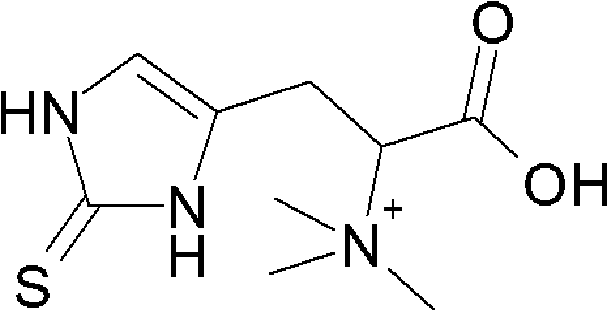
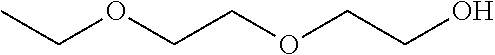
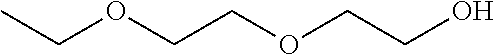
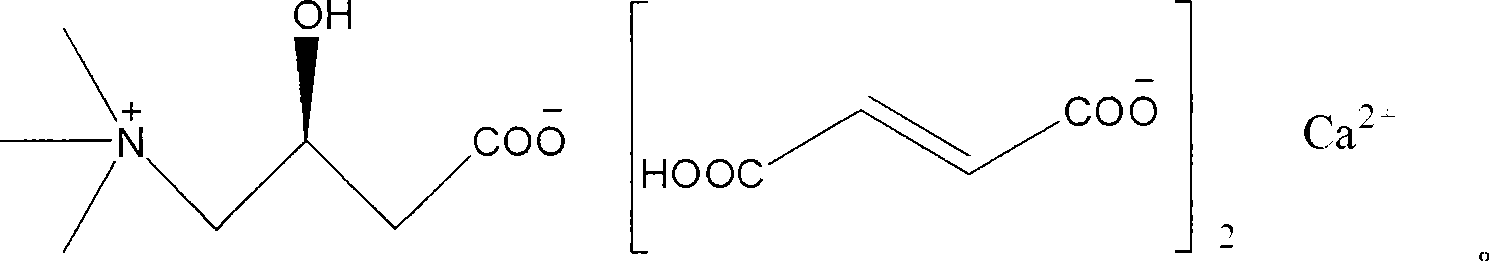
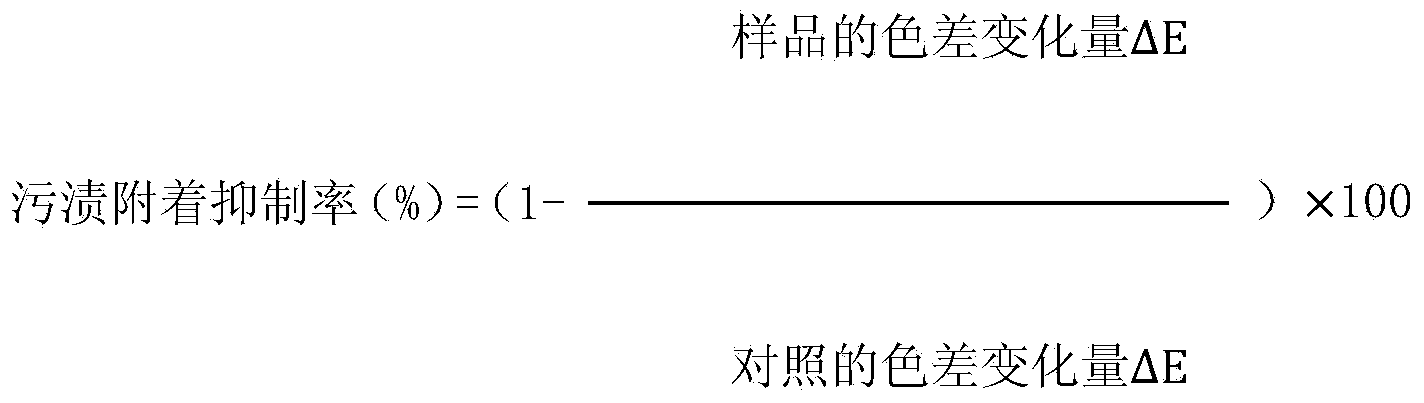
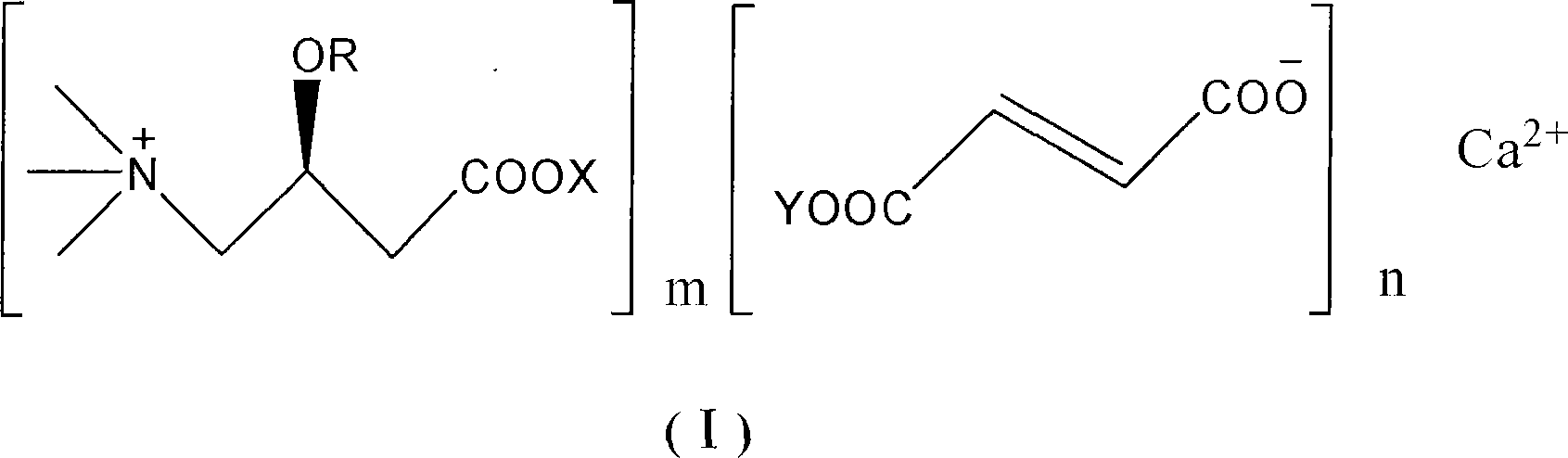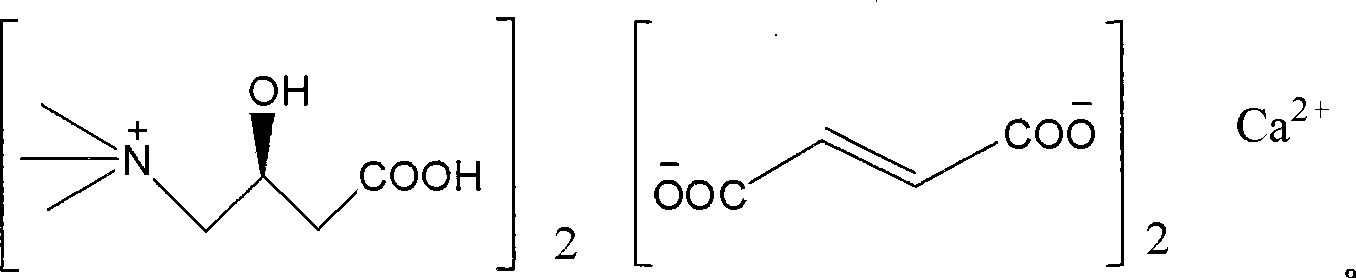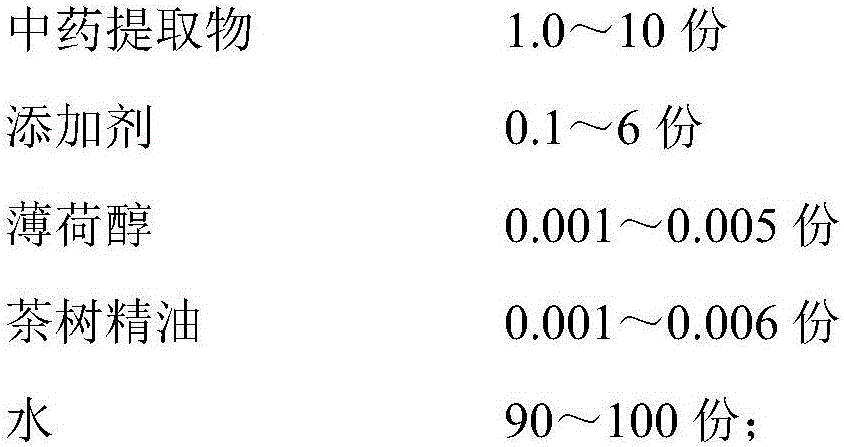Patents
Literature
119 results about "Liquid oral" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Substituted benzimidazole dosage forms and method of using same
InactiveUS7399772B2Easy to prepareImprove pharmacological activityBiocidePowder deliveryEffervescent PowderPharmaceutical formulation
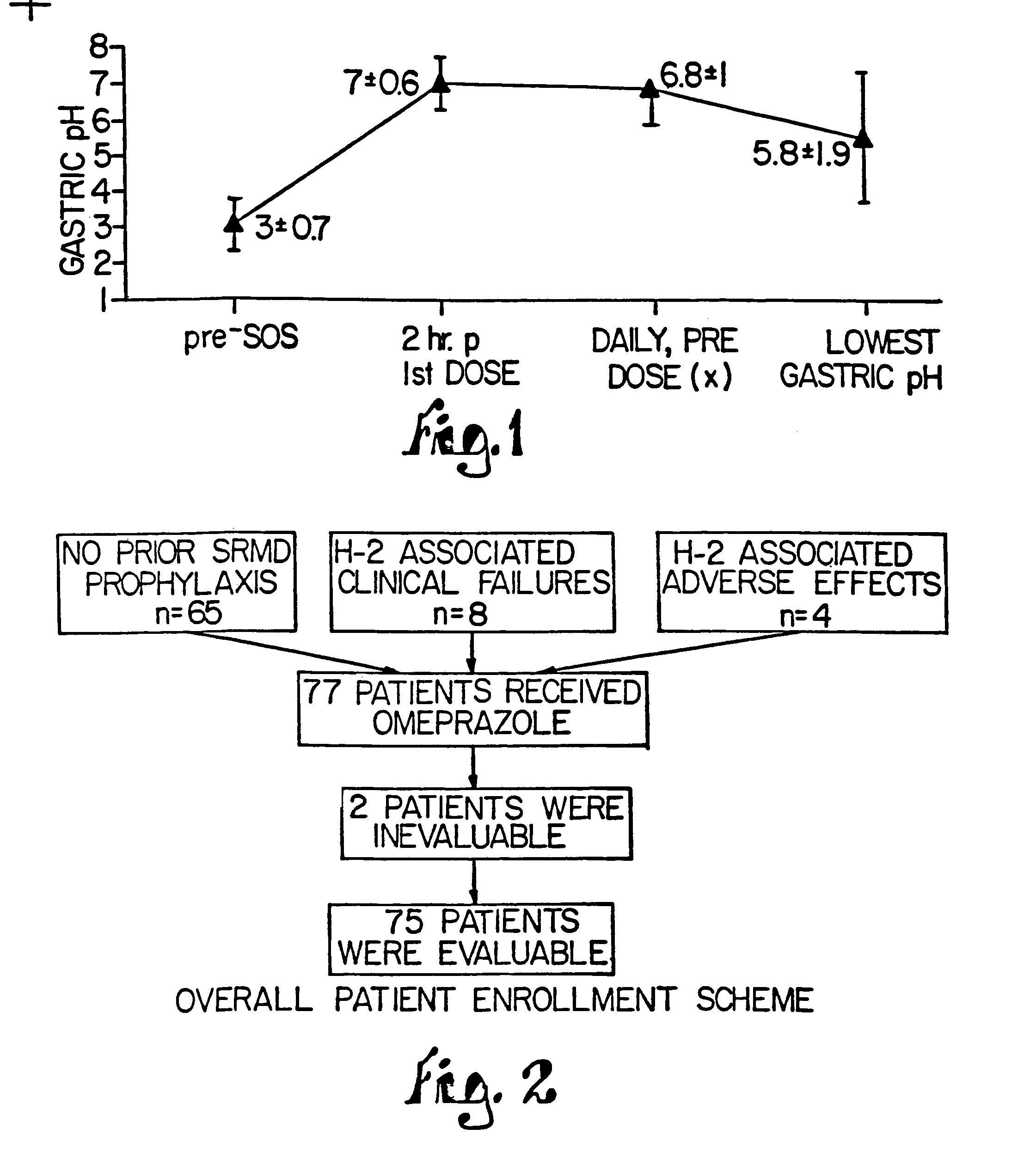
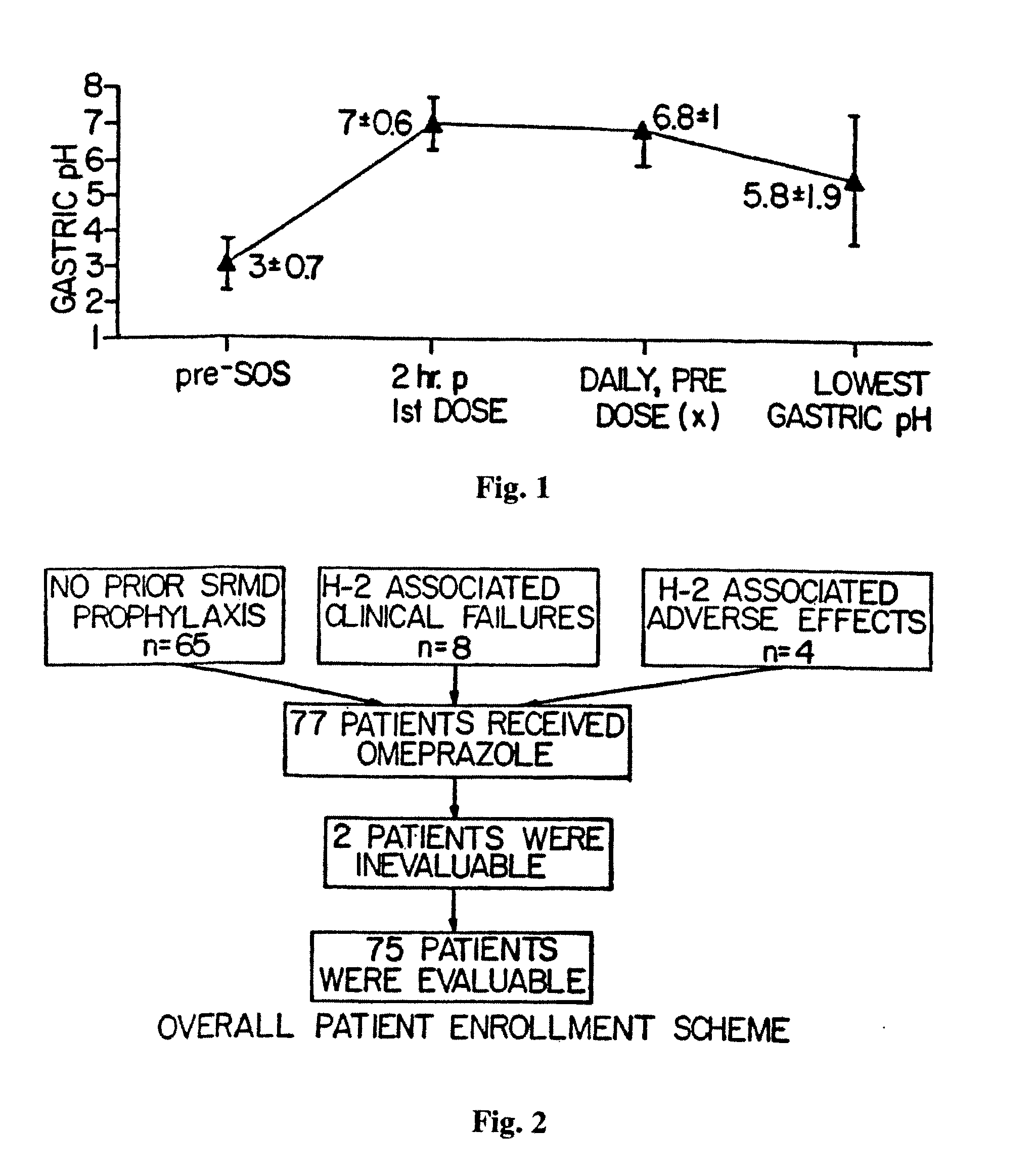
The present invention relates to pharmaceutical preparations comprising substituted benzimidazole proton pump inhibitors. There is provided a liquid or solid pharmaceutical composition consisting of a proton pump inhibitor and at least one buffering agent. Also provided is a pharmaceutical composition further comprising a parietal cell activator, an anti-foaming agent, a flavoring agent and combinations thereof; a method for treating acid-related gastrointestinal disorders by administering a solid pharmaceutical composition; and, a kit for the preparation of a liquid oral pharmaceutical composition. Dosage forms include: liquid, powder, tablet, capsule, effervescent powder, effervescent tablet, pellets, and granules.
