Patents
Literature
140 results about "Benzodiazine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Pyrrolobenzodiazepines
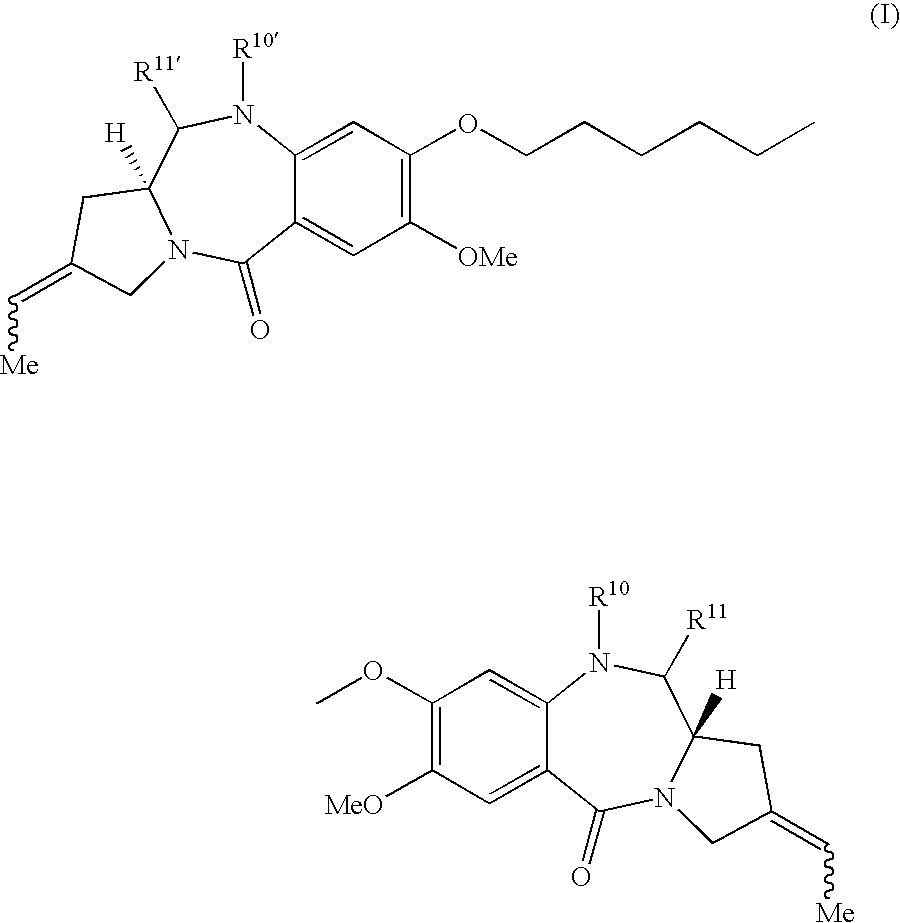
A compound of formula (I) and salts and solvates thereof, wherein: R10 is a nitrogen protecting group and R11 is either OH or O—R12, wherein R12 is an oxygen protecting group, or R10 and R11 together form a double bond between N10 and C11; and R10′ and R11′ are selected from the same options as R10 and R11 respectively.
Owner:MEDIMMUNE LTD
Non-cross-linking pyrrolo[2,1-c][1,4]benzodiazepines and process thereof
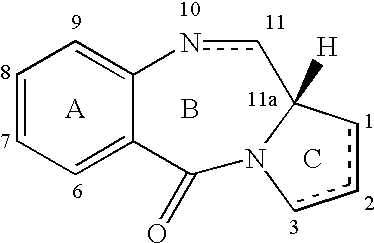
The present invention relates to novel pyrrolo[2,1-c][1,4]benzodiazepines compounds of general formula V as shown below, which are useful as potential antitumour agents and a process of preparing these compounds; particularly the present invention provides a process for the preparation of 7-methoxy-8-{n-[7-methoxy-(11aS)-1,2,3,10,11,11a-hexahydro-5H-pyrrolo[2,1-c][1,4]ben-zodiazepine-5-one-8-yloxy]alkyloxy}-(11aS)-1,2,3,11a-tetrahydro-5H-pyrrolo[2,1-c][1,4]benzodiazepin-5-one, with varying aliphatic chain length and its 2-hydroxy derivatives.
