Impurity A and impurity B of midazolam or pharmaceutical composition thereof and application thereof
A kind of technology of midazolam and composition, which is applied in the field of use in the pharmaceutical industry and can solve problems such as different impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
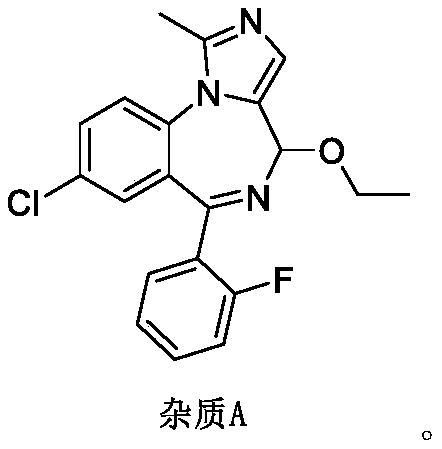
[0090] Example 1 8-chloro-4-ethoxy-6-(2-fluorophenyl)-1-methyl-4H-imidazo[1,5-α][1,4]benzodiazepine Preparation of (Impurity A)
[0091]
[0092] ① Preparation of midazolam nitrogen oxide (intermediate Ⅰ)
[0093] Add 65.0g of midazolam (self-made) and 1200mL of dichloromethane into a 2L four-neck flask, stir to dissolve, cool down in an ice bath, keep the temperature below 10°C, add 103.8g of m-chloroperoxybenzoic acid in batches, continue React at room temperature for 40 hours.
[0094] Add 1000mL dilute hydrochloric acid (1mol / L) to the reaction solution, stir and separate the liquids, extract the organic phase with dilute hydrochloric acid twice (1000mL / time), combine the water phase, add 1000mL dichloromethane to the water phase, and use Adjust the pH with ammonia water to 9-10, separate the liquids, extract the aqueous phase twice with dichloromethane (1000mL / time), combine the dichloromethane phases, wash once with 1000mL water, dry the organic phase with anhydrou...
Embodiment 2
[0107] Example 2 Preparation of 1-[4-chloro-2-(2-fluorobenzoyl)phenyl]-2-methyl-1H-imidazole-5-carbaldehyde (impurity B)
[0108]
[0109] ① Preparation of 4-hydroxy midazolam (intermediate III)
[0110] Prepare according to the method described in step ①~③ of Example 1.
[0111] ② Preparation of midazolam impurity B
