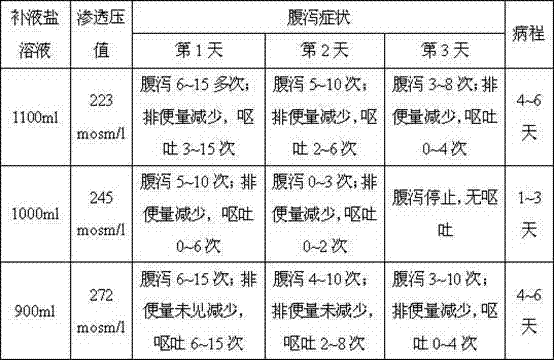
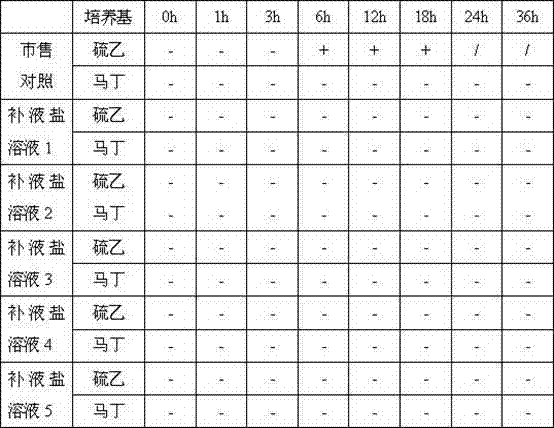
Liquid oral rehydration salt
A liquid rehydration salt and liquid technology, applied in the field of medicine, can solve the problems of difficulty in exerting a therapeutic effect, difficulty in accurate water consumption, and difficulty in mastering the dosage, and achieves the effects of medication safety, convenient storage, and dosage safety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 Liquid Oral Rehydration Salts
[0029] prescription:
[0030] Sodium chloride 2.6g potassium chloride 1.5g sodium citrate 2.9g anhydrous glucose 13.5g purified water 1000ml
[0031] Preparation:
[0032] 1. Take 80% of the amount of purified water and add it to the concentrated mixing tank, start stirring, add sodium chloride, potassium chloride, sodium citrate, and anhydrous glucose in sequence, and stir for 15 minutes to dissolve.
[0033] 2. Filter the above solution into the dilute mixing tank, add water to the full amount, and start stirring.
[0034] 3. Divide the prescription into 8 parts, one part is a solution with a pH value of 7.3 that is not adjusted with a pH regulator, and the rest is a solution that is adjusted to a different pH value with citric acid, filtered, and filled in a 100ml oral liquid bottle, 115 Sterilize at ℃ for 30 minutes, and measure the glucose content (optical rotation, liquid phase).
...
Embodiment 2
[0038] Example 2 Liquid Oral Rehydration Salts
[0039] prescription:
[0040] Sodium chloride 2.6g potassium chloride 1.5g sodium citrate 2.9g anhydrous glucose 13.5g stevia 2g purified water 1000ml
[0041] Preparation:
[0042] 1. Dissolve the weighed raw and auxiliary materials in 950ml of purified water, and stir to make them evenly mixed.
[0043] 2. Adjust the pH to 4.36 with phosphoric acid.
[0044] 3. Fill the above solution with water to full volume, filter, boil for 10 minutes, and pass in circulating water to rapidly cool down.
[0045] 4. Dispensed into 100ml oral liquid bottles under aseptic conditions.
Embodiment 3
[0046] Example 3 Liquid Oral Rehydration Salts
[0047] prescription:
[0048] Sodium chloride 2.6g potassium chloride 1.5g sodium citrate 2.9g anhydrous glucose 13.5g strawberry flavor 2g purified water 1000ml
[0049] Preparation:
[0050] 1. Dissolve the weighed raw and auxiliary materials in 950ml of purified water, and stir to make them evenly mixed.
[0051] 2. Adjust the pH to 4.35 with phosphoric acid.
[0052] 3. Add water to the full amount of the above solution, pass it through a 0.45μm filter membrane, stir evenly, and distribute it into 100ml oral liquid bottles.
[0053] 4. Sterilize at 115°C for 30 minutes in a high-temperature and high-pressure sterilizer, and then pass through circulating water to rapidly cool down and filter.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com