Owner:COUNCIL OF SCI & IND RES
11-hydroxy-5h-pyrrolo[2,1-c][1,4] benzodiazepin-5-one derivatives as key intermediates for the preparation of c2 substituted pyrrolobenzodiazepines
The present inventors have developed a key intermediate for the production of C2 substituted PBDs, which has a leaving group at the C2 position, a carbamate protecting group at the N10 position and a protected hydroxy group at the C11 position. In a first aspect, the present invention comprises a compound with a the formula (I), wherein: R10 is a carbamate-based nitrogen protecting group; R11 is an oxygen protecting group; and R2 is a labile leaving group. In a further aspect, the present invention comprises a method of synthesising a compound of formula (III), or a solvate thereof, from a compound of formula (I) as defined in the first aspect, R16 is either O—R11, wherein R11 is as defined in the first aspect, or OH, or R10 and R16 together form a double bond between N10 and C11; and R15 is R. The other substituents are defined in the claims. Further aspects of the present invention relate to compounds of formula (III) (including solvates thereof when R10 and R16 form a double bond between N10 and C11, and pharmaceutical salts thereof), pharmaceutical compositions comprising these, and their use in the manufacture of a medicament for the treatment of a proliferative disease.
Owner:MEDIMMUNE LTD
Pyrrolobenzodiazepines
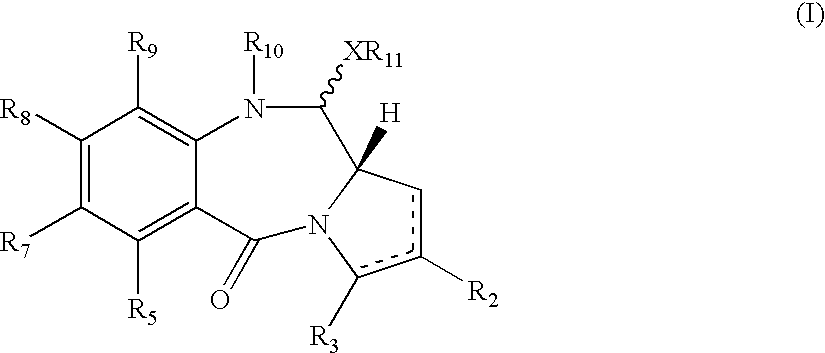
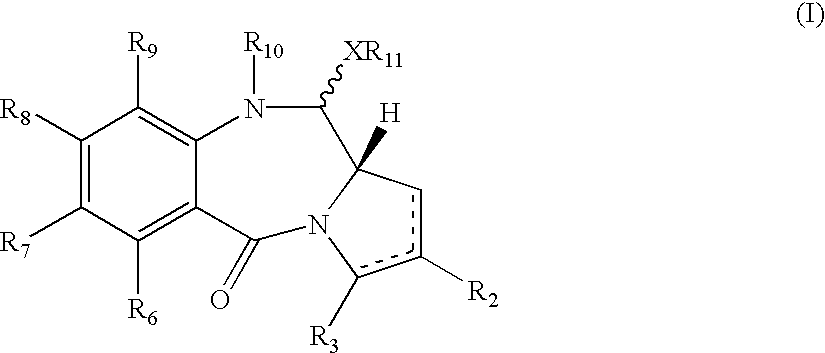
A compound with formula (I)where R10 is a therapeutically removable nitrogen protecting group; R2 and R3 are independently selected from: H, R, OH, OR, =O, =CH-R, =CH2, CH2-CO2R, CH2-CO2H, CH2-SO2R, O-SO2-R, CO2R, COR and CN; R6, R7 and R9 are independently selected from H, R, OH, OR, halo, amino, nitro, Me3Sn; X is S, O or NH; R11 is either H or R; and where there is optionally a double bond between C1 and C2 or C2 and C3; and R8 is selected from H, R, OH, OR, halo, amino, nitro, Me3Sn, or R7 and R8 together form a group -O-(CH2)p-O-, where p is 1 or 2. Such compounds may be used in methods of ADEPT, GDEPT, NPEPT or PDT.
