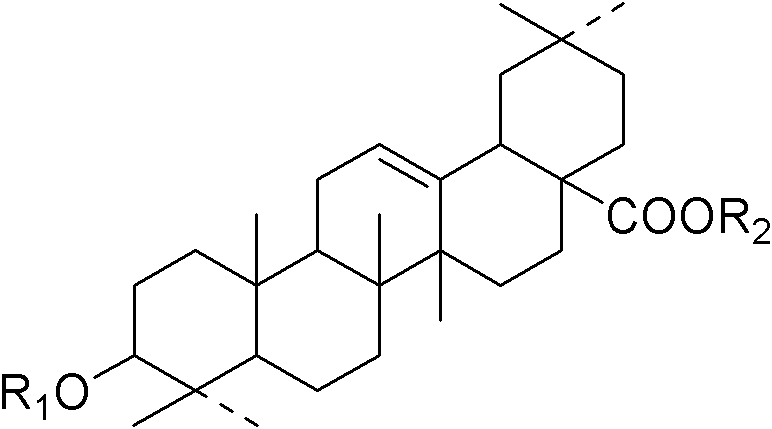
Method for preparing panax japonicus saponin IVa and application of panax japonicus saponin IVa in preparing a medicament for protecting liver and lowering transaminase
A technology for ginseng saponins and pharmaceutical preparations, applied in the directions of drug combinations, pharmaceutical formulations, steroids, etc., can solve the problems of complex processes and low purity of ginseng saponins IVa monomers, and achieve high purity, stable content, Curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] One, the preparation method of bamboo ginseng saponin IVa
[0030] The processing steps and technical conditions of this preparation method are as follows:
[0031] (1) Extraction Crush the raw medicinal material of Ginseng ginseng, extract the broken material with 5-40 times of water or a hydrophilic organic solvent, extract 1-3 times, each time for 1-3 hours, filter, and combine the filtrate; concentrate the filtrate, The concentrated solution is obtained, and the ratio of the concentrated solution is 1:2-1:3, that is, each milliliter of the concentrated solution contains 0.5-0.33 grams of the original medicinal material;
[0032] (2) Enrichment of total saponins The concentrated solution obtained in step (1) is adsorbed through a macroporous adsorption resin, and the ratio of resin to the original medicinal material is 1: 0.1-10 g / g, that is, the amount of the original medicinal material adsorbed by every gram of resin 0.1 to 1 gram), respectively eluting with 20%-7...
Embodiment 1
[0038] Pearl ginseng raw material 1Kg, pulverized, 20 times the amount of water extraction 2 times, 2 hours each time, the extract was filtered, concentrated to 1:2, the filtrate was enriched with total saponins through a macroporous adsorption resin chromatographic column, and mixed with water and 60% ethanol Elute with aqueous solution, collect the ethanol eluate, concentrate under reduced pressure, and dry to obtain 110 g of total saponins; the total saponins are separated by a 5000 g silica gel chromatography column, eluted with a 20-5:1 chloroform-methanol gradient, and the eluate is collected. Concentrate under reduced pressure and dry to obtain 10 g of crude bamboo ginseng saponin IVa; the eluate is purified by Sephadex LH-20, eluted with chloroform-methanol gradient (20-5:1), and the eluate is collected , recovered under reduced pressure, dried to obtain the crude product of bamboo ginseng saponin IVa; the crude product was recrystallized repeatedly with acetone-methano...
Embodiment 2
[0040] Ginseng raw material 1Kg, pulverized, extracted twice with 15 times the amount of ethanol, each time for 1.5h, the extract was filtered, concentrated to 1:2, the filtrate was enriched through a macroporous adsorption resin chromatographic column, and mixed with water and 60% methanol aqueous solution Elution, collect methanol eluate, concentrate under reduced pressure, and dry to obtain 120 g of total saponins; 6000 g of total saponins are separated by silica gel chromatography, eluted with 20~5:1 chloroform-methanol gradient, collect eluate, and depressurize Concentrate and dry to obtain 7 g of crude product of bamboo ginseng saponin IVa; the eluate is purified by Sephadex LH-20, eluted with chloroform-methanol gradient (20-5:1), collects the eluate, and subtracts The crude product of bamboo ginseng saponin IVa was obtained by pressing and drying; the crude product was repeatedly recrystallized with acetone-ethanol to obtain 1.2 g of bamboo ginseng saponin IVa crystals,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com