Owner:UNIVERSITY OF MISSOURI
Compositions of pharmaceutical actives containing diethylene glycol monoethyl ether or other alkyl derivatives
InactiveUS20140296191A1Less viscousLess denseBiocideOrganic chemistryDiethylene glycol monoethyl etherNasal spray
The present invention relates to pharmaceutical compositions of various pharmaceutical actives, especially lyophilic and hydrophilic actives containing Diethylene glycol monoethyl ether or other alkyl derivatives thereof as a primary vehicle and / or to pharmaceutical compositions utilizing Diethylene glycol monoethyl ether or other alkyl derivatives thereof as a primary vehicle or as a solvent system in preparation of such pharmaceutical compositions. The pharmaceutical compositions of the present invention are safe, non-toxic, exhibits enhanced physical stability compared to conventional formulations containing such pharmaceutical actives and are suitable for use as injectables for intravenous and intramuscular administration, as well as for use as a preformed solution / liquid for filling in and preparation of capsules, tablets, nasal sprays, gargles, dermal applications, gels, topicals, liquid oral dosage forms and other dosage forms.
Owner:THEMIS MEDICARE LTD
Novel substituted benzimidazole dosage forms and method of using same
InactiveUS20050042304A1Easy to prepareImprove pharmacological activityPowder deliveryBiocideATPaseOral suspensions
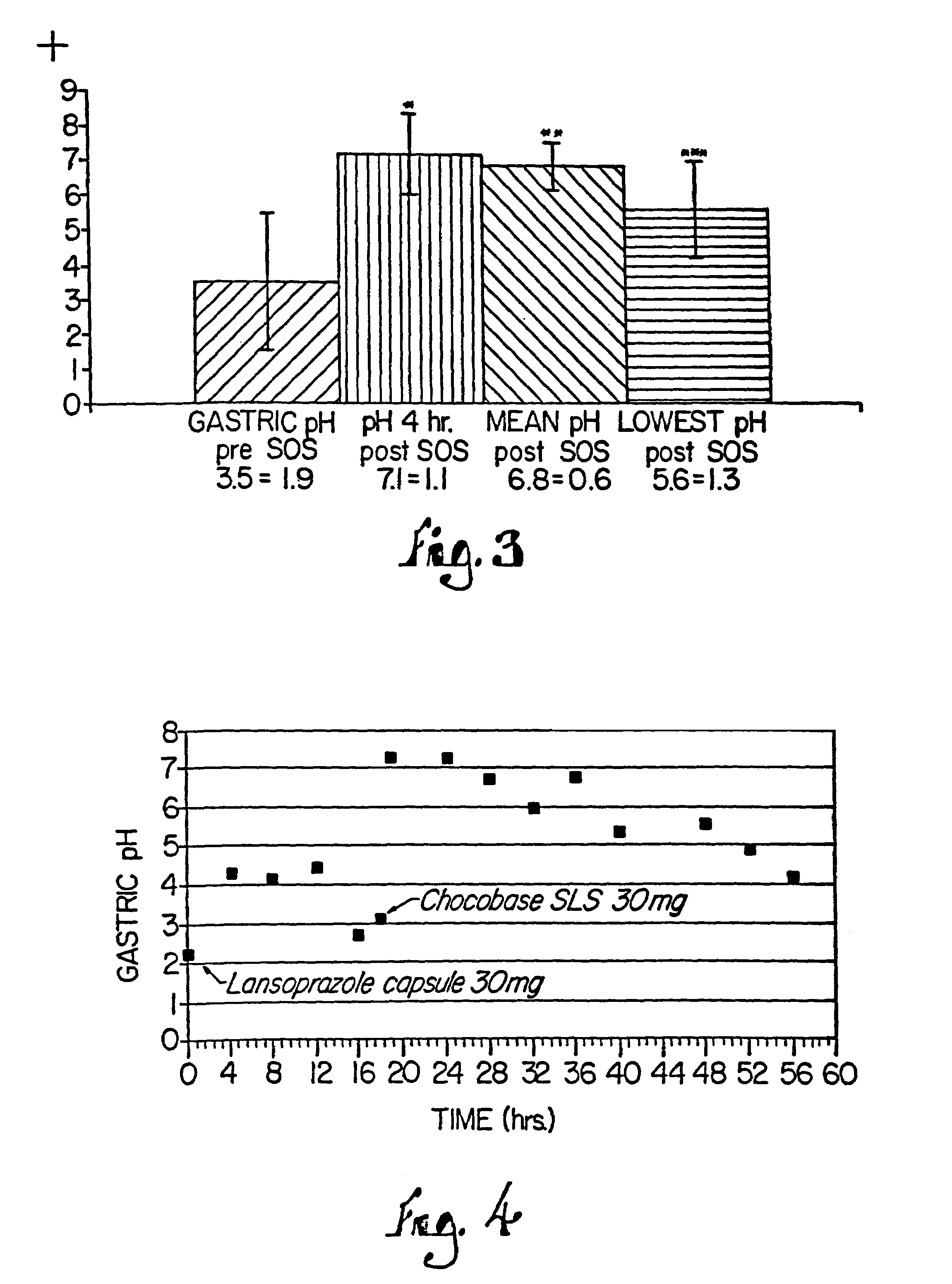
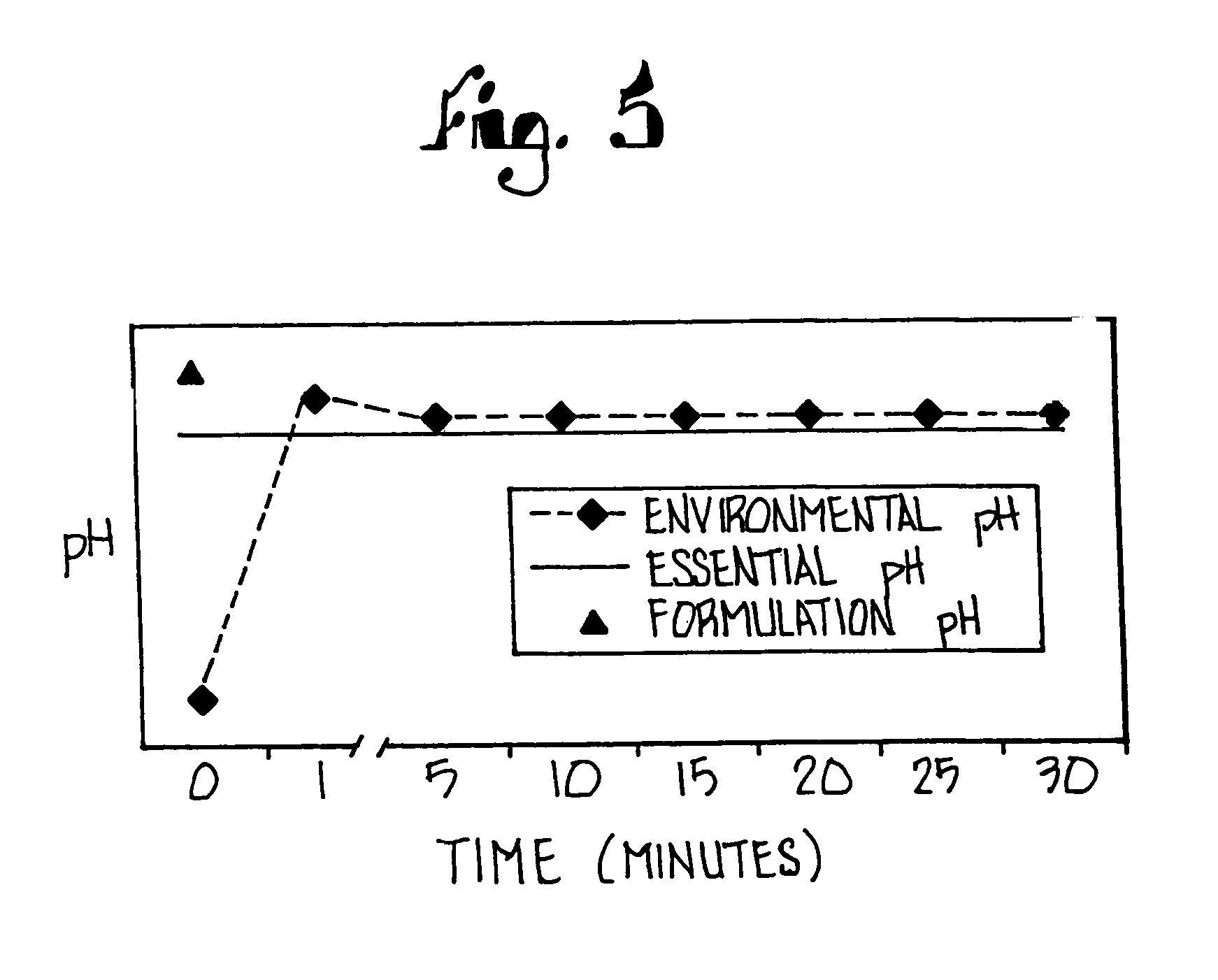
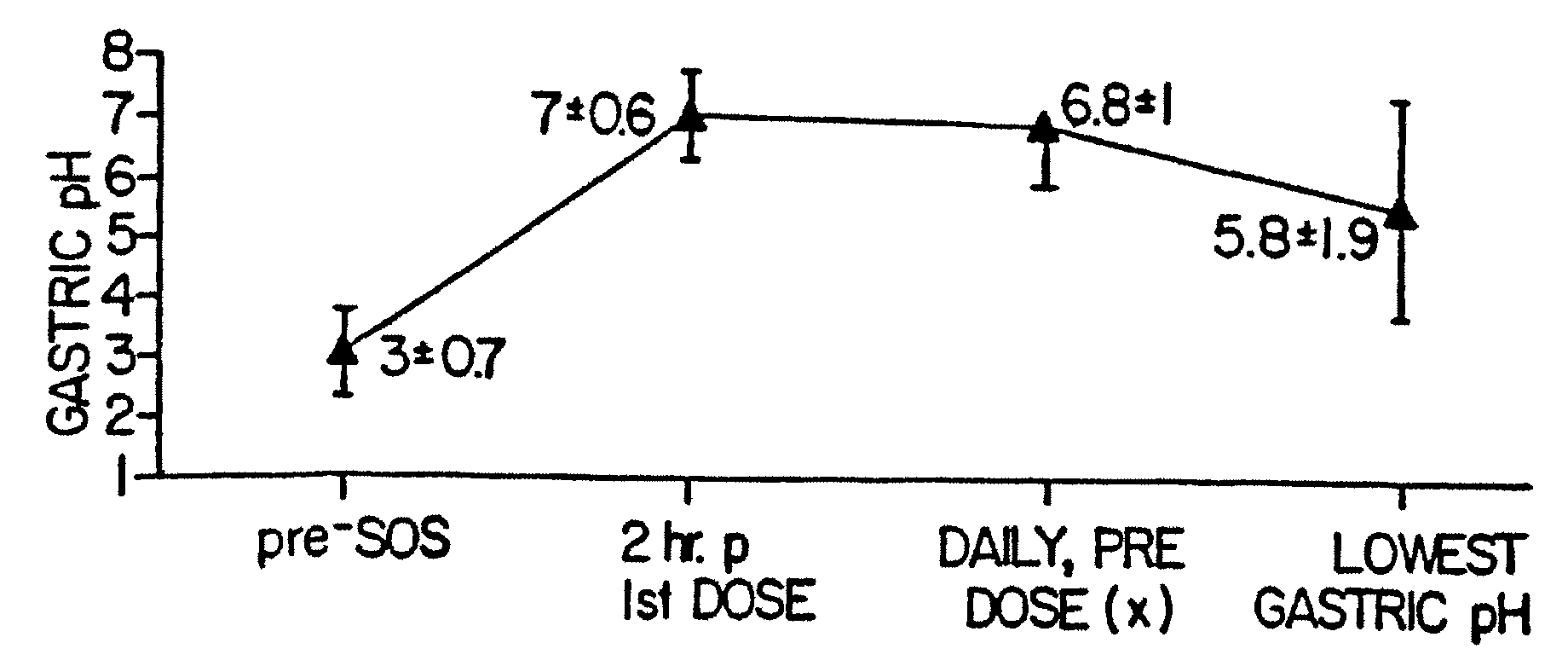
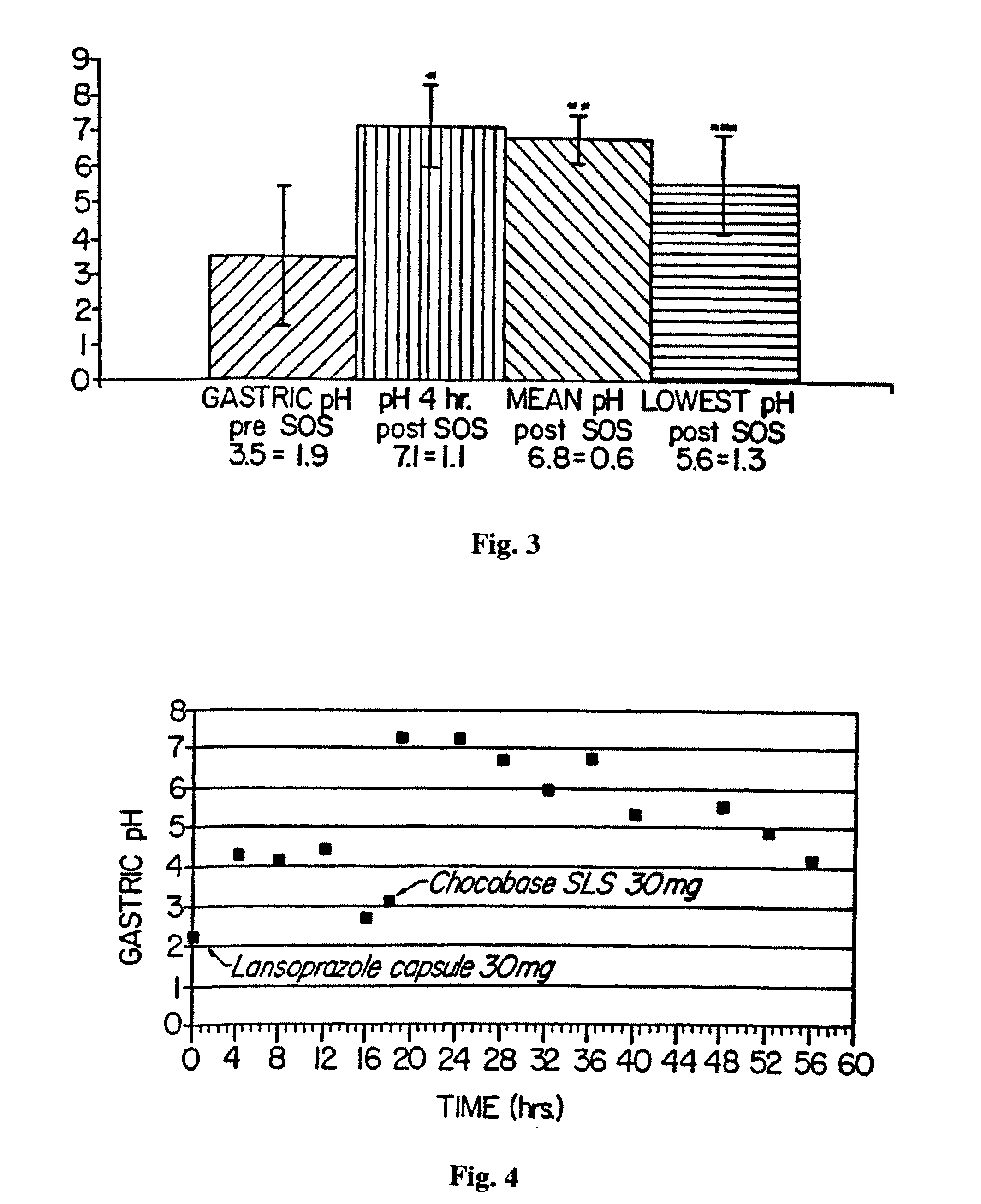
A method of treating gastric acid disorders by administering to a patient a pharmaceutical composition comprising a proton pump inhibitor (PPI) in a pharmaceutically acceptable carrier. The present invention provides an oral solution / suspension comprising a proton pump inhibitor and at least one buffering agent. The PPI can be any substituted benzimidazole compound having H+,K+-ATPase inhibiting activity and being unstable to acid. Omeprazole and lansoprazole are the preferred PPIs for use in oral suspensions in concentrations of at least greater than 1.2 mg / ml and 0.3 mg, respectively. The liquid oral compositions can be further comprised of parietal cell activators, anti-foaming agents and / or flavoring agents. The inventive compositions can alternatively be formulated as a powder, tablet, suspension tablet, chewable tablet, capsule, effervescent powder, effervescent tablet, pellets and granules. Such dosage forms are advantageously devoid of any enteric coating or delayed or sustained-release delivery mechanisms, and comprise a PPI and at least one buffering agent to protect the PPI against acid degradation. Similar to the liquid dosage form, the dry forms can further include anti-foaming agents, parietal cell activators and flavoring agents. Kits utilizing the inventive dry dosage forms are also disclosed herein to provide for the easy preparation of a liquid composition from the dry forms. In accordance