Owner:MEDIMMUNE LTD
Pyrrolobenzodiazepines and conjugates thereof
Conjugate compounds of formula (A):wherein:R2 iswhere R36a and R36b are independently selected from H, F, C1-4 saturated alkyl, C2-3 alkenyl, which alkyl and alkenyl groups are optionally substituted by a group selected from C1-4 alkyl amido and C1-4 alkyl ester or, when one of R36a and R36b is H, the other is selected from nitrile and a C1-4 alkyl ester;R6 and R9 are independently selected from H, R, OH, OR, SH, SR, NH2, NHR, NRR′, NO2, Me3Sn and halo;R7 is independently selected from H, R, OH, OR, SH, SR, NH2, NHR, NRR′, NO2, Me3Sn and halo;Y is selected from formulae A1, A2, A3, A4, A5 and A6:L is a linker connected to a cell binding agent;CBA is the cell binding agent;n is an integer selected in the range of 0 to 48;RA4 is a C1-6 alkylene group;either(a) R10 is H, and R11 is OH, ORA, where RA is C1-4 alkyl; or(b) R10 and R11 form a nitrogen-carbon double bond between the nitrogen and carbon atoms to which they are bound; or(c) R10 is H and R11 is OSOzM, where z is 2 or 3 and M is a monovalent pharmaceutically acceptable cation;R and R′ are each independently selected from optionally substituted C1-12 alkyl, C3-20 heterocyclyl and C5-20 aryl groups, and optionally in relation to the group NRR′, R and R′ together with the nitrogen atom to which they are attached form an optionally substituted 4-, 5-, 6- or 7-membered heterocyclic ring;wherein R16, R17, R19, R20, R21 and R22 are as defined for R6, R7, R9, R10, R11 and R2 respectively;wherein Z is CH or N;wherein T and T′ are independently selected from a single bond or a C1-9 alkylene, which chain may be interrupted by one or more heteroatoms e.g. O, S, N(H), NMe, provided that the number of atoms in the shortest chain of atoms between X and X′ is 3 to 12 atoms; andX and X′ are independently selected from O, S and N(H).
Owner:GENENTECH INC +1
Pyrrolobenzodiazepines and conjugates thereof
InactiveUS20130028917A1Organic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsDiseaseBenzodiazine
Conjugates and compounds for making conjugates which are PBD molecules linked via the N10 position are disclosed, along with the use of the conjugates for treating proliferative diseases, including cancer.
Owner:MEDIMMUNE LTD
C2-fluoro pyrrolo [2,1-c][1,4]benzodiazepine dimers
The present invention relates to novel 2-fluoro-pyrrolo[2,1-c][1,4]benzodiazepine dimers useful as antitumour agents and to a process for the preparation thereof.
