Development of Improved Cell-Permeable (iCP) Parkin Recombinant Protein as a Protein-Based Anti-Neurodegenerative Agent for the Treatment of Parkinson's Disease-Associated Phenotypes by Utilizing BBB-Penetrating Protein Delivery System MITT, Enabled by Advanced Macromolecule Transduction Domain (aMTD)
a technology of icp and parkin, which is applied in the field of new protein-based therapeutic agents, can solve the problems that cp-parkin is not clinically applicabl
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Construction of Expression Vectors for Recombinant Proteins
[0078]Our newly developed technology, aMTD-based MITT, has enabled us to improve the method for developing cell-permeable recombinant proteins. The expression vectors were designed for Parkin proteins fused with aMTD321 and solubilization domain A (SDA) or solubilization domain B (SDB). To acquire expression vectors for recombinant proteins, polymerase chain reaction (PCR) had been devised to amplify these recombinant proteins.
[0079]The PCR reactions (100 ng genomic DNA, 10 pmol each primer, each 0.2 mM dNTP mixture, 1× reaction buffer and 2.5 U Pfu(+) DNA polymerase (Doctor protein, Korea)) was digested on the restriction enzyme site between BamHI (5′) and HindIII (3′) involving 35 cycles of denaturation (95° C.) for 30 seconds, annealing (60° C.) for 30 seconds, and extension (72° C.) for 2 min each. For the last extension cycle, the PCR reactions remained for 5 minutes at 72° C. Then, they were cloned into the site of pET...
example 2
Purification and Preparation of Parkin Recombinant Proteins
[0080]Denatured recombinant proteins were lysed using denature lysis buffer (8 M Urea, 10 mM Tris, 100 mM NaH2PO4) and purified by adding Ni-NTA resin. Resin bound to proteins were washed 3 times with 30 mL of denature washing buffer (8 M Urea, 10 mM Tris, 20 m imidazole, 100 mM NaH2PO4). Proteins were eluted 3 times with 30 mL of denature elution buffer (8 M Urea, 10 mM Tris, 250 mM imidazole). After purification, they was dialyzed twice against a refolding buffer (550 mM Guanidine-HCl, 440 mM L-Arginine, 50 mM Tris, 100 mM NDSB, 150 mM NaCl, 2 mM reduced glutathione and 0.2 mM oxidized glutathione). Finally, they were dialyzed against a physiological buffer such as DMEM at 4° C. until the dialysis was over 300×105 times. Concentration of purified proteins was quantified using Bradford assay according to the manufacturer's instructions. After purification, they were dialyzed against DMEM as indicated above. Finally, SDS-PAG...