with the present invention, there is further provided a method of treating gastric acid disorders by administering to a patient a pharmaceutical composition comprising a proton pump inhibitor in a pharmaceutically acceptable carrier and at least one buffering agent wherein the administering step comprises providing a patient with a single dose of the composition without requiring further administering of the buffering agent. Additionally, the present invention relates to a method for enhancing the pharmacological activity of an intravenously administered proton pump inhibitor in which at least one parietal cell activator is orally administered to the patient before, during or after the intravenous administration of the proton pump inhibitor.
Owner:UNIVERSITY OF MISSOURI
Taste masked pharmaceutical compositions comprising bitter drug and pH sensitive polymer
InactiveUS20050136114A1Improve palatabilityA large amountPowder deliveryDispersion deliveryPH-sensitive polymersLiquid oral
The present invention discloses pharmaceutical compositions comprising of pH sensitive polymers used for taste masking highly bitter drugs. The pH sensitive polymer acts as a reverse enteric coating, which is soluble in the acidic pH range 1.0 to 3.0 normally found in the stomach but is insoluble in the pH range 3.5 to 7 thus inhibiting the release of the bitter drug at the pH of saliva and also at the pH of reconstitution medium in case of liquid orals.
Owner:COUNCIL OF SCI & IND RES
Therapeutic responsive dental gel composition
InactiveUS20050026107A1High viscosityLong period of timeImpression capsTeeth fillingWater solubleLiquid oral
A liquid oral therapeutic dental composition, that increases in viscosity upon contact with moisture following application to an oral cavity surface, is disclosed. The composition comprises a moisture responsive gel carrier comprising a moisture sensitive polymer complex and a water soluble salt and a therapeutic agent dispersed in the responsive gel carrier. Methods and devices for administering the composition are also disclosed.
Owner:DISCUS DENTAL LLC
Liquid matrix undergoing phase transfer in vivo and liquid oral preparations
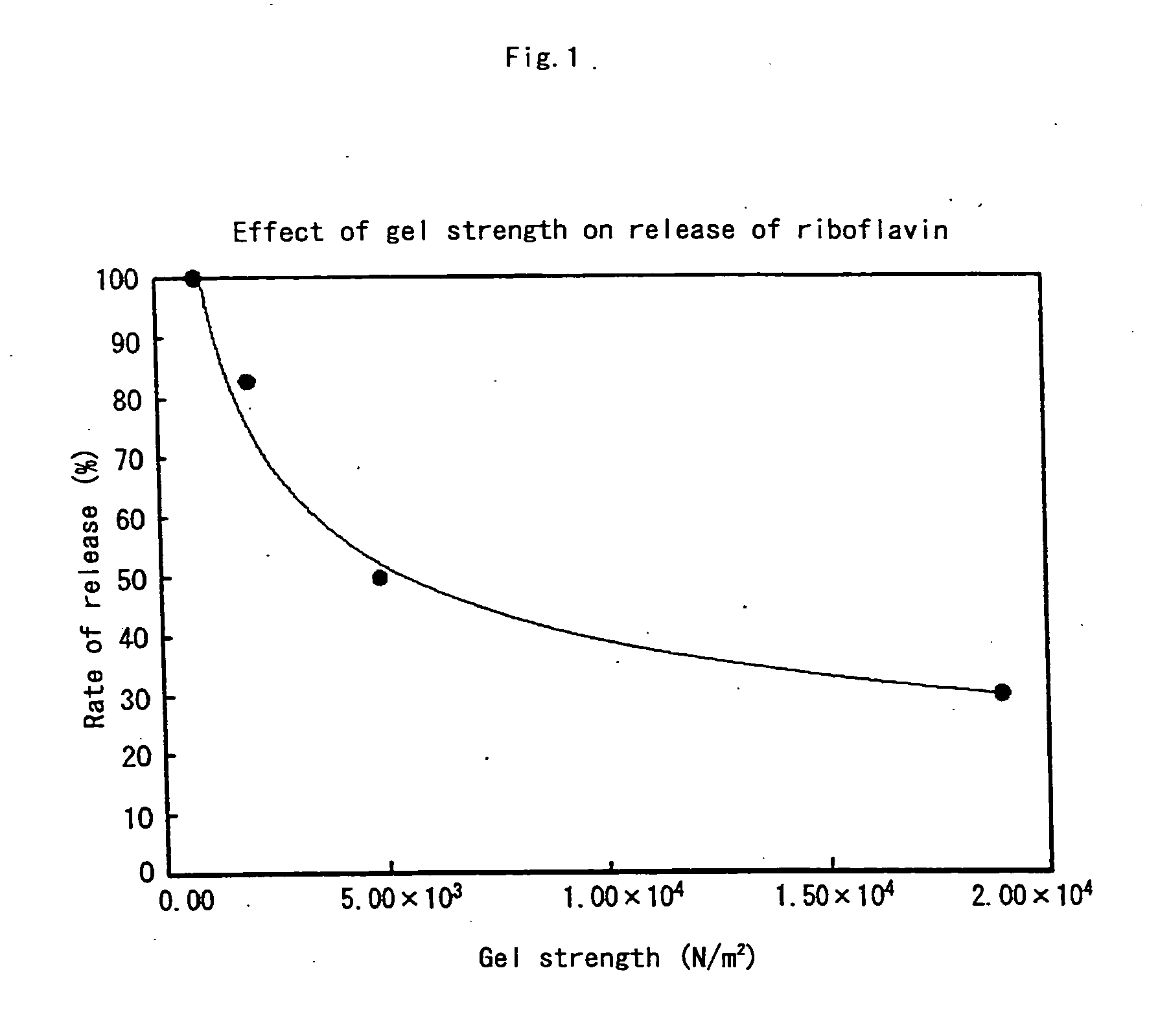
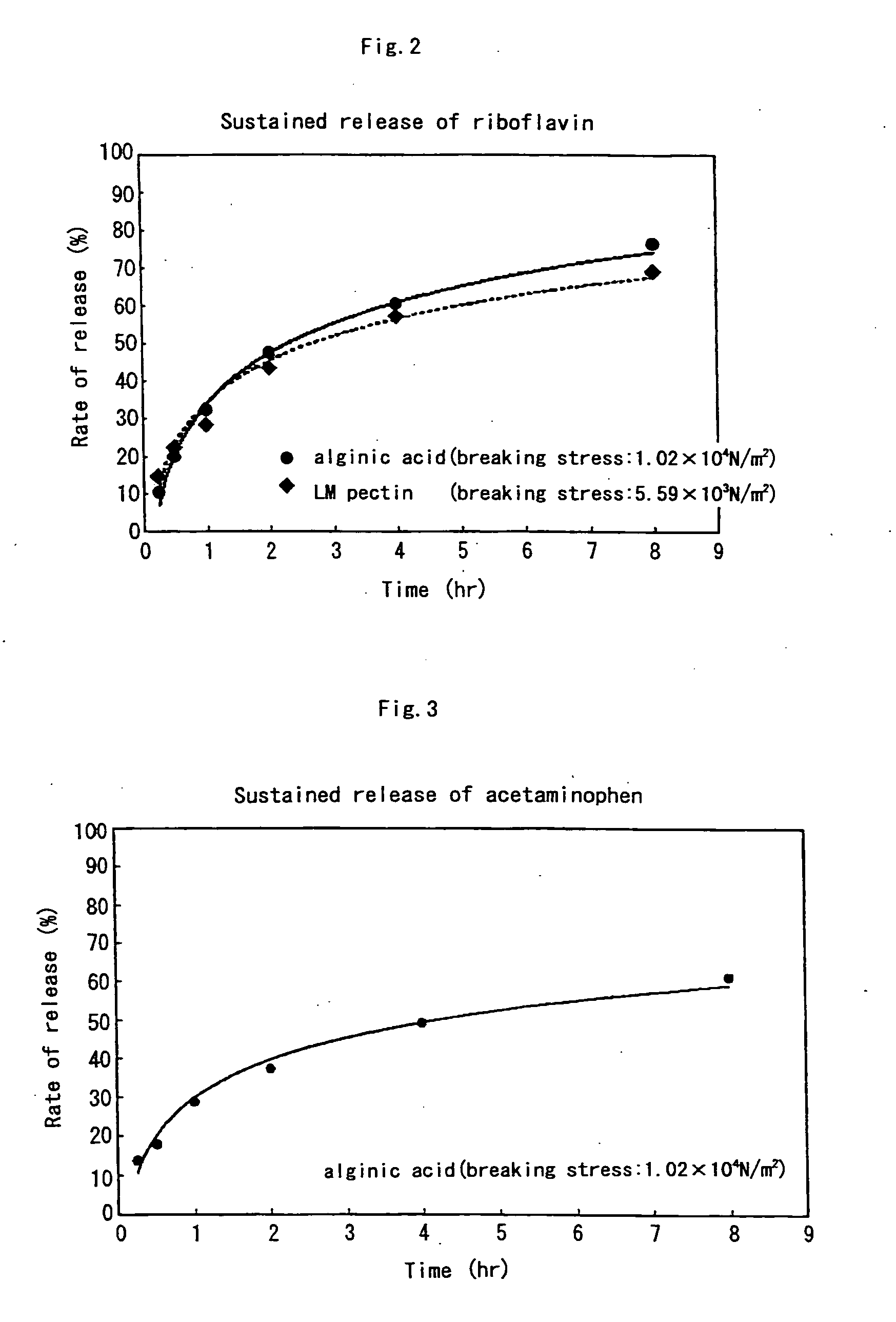
It is intended to provide a liquid matrix for medicinal use in which medicine can be easily solubilized, dispersed or suspended and which can be easily swallowed because of being liquid, has favorable working properties in sterilization and so on and a high stability, also exhibits an effect of masking bitterness, and gels in vivo so as to control the release speed of the medicine, and liquid oral preparations using the same. Namely, a liquid matrix which is a liquid assistant for facilitating swallowing medicine characterized in comprising a water-soluble polymer gelling under acidic conditions, and the breaking stress of the gel is about 3.00×103 N / m2 or more. Liquid oral preparations have favorable slow release properties even though being a liquid.