Owner:COUNCIL OF SCI & IND RES
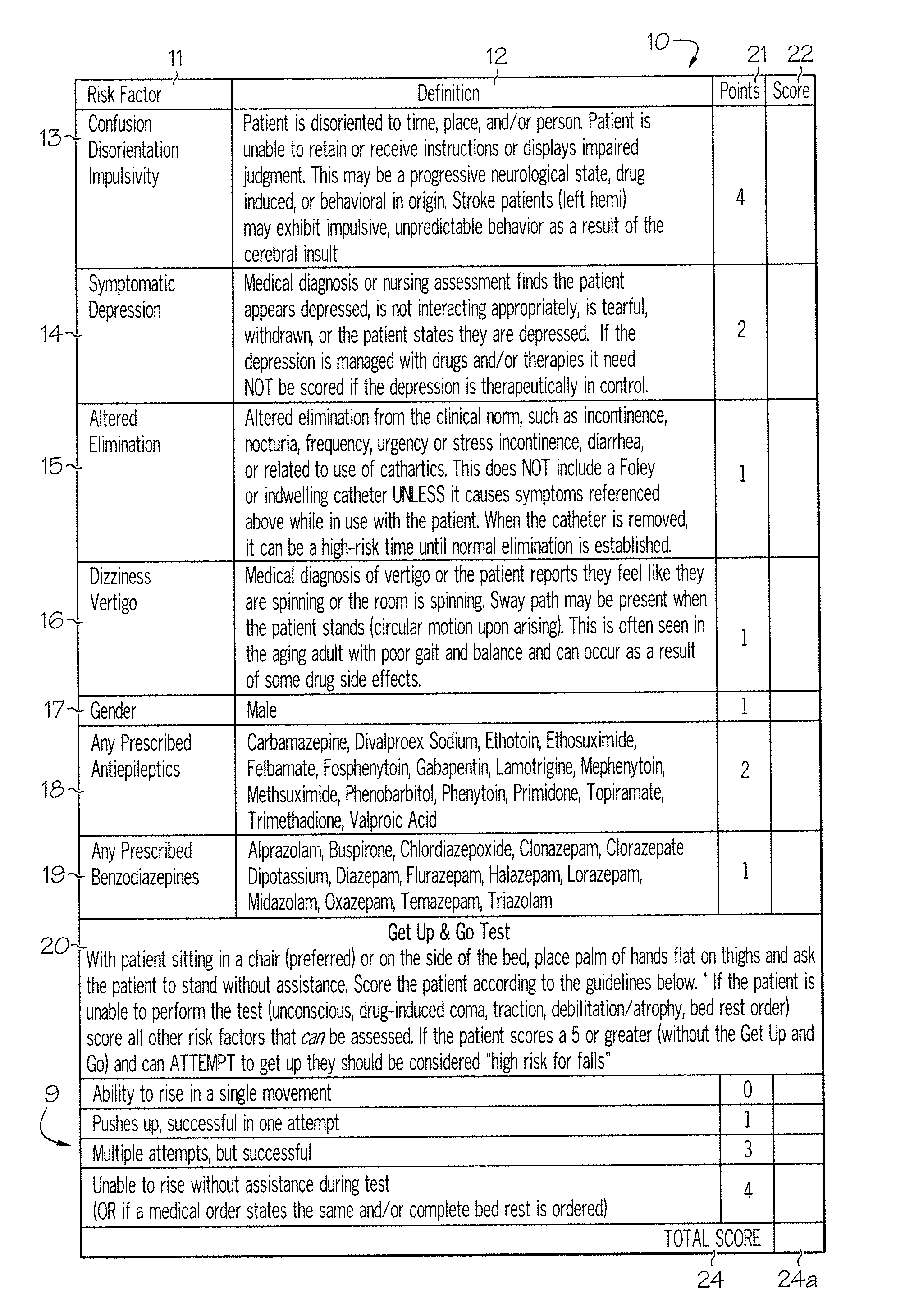
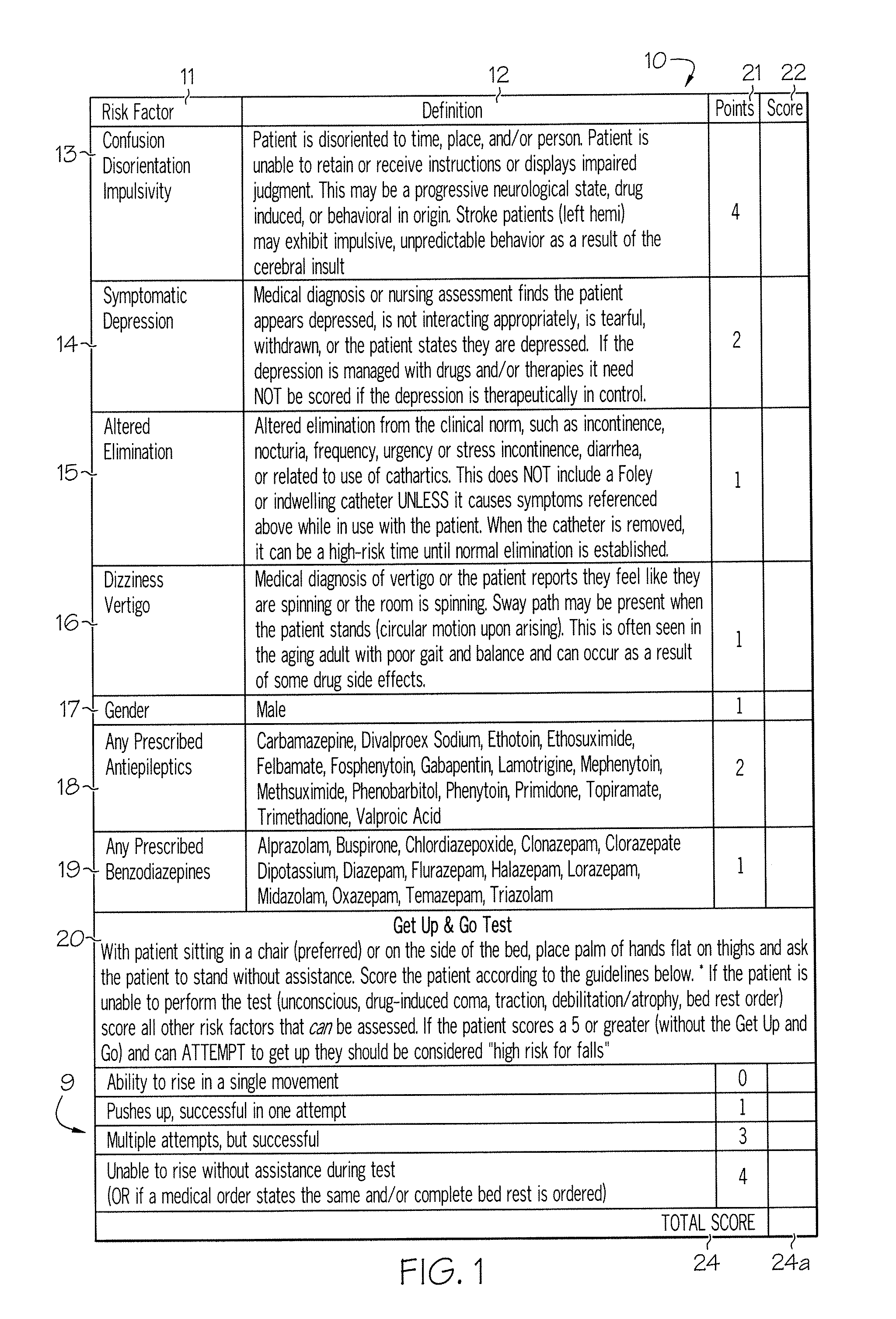
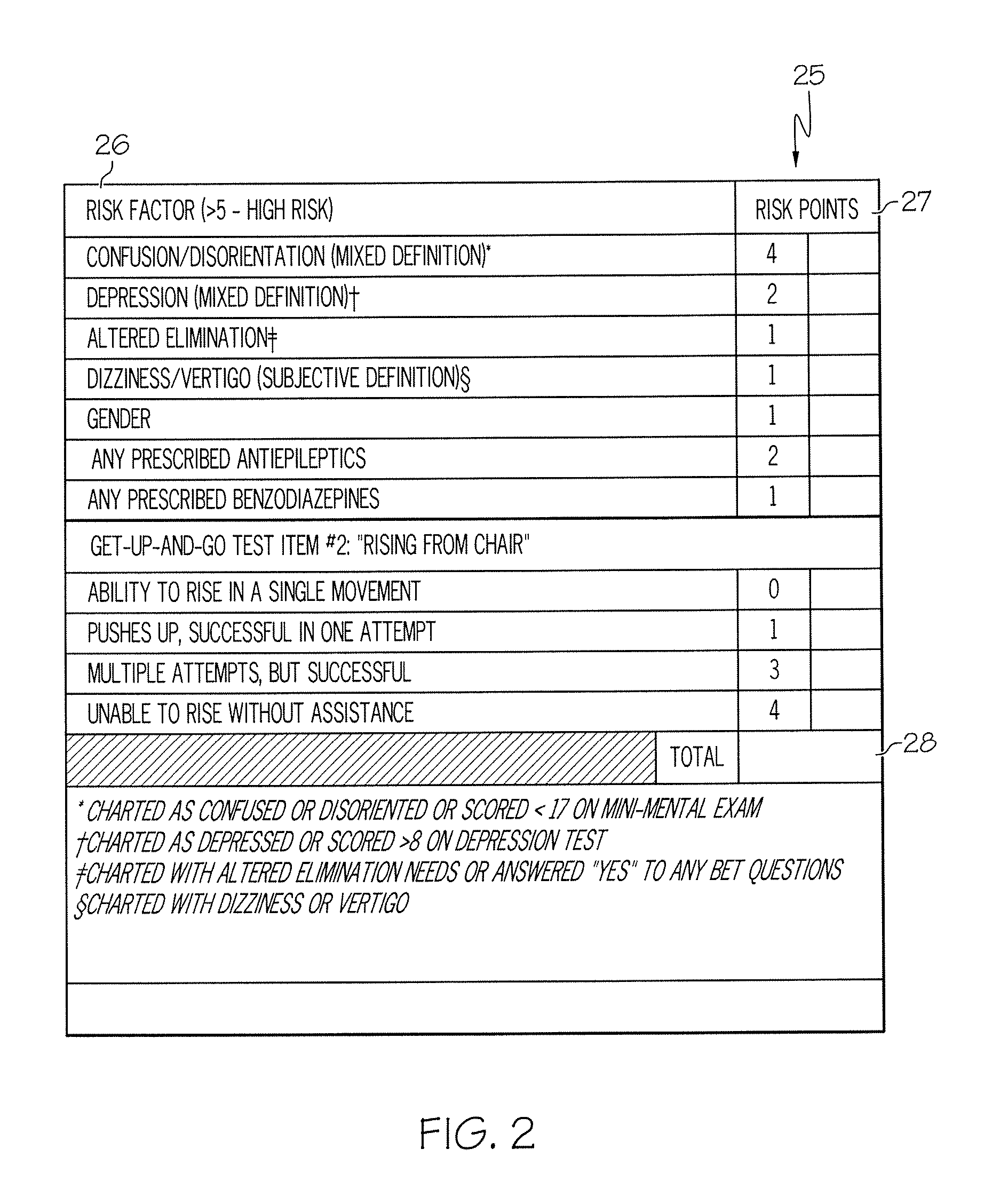
Method and system for assessing fall risk
ActiveUS20080009686A1Easy to addAccurate predictionData processing applicationsTherapiesBenzodiazepineTotal risk
A method and system for determining the fall risk of a patient is provided. The method includes the evaluation of a patient to determine whether the patient exhibits one or more intrinsic fall risk factors selected from a group consisting of confusion, depression, altered elimination, dizziness, male gender, antiepileptic / anticonvulsant prescriptions and benzodiazepine prescriptions. A specific point value is assigned to each of the intrinsic risk factors found to be exhibited by the patient. A mobility test is also performed on the patient to evaluate the patient's ability to rise from a seated position, and a specific mobility test point value is assigned to the patient based upon the patient's performance of the mobility test. Each intrinsic risk factor's specific point value is then summed together with the specific mobility test point value to achieve a total risk score, and the patient's fall risk is determined based on the total risk score. An intervention process may be developed for the patient based on the patient's fall risk.