[0112] Add 2.40g of 4-hydroxymidazolam and 50mL of absolute ethanol into a 500mL reaction flask, add 24mL of dilute hydrochloric acid (2mol / L) dropwise with stirring, and react at room temperature for 2.5 hours. The reaction is complete as monitored by TLC.
[0113] Add 250mL of purified water to the reaction liquid, evaporate about half of the solvent under reduced pressure at a water bath temperature of 35±2°C, add 150mL of dichloromethane to it, add ammonia water dropwise under stirring to adjust the pH to 9-10, separate liquid, water phase was extracted twice with dichloromethane (150mL / time), the combined dichloromethane phases were washed once with 10...
Embodiment 3
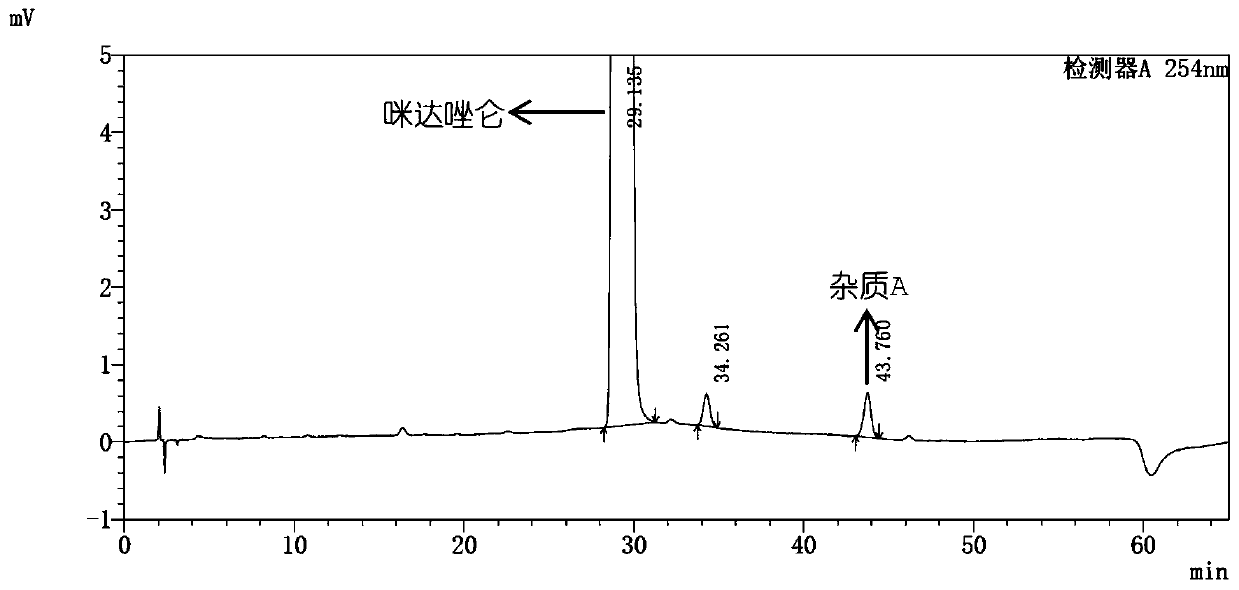
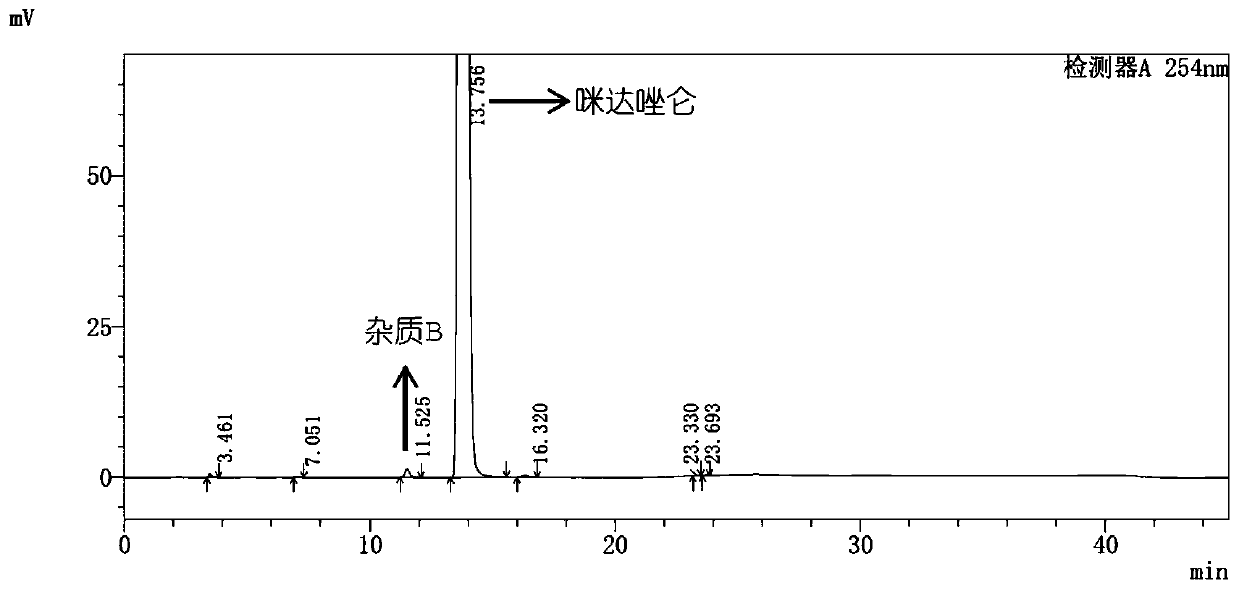
[0117] Embodiment 3, the HPLC determination of impurity A, impurity B in midazolam
[0118] Get midazolam, add methanol-water (80:20) to dissolve and dilute to make the solution that contains 1mg midazolam in every 1mL as need testing solution; ) diluted to a solution containing 1 μg per 1 mL, as a control solution. Take an appropriate amount of impurity A and impurity B reference substances, add methanol to dissolve and make each impurity stock solution containing 0.1mg per 1mL. Take an appropriate amount of midazolam reference substance, add an appropriate amount of impurity stock solution, add methanol-water (80:20) to dissolve and make a solution containing about 1 mg of midazolam and about 1 μg of each impurity in 1 mL, as a system Suitability solution.
[0119] According to the high-performance liquid chromatography (Chinese Pharmacopoeia 2015 edition four general rules 0512) test, use octylsilane bonded silica gel as filler (Angilent Eclipse XDB C8, 4.6mm×250mm, 5μm c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com