Owner:MEDRX CO LTD
Compositions of pharmaceutical actives containing diethylene glycol monoethyl ether or other alkyl derivatives
ActiveUS20180071390A1Less viscousLess denseOrganic active ingredientsAerosol deliveryUse medicationDiethylene glycol monoethyl ether
The present invention relates to pharmaceutical compositions of various pharmaceutical actives, especially lyophilic and hydrophilic actives containing Diethylene glycol monoethyl ether or other alkyl derivatives thereof as a primary vehicle and / or to pharmaceutical compositions utilizing Diethylene glycol monoethyl ether or other alkyl derivatives thereof as a primary vehicle or as a solvent system in preparation of such pharmaceutical compositions. The pharmaceutical compositions of the present invention are safe, non-toxic, exhibits enhanced physical stability compared to conventional formulations containing such pharmaceutical actives and are suitable for use as injectables for intravenous and intramuscular administration, as well as for use as a preformed solution / liquid for filling in and preparation of capsules, tablets, nasal sprays, gargles, dermal applications, gels, topicals, liquid oral dosage forms and other dosage forms.
Owner:THEMIS MEDICARE LTD
Preparation method of functional oral preparation rich in erythrothioneine
ActiveCN103181933AIncrease profitImprove securityOrganic active ingredientsAntinoxious agentsSubmerged fermentationSulfur
The invention discloses a preparation method of a functional oral preparation rich in erythrothioneine, which comprises the following steps: firstly, edible fungus mycelium rich in erythrothioneine is mixed with water, and under the temperature of 80 to 100 DEG C, the mixture is stirred and leached; secondly, a concentrated solution is obtained through concentration, and acceptable additives of food are added to the concentrated solution, so that the liquid oral preparation is obtained. The edible fungus mycelium rich in the erythrothioneine obtained by adopting a submerged fermentation biosynthesis method of the edible fungus mycelium is used as a raw material; and after treated by the process steps of the method, the obtained functional oral preparation, prepared from the concentrated solution or a dried material of the concentrated solution is high in product safety. The process provided by the invention has the advantages that all elements of the edible fungus mycelium rich in the erythrothioneine are comprehensively utilized; yield loss caused by extraction of functional elements through purification process is zero; the steps of the process are simple; the energy consumption is less; the erythrothioneine in the body of the mycelium is leached out of cells; and the usage efficiency of the erythrothioneine is high.
Owner:TIANJIN INST OF IND BIOTECH CHINESE ACADEMY OF SCI
Compositions of pharmaceutical actives containing diethylene glycol monoethyl ether or other alkyl derivatives
InactiveUS9827315B2Less viscousLess denseOrganic active ingredientsBiocideUse medicationDiethylene glycol monoethyl ether
The present invention relates to pharmaceutical compositions of various pharmaceutical actives, especially lyophilic and hydrophilic actives containing Diethylene glycol monoethyl ether or other alkyl derivatives thereof as a primary vehicle and / or to pharmaceutical compositions utilizing Diethylene glycol monoethyl ether or other alkyl derivatives thereof as a primary vehicle or as a solvent system in preparation of such pharmaceutical compositions. The pharmaceutical compositions of the present invention are safe, non-toxic, exhibits enhanced physical stability compared to conventional formulations containing such pharmaceutical actives and are suitable for use as injectables for intravenous and intramuscular administration, as well as for use as a preformed solution / liquid for filling in and preparation of capsules, tablets, nasal sprays, gargles, dermal applications, gels, topicals, liquid oral dosage forms and other dosage forms.
Owner:THEMIS MEDICARE LTD
Levulorotation carnitine calcium fumarate and its preparing method and use
ActiveCN101209975ASuitable for oralNot easy to absorb moistureNervous disorderPeptide/protein ingredientsSolubilityFiltration
The invention relates to an L-carnitine calcium fumarate and a preparation method and usages thereof. The L-carnitine calcium fumarate is characterized by oral intake, stability and no-hygroscopicity, and has stronger and more functions of nutrition and treatment, compared with corresponding internal salt and good water solubility; the preparation method is: the calcium furmarate is dissolved in the water and added with calcic alkali for reaction with the temperature increasing to 70 to 90 DEG C for 2 to 8 hours and then water is evaporated by reducing pressure. The solid obtained by drying is added into ethanol and evenly mixed, with the L-carnitine added for reaction with the temperature at 60 to 70 DEG C for 1 to 6 hours, then the mixture is put into a refrigerator for refrigeration for 2 to 8 hours and the L-carnitine calcium fumarate is finally obtained by suction and filtration; the composition containing the L-carnitine calcium fumarate can be made into one or more excipients acceptable on pharmacology, particularly solid and liquid oral intake preparation, such as powdered drug, granules, tablets, capsules, oral liquid, etc., is preferred and the solid and liquid oral intake preparation can be used for food / nutrition additives for people, or feed additives for animals, including additives for calcium supplement.
Owner:リャオニンコンセプヌトラシーオーエルティーディー
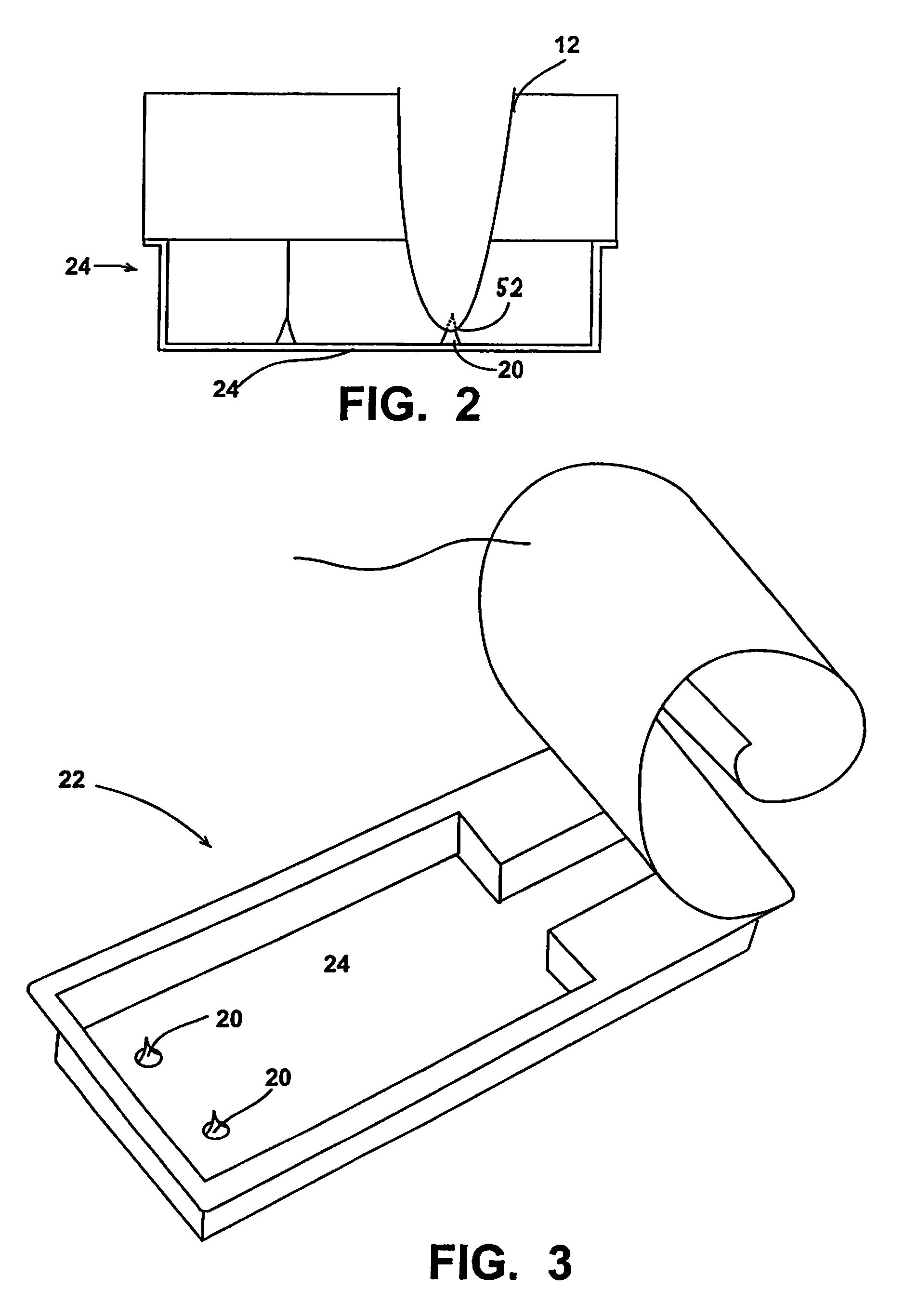
Atomized liquid oral cleaning appliance
The oral cleaning appliance includes an assembly for generating low-pressure bursts of gas in the range of 2-7 bar, directed to a mixing chamber portion of the appliance. A pump provides successive doses of liquid to the mixing chamber. The mixing chamber includes an outlet for liquid droplets produced by the interaction of the gas and the liquid in the mixing chamber. The gas inlet line to the mixing chamber has an internal diameter in the range of 1 -5 mm. The center line of the gas inlet line is offset from the center line of the liquid droplet outlet line by a distance in the range of 1 -5 mm.
Owner:KONINKLIJKE PHILIPS ELECTRONICS NV
Application of forsythin in the process for preparing adiposis treating oral medicine and healthy food
InactiveCN1602855ALess weight gainReduce fat indexMetabolism disorderFood preparationOral medicineSide effect
The invention relates to the application of effective component forsythin in preparation of tablet to swallow for curing adiposis and Health-care food. Forsythin is an effective component abstracted from fruit or leaf of Chinese medicine forsythia, it is mixed with organic or inorganic, solid or liquid excipient to form solid preparation to swallow such as tablet, capsule, particle, powder and pill, and it can also be added into food to produce Health-care food. The poisonous experiment has proved that the LD50 of rat is 8.30g / Kg, it has smaller poisonous side effect, and medicine effect experiment has proved that it can reduce the weight rate of growth, coefficients of fat and lee's index of nutritional fat rat, make the cell of fat rat become smaller by decreasing cholesterin and triglyceride content in serum, so it has better effect for nutritional fat rat.
Owner:SHAANXI NORMAL UNIV
Liquid oral rehydration salt
ActiveCN103479664AAccurate dosageSolve the inconvenienceOrganic active ingredientsMetabolism disorderModerate dehydrationOlder people
The invention provides a liquid oral rehydration salt which comprises the following components in percentage by weight: 0.056-0.96% of sodium chloride, 0.034-0.042% of potassium chloride, 0.065-0.079% of sodium citrate, 0.31-0.37% of glucose and 94.5-104.5% of water. The liquid oral rehydration salt is used for treating mild and moderate dehydration caused by diarrhea and dehydration caused by children diarrhea. Through accurate control of water dosage, asepsis operation and other treatment, the high liquid oral rehydration salt is stable in quality, more convenient to use and suitable for being taken by old people and children under water shortage conditions in the open air and in case of an emergency, and can better express the efficacy.