Owner:AHI OF INDIANA
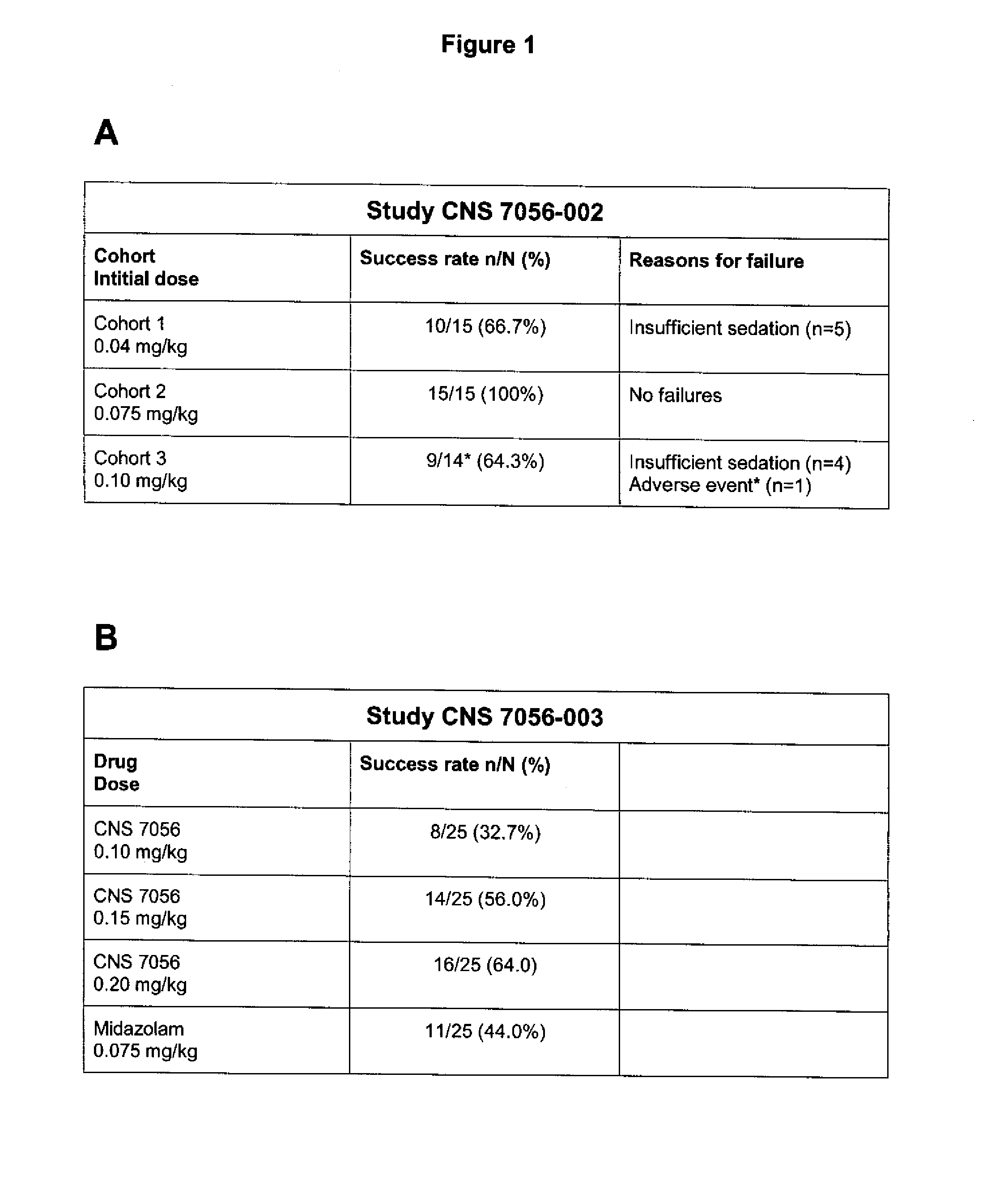