example 3
Determination of Solubility / Yield of Parkin Recombinant Proteins
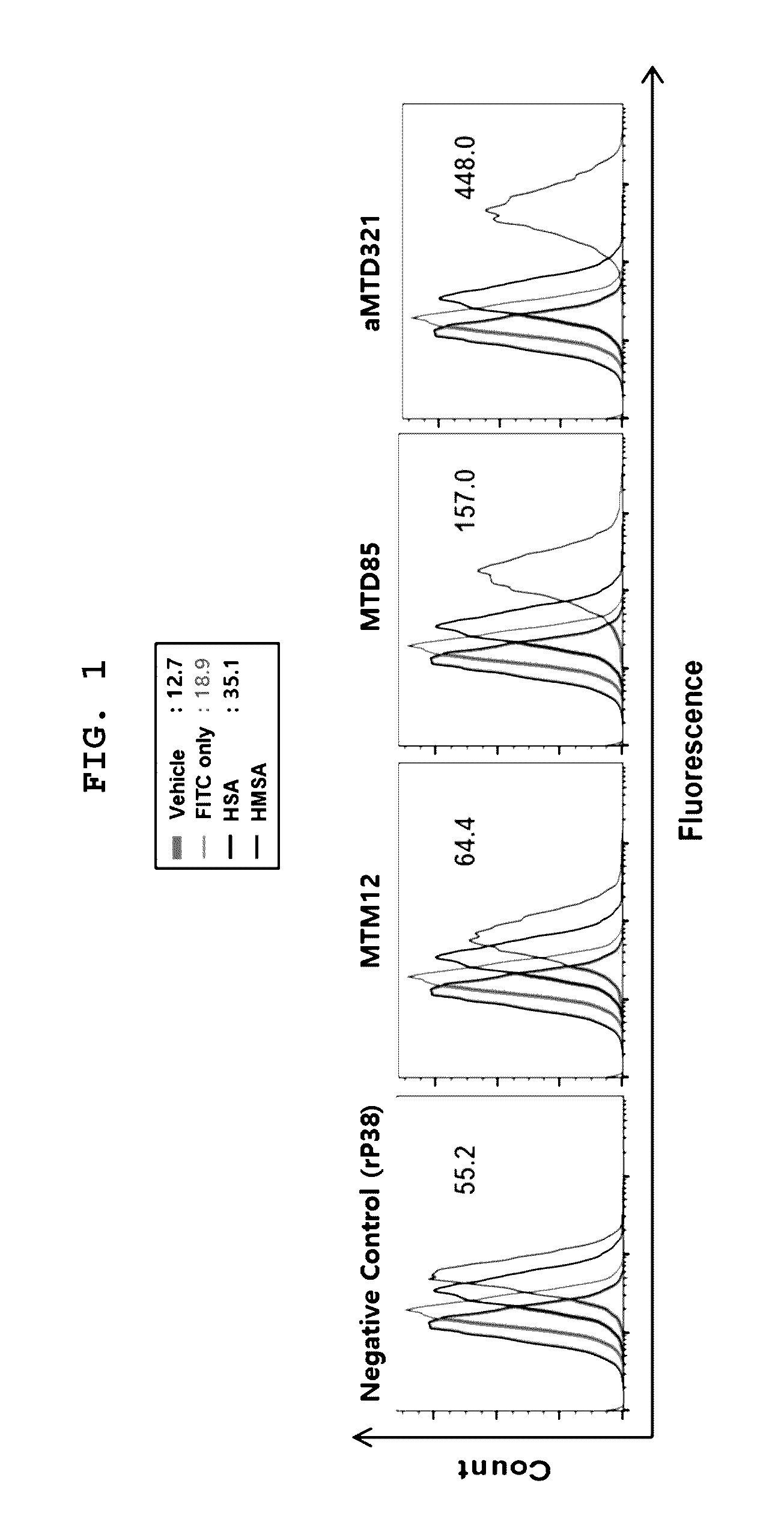
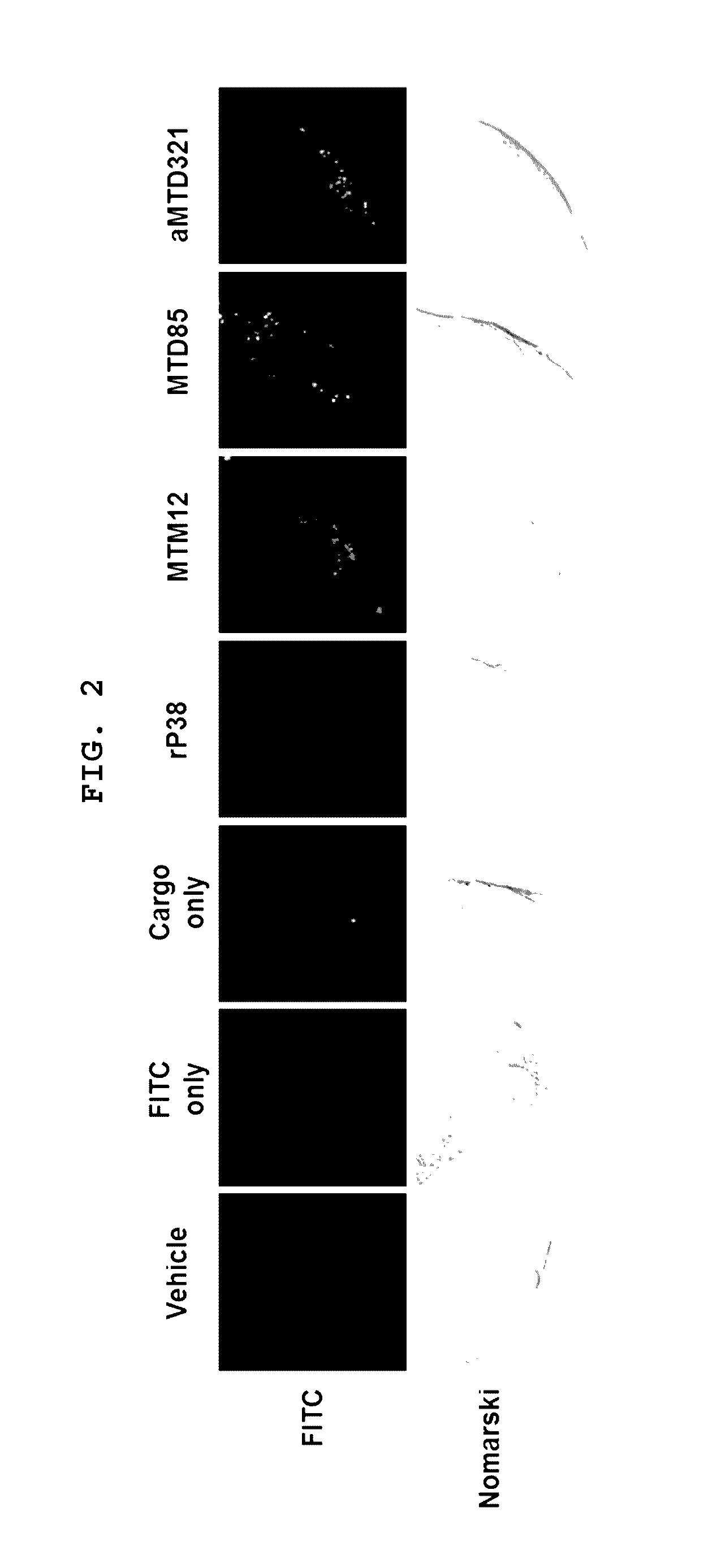
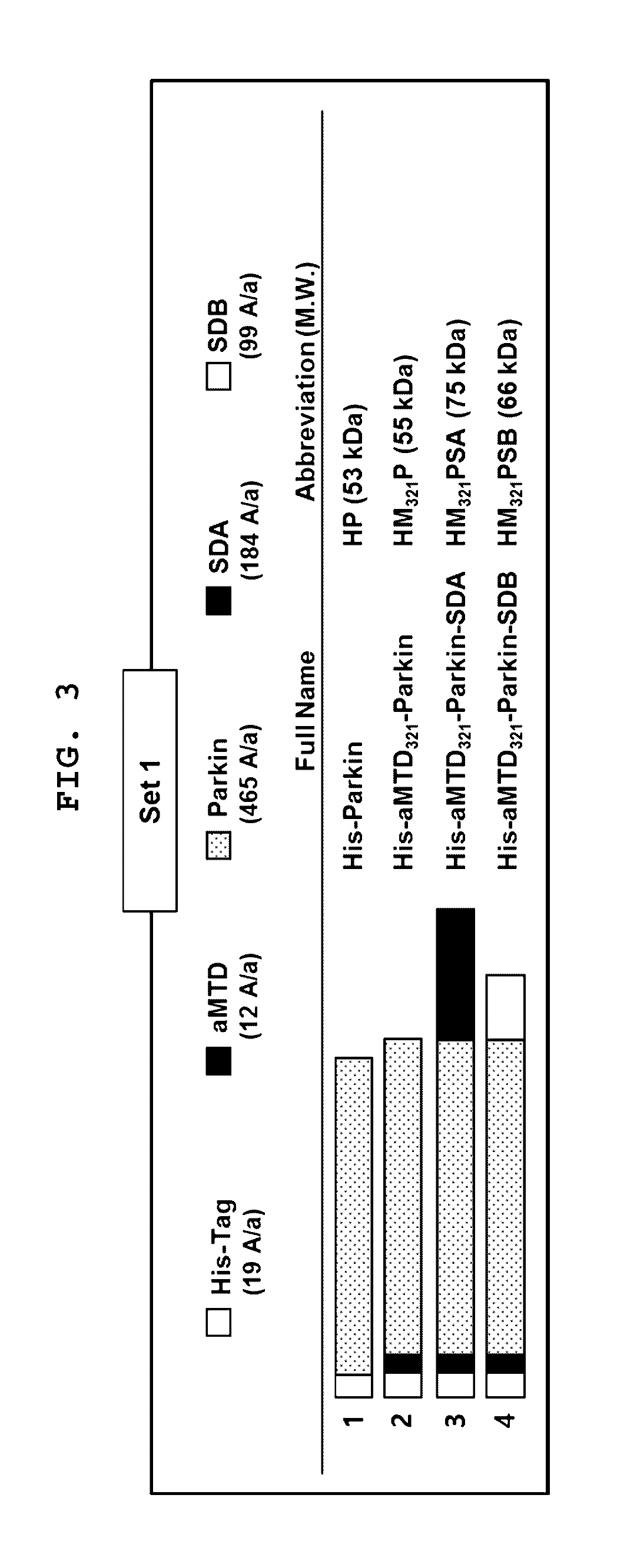
[0081]The aMTD321-fused Parkin proteins containing SDA or SDB are cloned, expressed, purified, and prepared in a soluble form. Each recombinant protein fused to aMTD and / or SD was determined for their solubility and yield. Solubility was scored on a 5-point scale ranging from highly soluble proteins with little tendency to precipitate (*****) to largely insoluble proteins (*) by measuring their turbidity (A450). Yield (mg / L) in physiological buffer condition of each recombinant protein was also determined. The cell-permeable Parkin recombinant proteins were observed as a single band, where the amount of the final purified protein was 13 mg / L (FIG. 3).
[0082]Recombinant proteins purified under the denatural condition were analyzed on 10% SDS-PAGE gel and stained with Coomassie Brilliant Blue.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| hydrophobic | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com