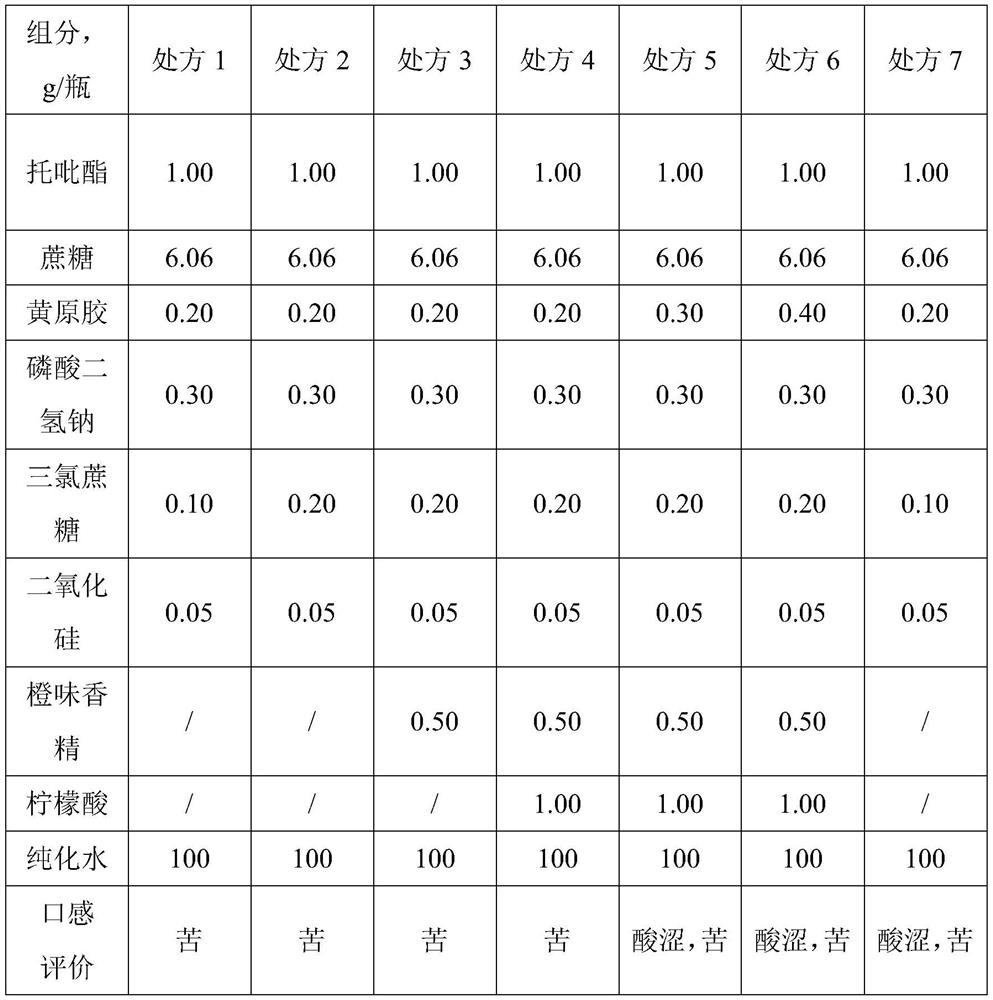
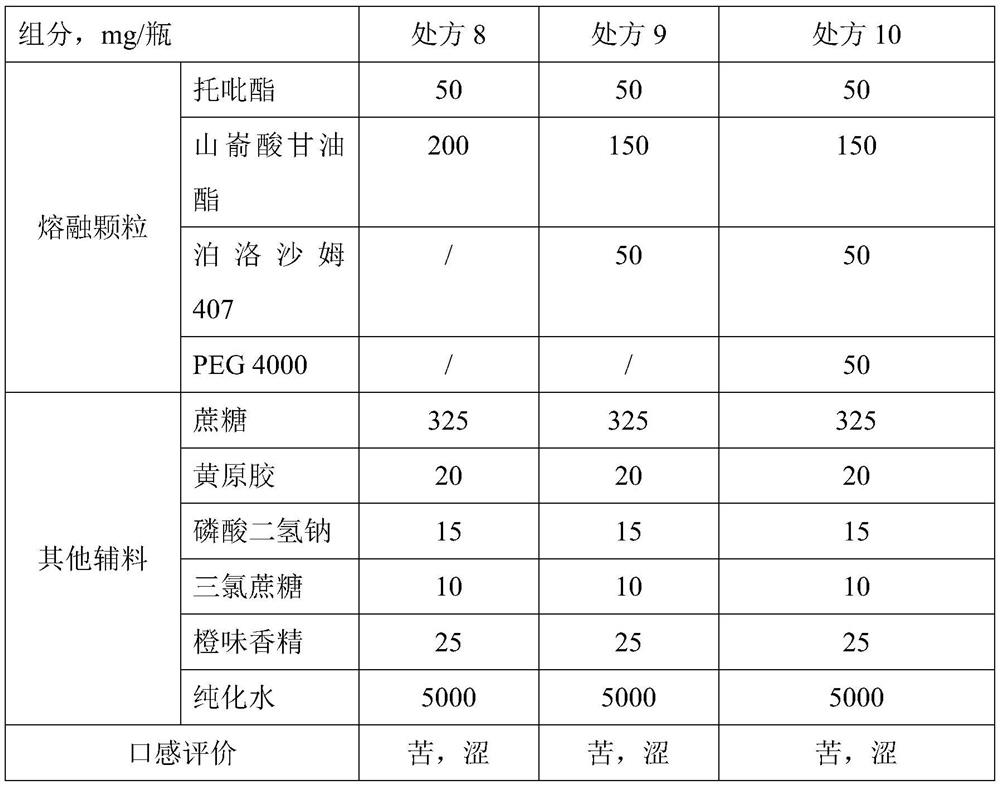
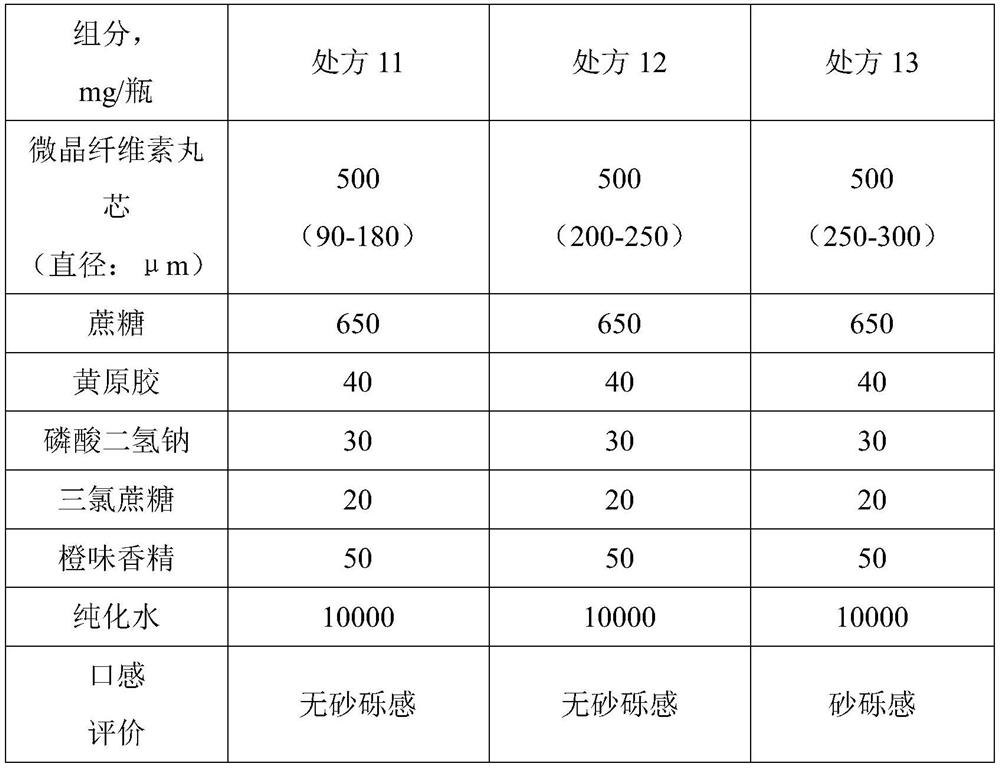
Topiramate dry suspension as well as preparation method and application thereof
A technology of dry suspension and topiramate, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc. Problems such as low drug loading capacity of the resin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0092] Table 8 Preparation of taste-masked pellets
[0093] components mg / bag Topiramate (Aurobindo, content = 99%) 100 Microcrystalline Cellulose Core (Asahi Kasei, CP102) 500 Talc powder (Merck, 108070) 50 Cellulose acetate (Colorcon, Opadry CA) 270 Povidone (Basf, PVP K30) 90
[0094] The specific steps are:
[0095] Take the topiramate bulk drug and all auxiliary materials of prescription quantity. Add absolute ethanol to a suitable container. Add the prescribed amount of topiramate and mix the resulting mixture until the topiramate is completely dissolved. The prescribed amount of talc was added and the resulting mixture was mixed for at least 15 minutes to obtain a topiramate suspension.
[0096] The prescribed amount of microcrystalline cellulose spheres was added to the fluidized bed equipped with a Wurster column. Fluidize the microcrystalline cellulose balls, and spray the full amount of topiramate suspension through t...
Embodiment 2
[0112] The topiramate dry suspension obtained in Example 1 is packed in a composite film bag. Place it under accelerated conditions of 40°C and 60% RH for 3 months to investigate the stability. All tests met the limit requirements, and the results are shown in the table below:
[0113] Table 13 Topiramate dry suspension stability investigation
[0114]
Embodiment 3
[0116]Take topiramate taste-masking pellets, suspending components, and silicon dioxide in Example 1, mix them in the hopper according to the following table, and mix for 10 minutes to prepare topiramate dry suspension.
[0117] The preparation of table 14 topiramate dry suspension
[0118] components mg / bag Topiramate taste masked pellets 1010 Suspending component 215 Silica (Evonik, Aerosil 200) 10
[0119] The topiramate dry suspension is packed in a composite film bag. Place it under accelerated conditions of 40°C and 60% RH for 3 months to investigate the stability. All tests met the limit requirements, and the results are shown in the table below:
[0120] Table 15 Stability investigation of microcrystalline cellulose-free topiramate dry suspension
[0121]
[0122] Without microcrystalline cellulose, the content of the suspension is obviously low, but the stability of this product is good after the addition of microcrystalline cell...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com