Owner:北京亿灵医药科技发展有限公司
Method for preparing panax japonicus saponin IVa and application of panax japonicus saponin IVa in preparing a medicament for protecting liver and lowering transaminase
InactiveCN102134268AHigh purityEasy to operateOrganic active ingredientsDigestive systemChromatographic separationSolvent
The invention discloses a method for preparing panax japonicus saponin IVa and an application of panax japonicus saponin IVa in preparing a medicament for protecting liver and lowering transaminase. The preparation method mainly comprises the following steps: crushing panax japonicus saponin IVa bulk pharmaceutical chemicals, extracting with water or a hydrophilic organic solvent, filtering and concentrating extract; passing the concentrated solution through a macroporous adsorption resin column chromatography column so as to enrich total saponin, eluting with water and an alcohol-water mixedliquid in turn, collecting the alcohol-water eluent, performing reduced pressure concentration at a low temperature, and drying to obtain the total saponin; and carrying out chromatograph separation on the total saponin, eluting with a solvent, recovering the solvent at reduced pressure, and drying so as to obtain crude panax japonicus saponin IVa; and repeatedly recrystallizing the crude productwith an organic solvent so as to obtain pure panax japonicus saponin IVa. According to verification, the panax japonicus saponin IVa has bioactivity of protecting liver and lowering transaminase, andcan be mixed with one or more of pharmaceutically acceptable carriers / auxiliary materials so as to be prepared into solid and liquid oral preparations with effects of protecting liver and lowering transaminase.
Owner:SHAANXI UNIV OF CHINESE MEDICINE
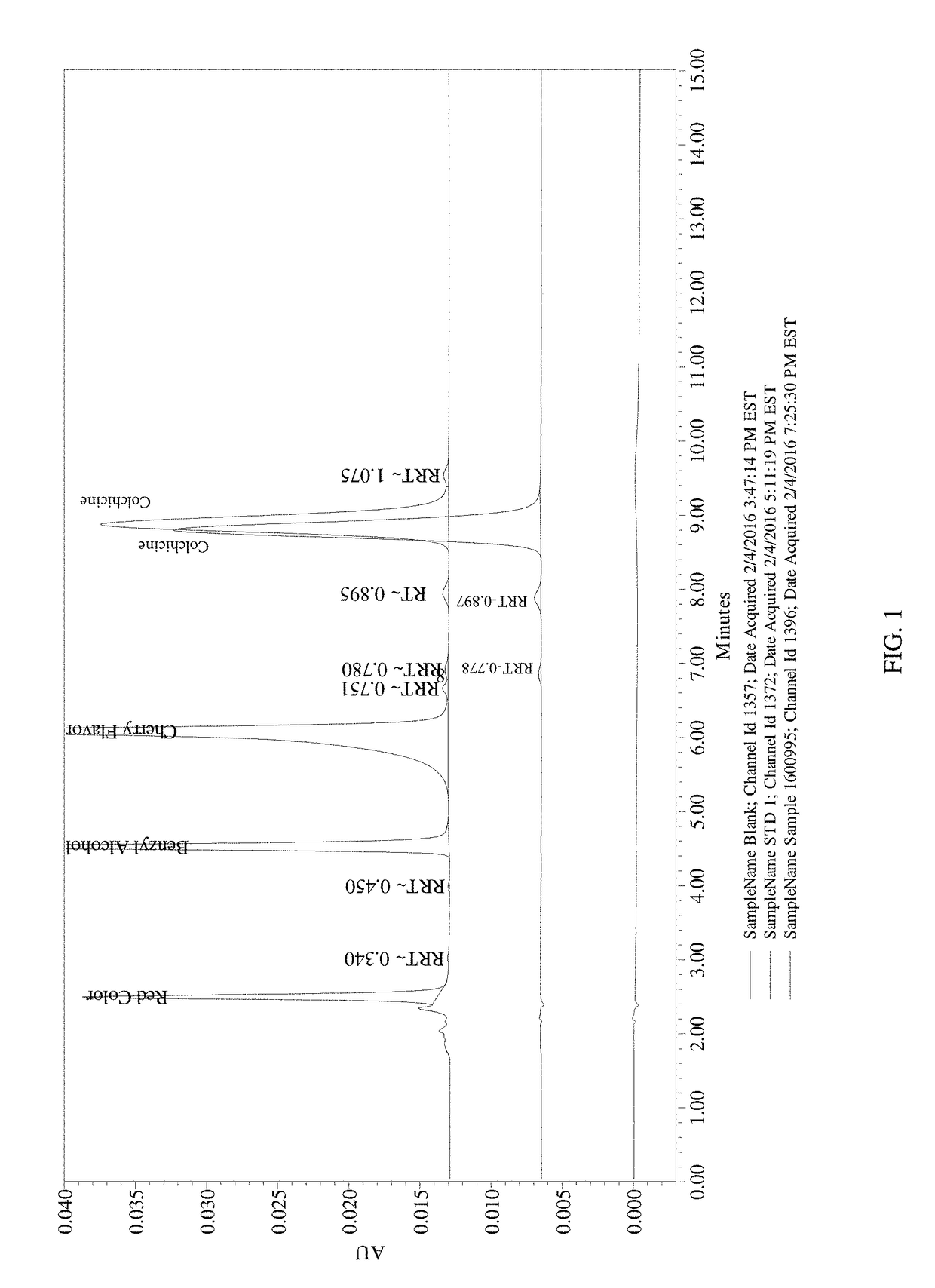
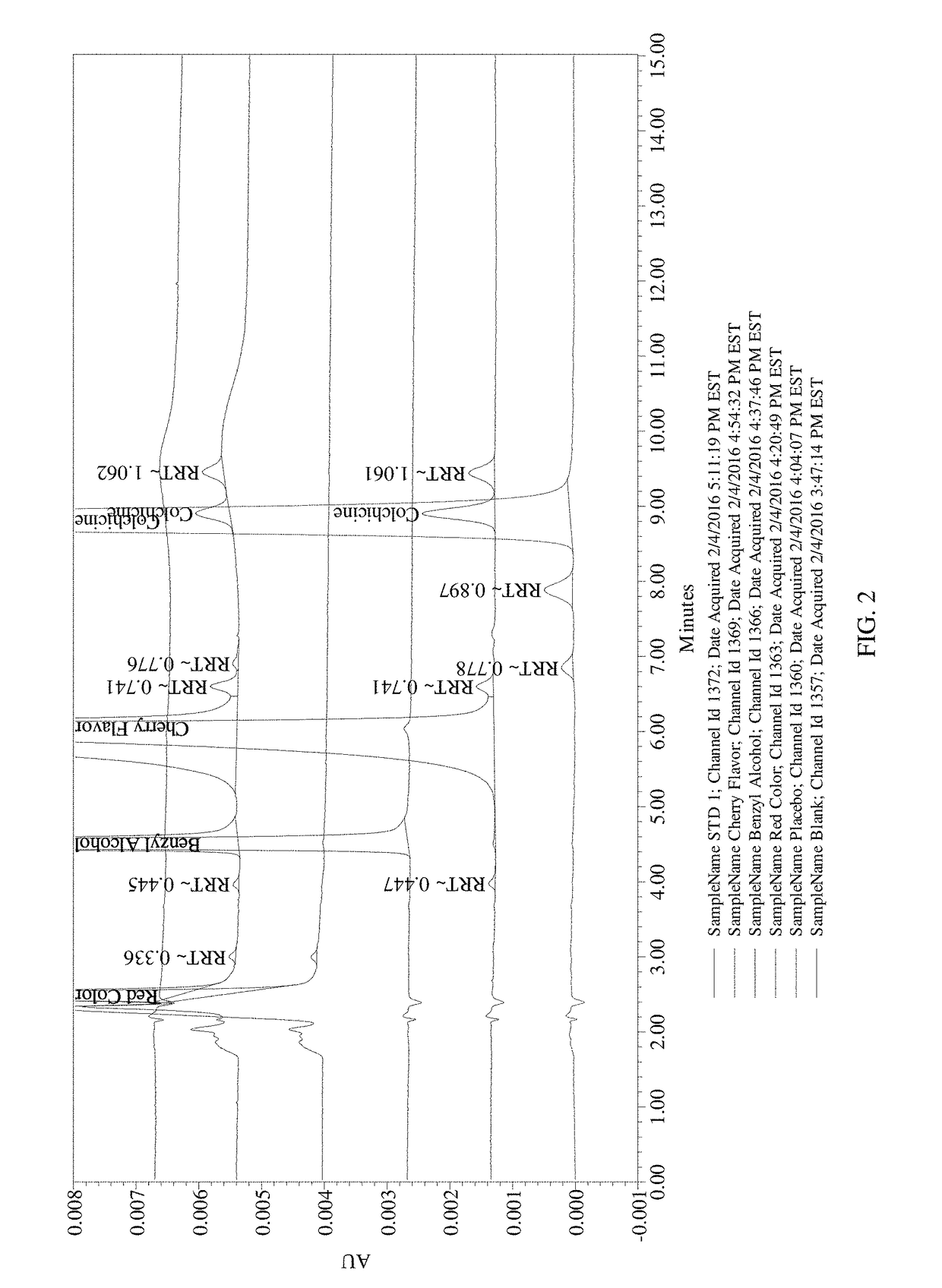
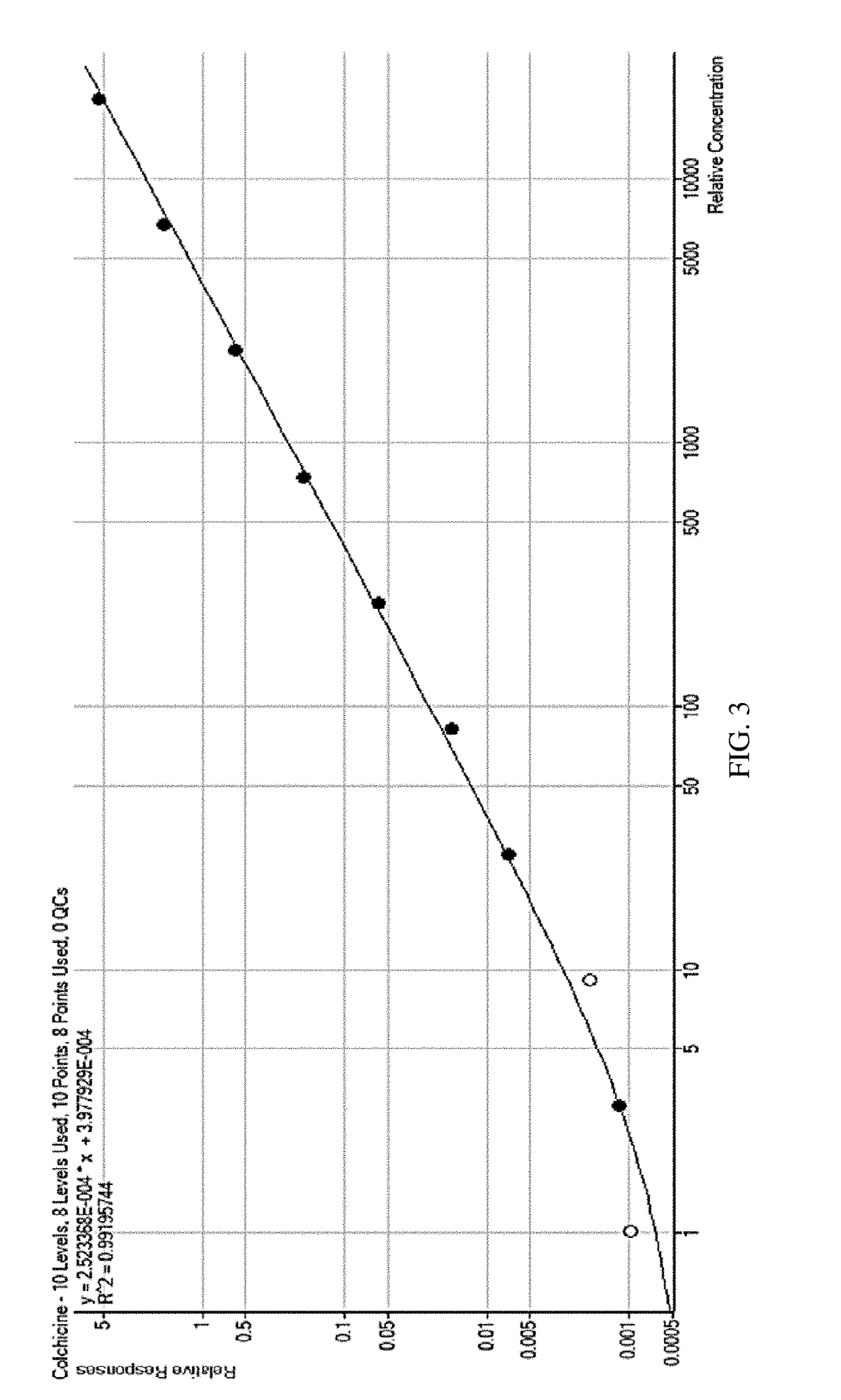
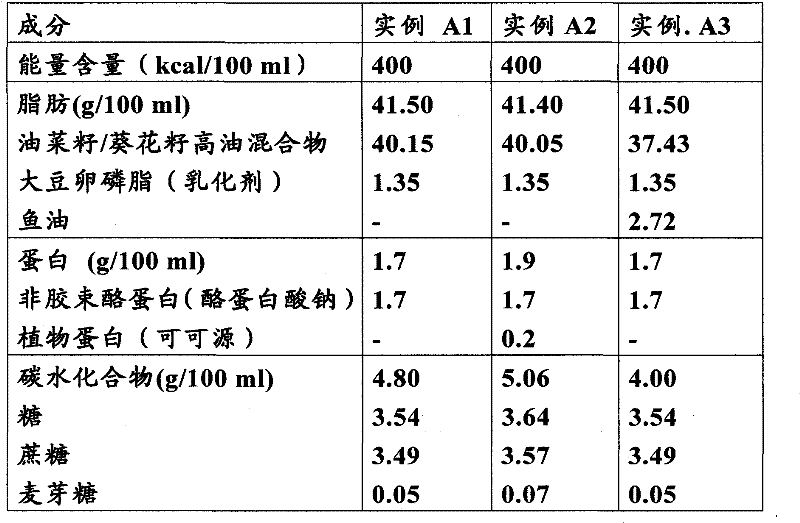
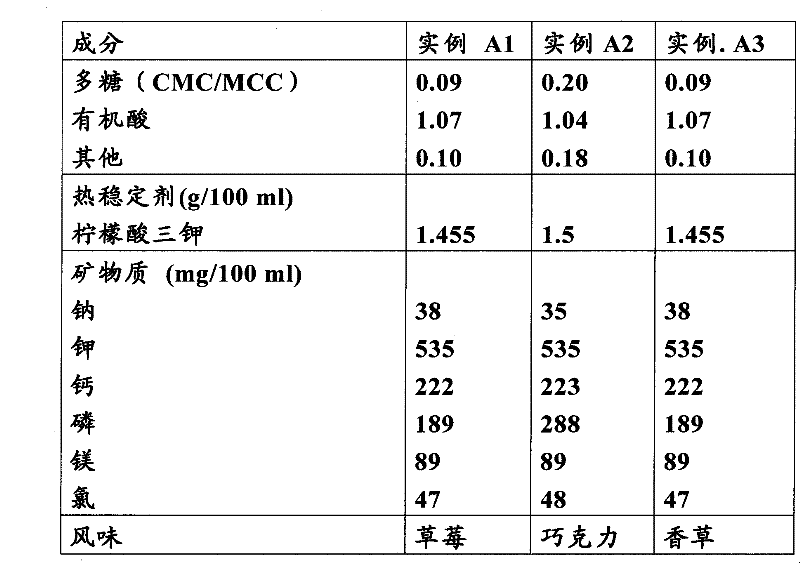
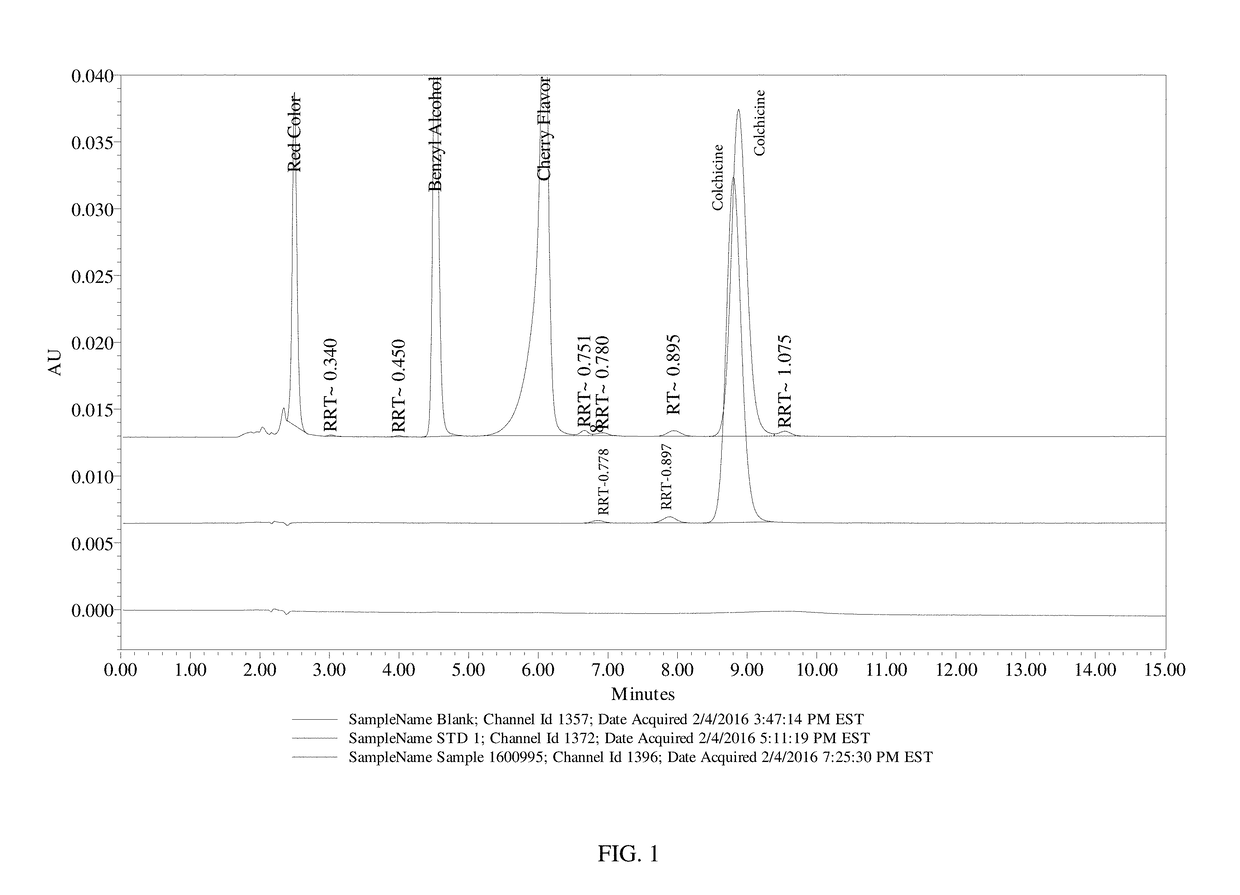
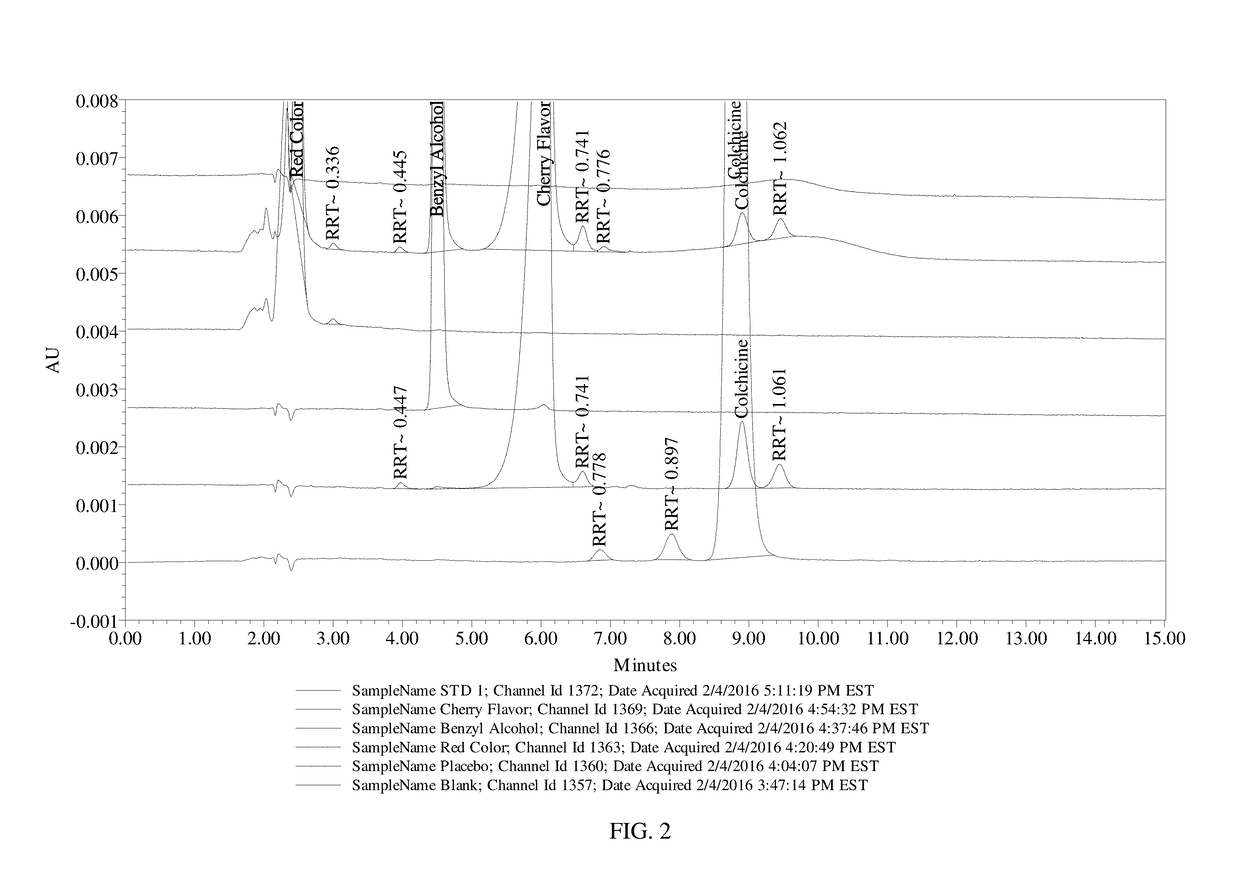
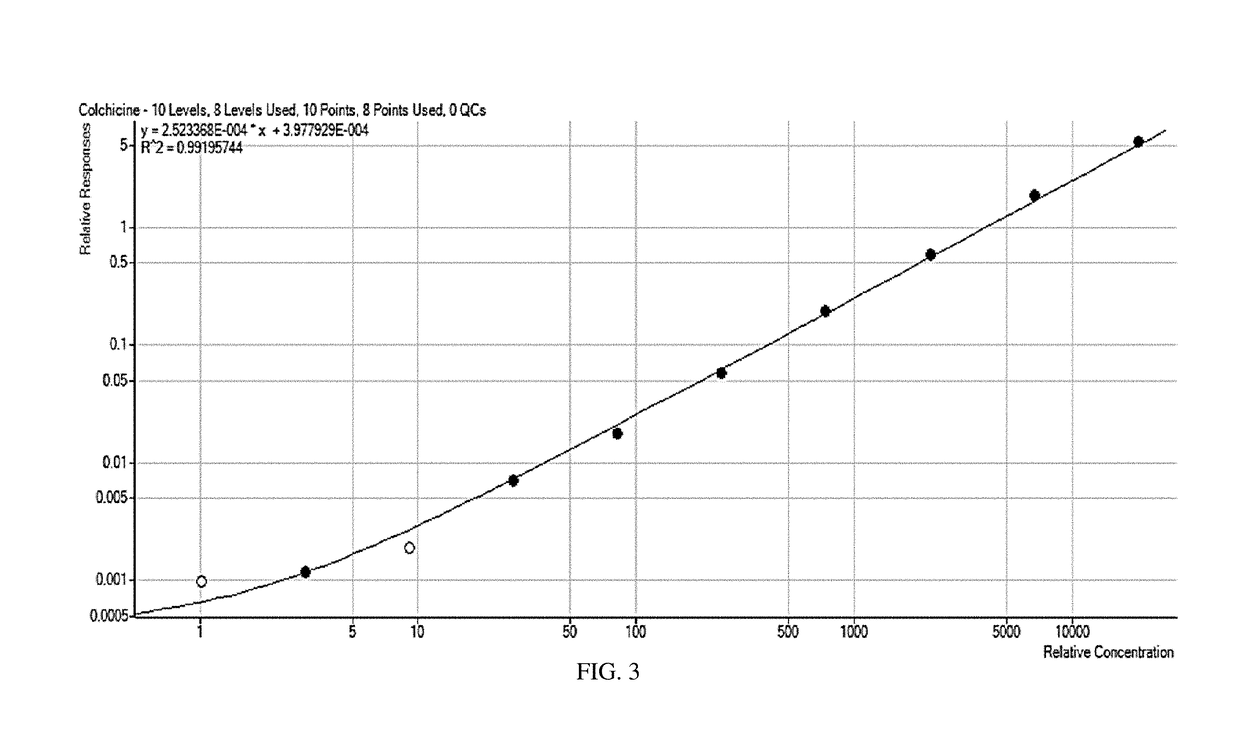
Composition and method of use of colchicine oral liquid
