derivatives and related compounds as inhibitors of the human dopamine-active-transporter (dat) protein for the treatment of e.g. attention deficit disorder (add) 2-[bis(4-fluorophenyl)methyl]-2,7-diazaspiro[4.5]decan-10-one derivatives and related compounds as inhibitors of the human dopamine-active-transporter (dat) protein for the treatment of e.g. attention deficit disorder (add)](https://images-eureka.patsnap.com/patent_img/5b6fdd24-358e-4d3b-b170-d679916f0f4f/BDA0001275465780000061.png)
![2-[bis(4-fluorophenyl)methyl]-2,7-diazaspiro[4.5]decan-10-one derivatives and related compounds as inhibitors of the human dopamine-active-transporter (dat) protein for the treatment of e.g. attention deficit disorder (add) 2-[bis(4-fluorophenyl)methyl]-2,7-diazaspiro[4.5]decan-10-one derivatives and related compounds as inhibitors of the human dopamine-active-transporter (dat) protein for the treatment of e.g. attention deficit disorder (add)](https://images-eureka.patsnap.com/patent_img/5b6fdd24-358e-4d3b-b170-d679916f0f4f/BDA0001275465780000101.png)
![2-[bis(4-fluorophenyl)methyl]-2,7-diazaspiro[4.5]decan-10-one derivatives and related compounds as inhibitors of the human dopamine-active-transporter (dat) protein for the treatment of e.g. attention deficit disorder (add) 2-[bis(4-fluorophenyl)methyl]-2,7-diazaspiro[4.5]decan-10-one derivatives and related compounds as inhibitors of the human dopamine-active-transporter (dat) protein for the treatment of e.g. attention deficit disorder (add)](https://images-eureka.patsnap.com/patent_img/5b6fdd24-358e-4d3b-b170-d679916f0f4f/BDA0001275465780000111.png)