Oral liquid colchicine formulations are described herein. Methods of using the oral liquid colchicine formulations are also provided.
Owner:RXOMEG THERAPEUTICS LLC
Preparation method of no-alcohol type Shexiang Qushu liquid oral preparation
The invention relates to a preparation method of a nonalcoholic ageratum summer-heat clearing liquid oral preparation. The raw materials are chosen according to the raw materials which are specified by 'ageratum summer-heat clearing water' recorded by ministerial traditional Chinese medicine standard and the dosage proportion thereof. The particular method of the invention is as follows: patchouly, elsholtzia haichowensis, angelica dahurica, perilla leaf, rhizoma atractylodis, clove, dried orange peel, shell of areca nut, rhizoma pinellinae praeparata, tuckahoe, ginger and liquorice are chosen; firstly, the patchouly, the elsholtzia haichowensis, the angelica dahurica, the perilla leaf, the rhizoma atractylodis, the clove, the dried orange peel and the ginger which contain volatile oil are extracted to obtain the volatile oil; solubilizer is added into distilment to lead the volatile oil and aromatic water to be well blended; the water part is steamed with water, and clear paste is remained; the residue after the volatile oil is extracted, shell of areca nut, rhizoma pinellinae praeparata, tuckahoe, and liquorice are extracted by ethanol; after the ethanol is volatilized, the two clear pastes are combined and are blended with the volatile oil; finally flavouring agent and specified amount of water are added to prepare nonalcoholic ageratum summer-heat clearing liquid oral preparation. Compared with ageratum summer-heat clearing liquid which contains 40-45% of ethanol recorded by ministered standard, the invention does not contain ethanol, thus avoiding the side effect such as irritating gastrointestinal tract and the like, being accepted by patients easily, better taking the healing effect, expanding the scope of treated subjects. In addition, compared with the original ageratum summer-heat clearing water, the amount of the ethanol is reduced in the course of production, and the cost is reduced.
Owner:SINOMEDICINE BEIJING PHARMA SCI
Pre-dosed oral liquid medication dispensing system
InactiveUS7452350B2Avoid placingStable deliveryTeeth fillingDiagnosticsOral medicationDrug dispensing
A liquid oral medication dispensing system made up of an ampule configured to hold a pre-measured quantity of the selected medication and to dispense that quantity of oral medication through an opening in the ampule formed by a puncturing device. This dispensing system provides a system for storing and delivering oral medications that can be utilized in a broad variety of circumstances by individuals with little or no medical training and provides safe, effective use of the medication.
Owner:YEAKLEY ROURKE M
Taste masked pharmaceutical compositions comprising bitter drug and pH sensitive polymer
The present invention discloses pharmaceutical compositions comprising of pH sensitive polymers used for taste masking highly bitter drugs. The pH sensitive polymer acts as a reverse enteric coating, which is soluble in the acidic pH range 1.0 to 3.0 normally found in the stomach but is insoluble in the pH range 3.5 to 7 thus inhibiting the release of the bitter drug at the pH of saliva and also at the pH of reconstitution medium in case of liquid orals.
Owner:COUNCIL OF SCI & IND RES
Calcium phosphate dispersion composition
ActiveUS20150010481A1Promote remineralizationEnhances rich textureCosmetic preparationsMilk preparationCalcium biphosphateApatite
It is intended to provide a liquid oral composition and a liquid food composition in which calcium phosphate such as hydroxyapatite or tricalcium phosphate can be stably dispersed and prevented from being precipitated or separated out even in long-term storage. Calcium phosphate mixed with xanthan gum and polyglycerin fatty acid ester can be stably dispersed even in long-term storage and prevented from being precipitated or separated out even when used in liquid oral compositions such as mouthwashes, liquid dentifrices, and oral detergents or in liquid food compositions such as milk, soy milk, yoghurt, and refreshing beverages. In addition, calcium phosphate mixed with xanthan gum and polyglycerin fatty acid ester further supplemented with an amphoteric surfactant can be more effectively prevented from being precipitated or separated out in liquid oral compositions such as mouthwashes, liquid dentifrices, and oral detergents.
Owner:SANGI CO LTD
Liquid oral compositions
A suspension which is suitable for oral administration, comprising simvastatin, at least one suspending agent, and at least one preservative, wherein at least 90 wt % of the particles of simvastatin are less than 100 μm in diameter. The present invention also includes uses of the suspension and methods of making the suspension.
Owner:ROSEMONT PHARMA LTD
Methods of treating rett syndrome using fenfluramine
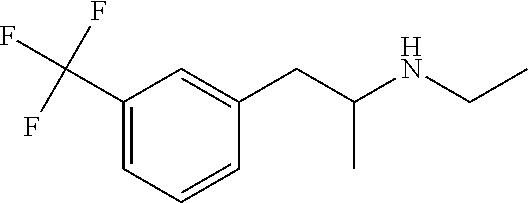
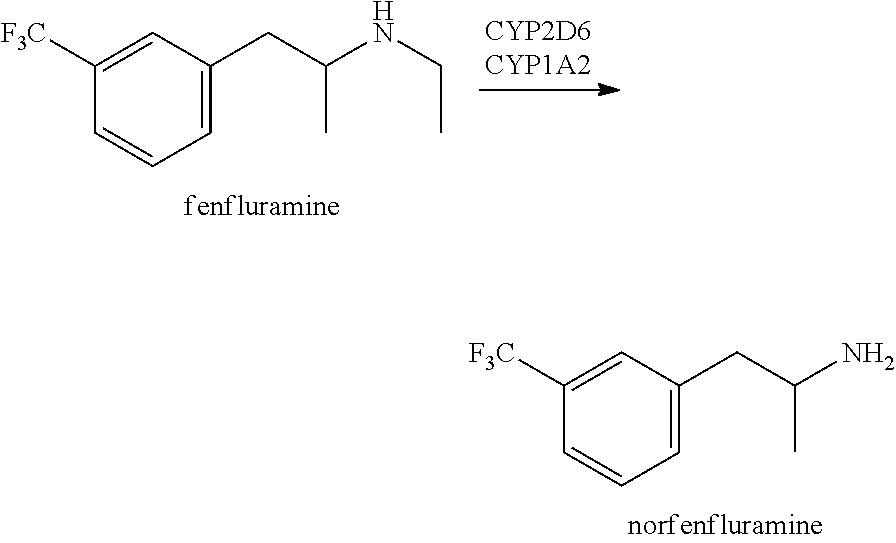
PendingUS20220008389A1Improving cognitive deficitExtended durationNervous disorderHydroxy compound active ingredientsFenfluramineRett syndrome
A method of treating and / or preventing symptoms of Rett syndrome (RTT) in a patient such as a patient previously diagnosed with Rett syndrome, by administering an effective dose of a 5-HT1D, 5-HT2A, 5-HT2C or sigma-1 receptor agonist (e.g., fenfluramine or its pharmaceutically acceptable salt) to that patient. RTT patients are treated at a preferred dose of less than about 1.0 mg / kg / day and may be administered as fenfluramine in an amount of between 0.2 to 0.8 mg / kg / day, to a maximum of 30 mg / day in a liquid oral dose.
Owner:ZOGENIX INT
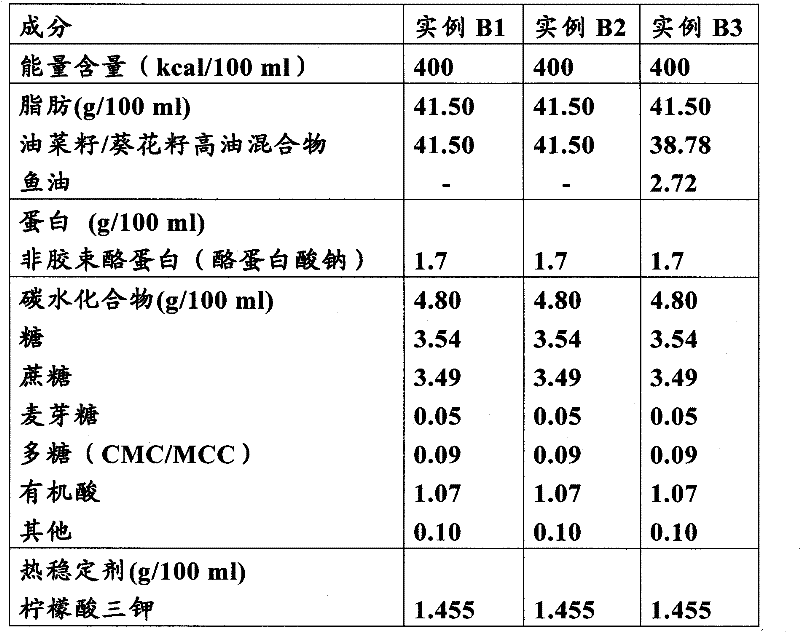
Liquid high-fat protein composition
The present invention relates to nutritional compositions for use in people with a (partially) functional gastrointestinal tract who are unwilling and / or unable to consume sufficient amounts of conventional foods to meet their nutritional needs, such as being malnourished or at risk of developing malnutrition People who are in need of liquid oral nutrition. The shelf-stable liquid aqueous nutritional composition contains at least non-micellar casein, a substantial amount of fat, and optionally a heat stabilizing system.
Owner:NUTRICIA
Composition and method of use of colchicine oral liquid
Oral liquid colchicine formulations are described herein. Methods of using the oral liquid colchicine formulations are also provided.
Owner:RXOMEG THERAPEUTICS LLC
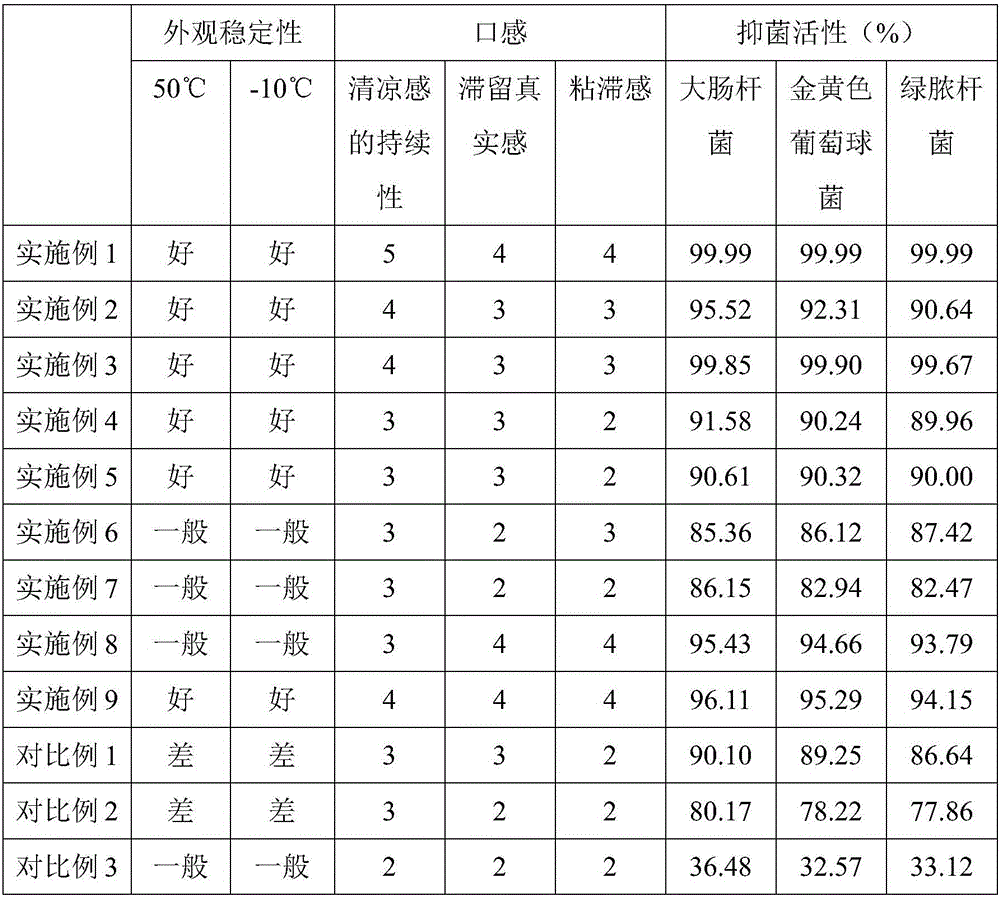
Liquid oral composition containing TCM active substances and tea tree essential oil
ActiveCN106562921AGreat tasteImprove MicroecologyCosmetic preparationsToilet preparationsMentholLamium album
The invention provides a liquid oral composition containing TCM active substances and the tea tree essential oil. The liquid oral composition comprises the following materials in parts by weight: 1.0 to 10 parts of traditional Chinese medicine extracts, 0.1-6 parts of additives, 0.001-0.005 part of menthol, 0.001-0.006 part of tea tree essential oil, and 90-100 parts of water. The traditional Chinese medicine extracts are composed of green tea extract, affine cudweed extract, plantain herb extract, Lamium album extract, tea tree root extract, gallnut, honeysuckle and orange peel mixed extract, and violet extract. The additives are composed of a wetting agent, a pH regulator, a preservative, essence and a flocculating agent.
Owner:李浩然
Novel Substituted Benzimidazole Dosage Forms and Method of Using Same
InactiveUS20090022796A1Easy to prepareImprove pharmacological activityBiocidePowder deliveryOmeprazoleEffervescent Powder
The present invention relates to pharmaceutical preparations comprising substituted benzimidazole proton pump inhibitors. There is provided a liquid or solid pharmaceutical composition consisting of a proton pump inhibitor, including preparations of s-omeprazole, and at least one buffering agent. Also provided is a pharmaceutical composition comprising a parietal cell activator, an anti-foaming agent, a flavoring agent and combinations thereof; a method for treating acid-related gastrointestinal disorders by administering a solid pharmaceutical composition; and a kit for the preparation of a liquid oral pharmaceutical composition. Dosage forms include: liquid, powder, tablet, capsule, effervescent powder, effervescent tablet, pellets, and granules
Owner:UNIVERSITY OF MISSOURI
Chitosan oligosaccharide liquid oral administration preparation and preparation method thereof
InactiveCN108157971AHypoglycemicWith hypolipidemicNatural extract food ingredientsFood ingredient functionsNormal peopleOral medication
The invention discloses a chitosan oligosaccharide liquid oral administration preparation and a preparation method thereof. The oral administration preparation comprises chitosan oligosaccharide, hawcondensed juice, fructo-oligosaccharide, vitamin C, steviol glycoside, citric acid and water. The chitosan oligosaccharide liquid oral administration preparation disclosed by the invention has the effects of reducing blood sugar, reducing blood lipid and enhancing organism immunity after being drunk by normal people; and the preparation method of the chitosan oligosaccharide liquid oral administration preparation is simple, and the oral administration preparation prepared by the preparation method facilitates absorption by human bodies and is good in mouth feel.
Owner:沈勇子
Oral pharmaceutical composition for soft capsules containing vinorelbine and method of treatment
The invention described herein relates to a pharmaceutical composition containing vinorelbine as an active ingredient which is suitable for encapsulation in soft capsules. The liquid oral pharmaceutical composition suitable for a liquid fill composition for a soft capsule dosage form comprises: vinorelbine or a pharmaceutically acceptable salt thereof; ethanol; water; glycerol; and polyethylene glycol. In a preferred embodiment, the tartrate salt form of vinorelbine is used in the composition. The invention also provides for a method of treating cancer comprising orally administering, to a patient in need of treatment thereof, a soft capsule comprising the pharmaceutical composition of the invention.
Owner:R P SCHERER TECH INC
Liquid oral composition
ActiveCN104363881AInhibition formationEasy to useCosmetic preparationsToilet preparationsCelluloseIrritation
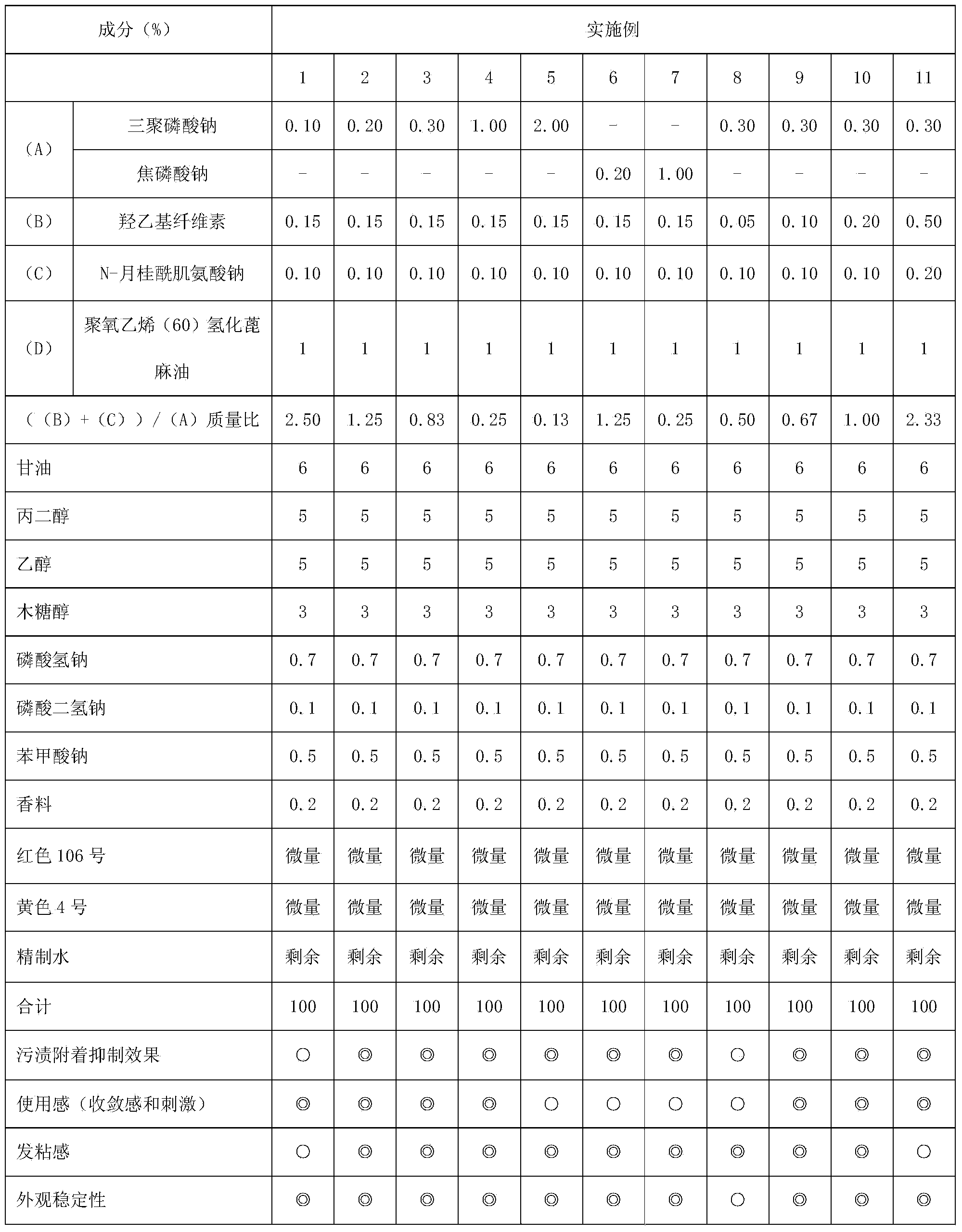
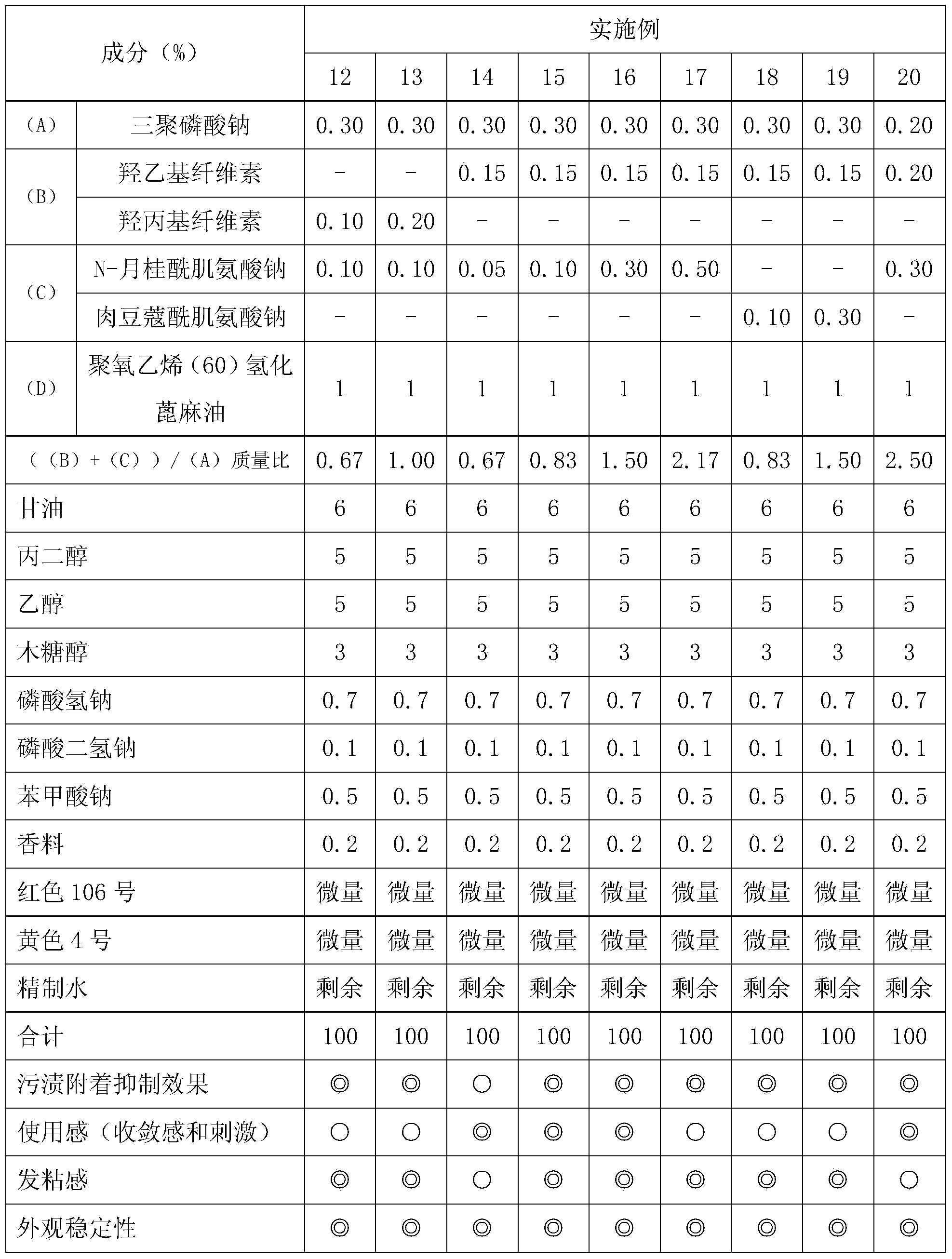
Provided is a liquid oral composition which has an improved effect of preventing the adhesion of stain onto teeth, is free from a sensation of astringency or irritation, does not render the inside of an oral cavity sticky after being applied to the oral cavity, can leave a good sensation particularly after the washing of the mouth with the liquid oral composition, and has a highly clear preparation appearance. A liquid oral composition characterized by comprising (A) a condensed phosphoric acid salt, (B) a hydroxyalkyl cellulose, (C) an acylsarcosine salt and (D) a nonionic surfactant.
Owner:LION CORP
Making method of health-care food of coenzyme Q10 liposome
ActiveCN109463751ASolve the problem of too small particle sizeGood coagulationFood scienceAcetic acidCholesterol
The invention belongs to the technical field of health-care foods, and particularly relates to a health-care food of a coenzyme Q10 liposome and a making method of the health-care food. The coenzyme Q10 liposome is a liquid orally-taken health-care product, coenzyme Q10, lecithin, cholesterol, tween-80 and VE acetate dissolve when being heated with ethanol, and reduction vaporization is performed,so that a smooth film is formed; after vacuum drying, polyvinylpyrrolidone and glycerine are added, an aqueous phase medium hydration film is added to obtain emulsifying liquid, and ultrasonic treatment is performed on the emulsifying liquid to obtain the coenzyme Q10 liposome of which the particle diameter is nanoscale, wherein the concentration of the coenzyme Q10 can achieve 30-40mg / mL. The coenzyme Q10 is packed in the liposome, so that the problem that the coenzyme Q10 liquid oral liquid is unstable can be solved, the bioavailability of the coenzyme Q10 in bodies can be reinforced, and the passive target action of the coenzyme Q10 is realized. The prepared coenzyme Q10 liposome has the effects of improving the immunity of human bodies, and increasing functions of resisting oxidation,delaying senescence, and the like.
Owner:JIANGSU ALAND NOURISHMENT
Liquid oral composition and method for producing same
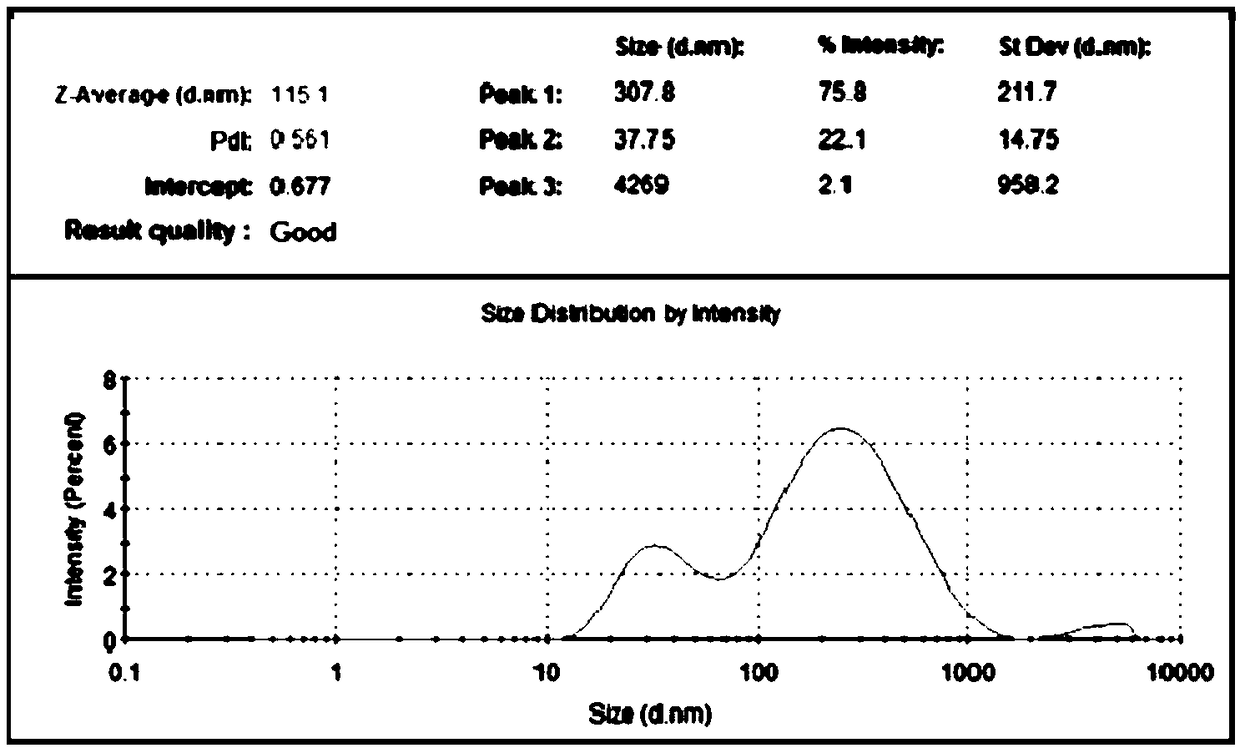
ActiveCN102811702AExcellent appearance stabilityNo unpleasant smellCosmetic preparationsToilet preparationsPolyethylene glycolGlycerol
Disclosed is a liquid oral composition that has excellent stability in appearance, is perceived to be mild and refreshing when used in the oral cavity, has an excellent lasting sensation of coolness and retention, and is non-ethanol-based. Further disclosed is a method for producing same. The liquid oral composition that contains virtually no ethanol is mixed with: (A) a polyoxyethylene-cured castor oil having an E.O. average added mole number of 40-100 moles; (B) an emulsion comprising a decaglycerol mono-fatty acid ester, a tri-fatty acid glyceryl, glycerol, and water, and having an average particle size of 50-500 nm; (C) L-menthol; (D) 3-(L-menthoxy) propane-1,2-diol; and (E) a polyvalent alcohol selected from glycerol, propylene glycol, and a polyethylene glycol that has an average molecular weight of 190-630. In the method for producing the abovementioned oral composition, after combining the abovementioned components A, C, D, and E, component B is added.
Owner:LION CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com