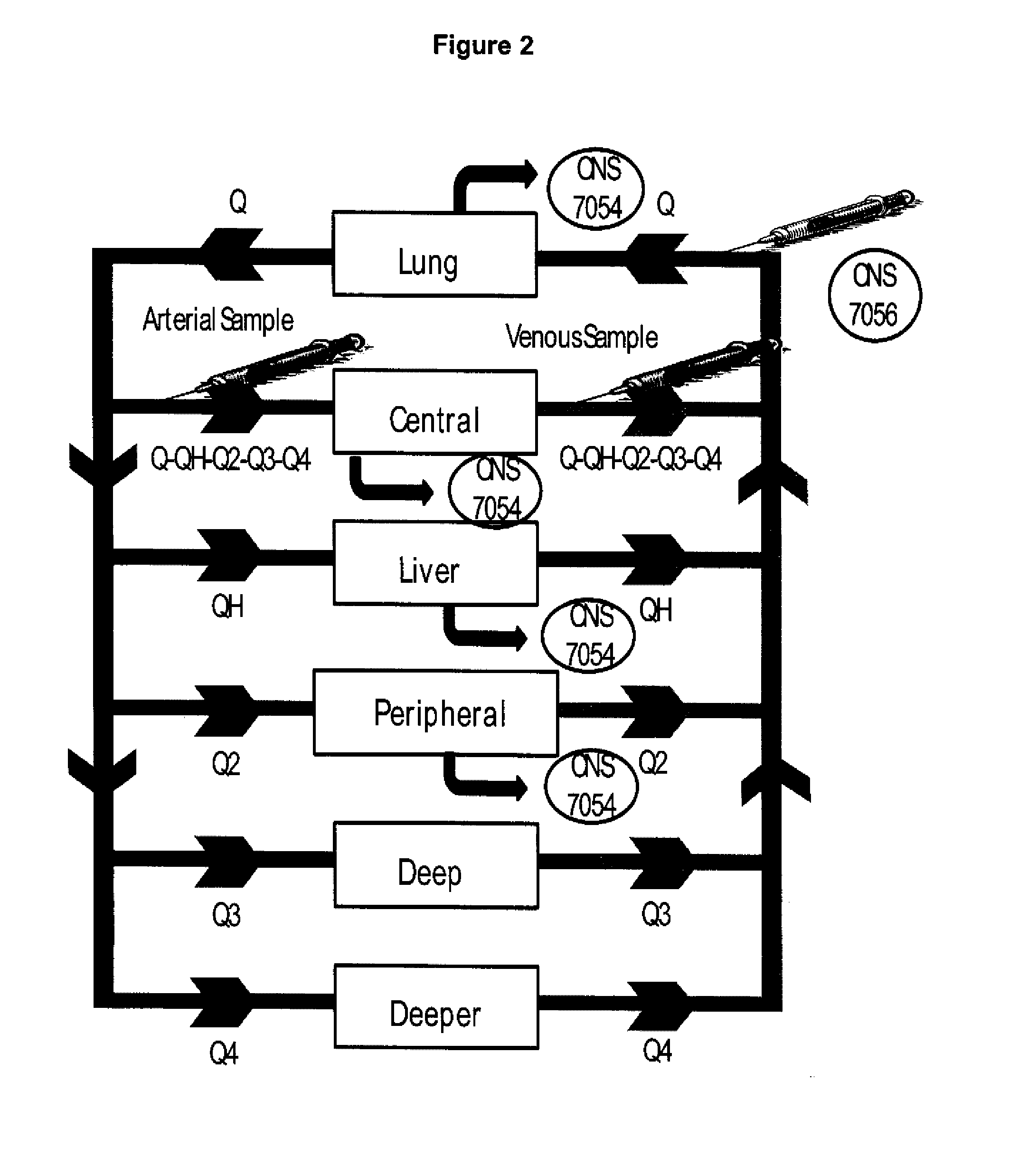
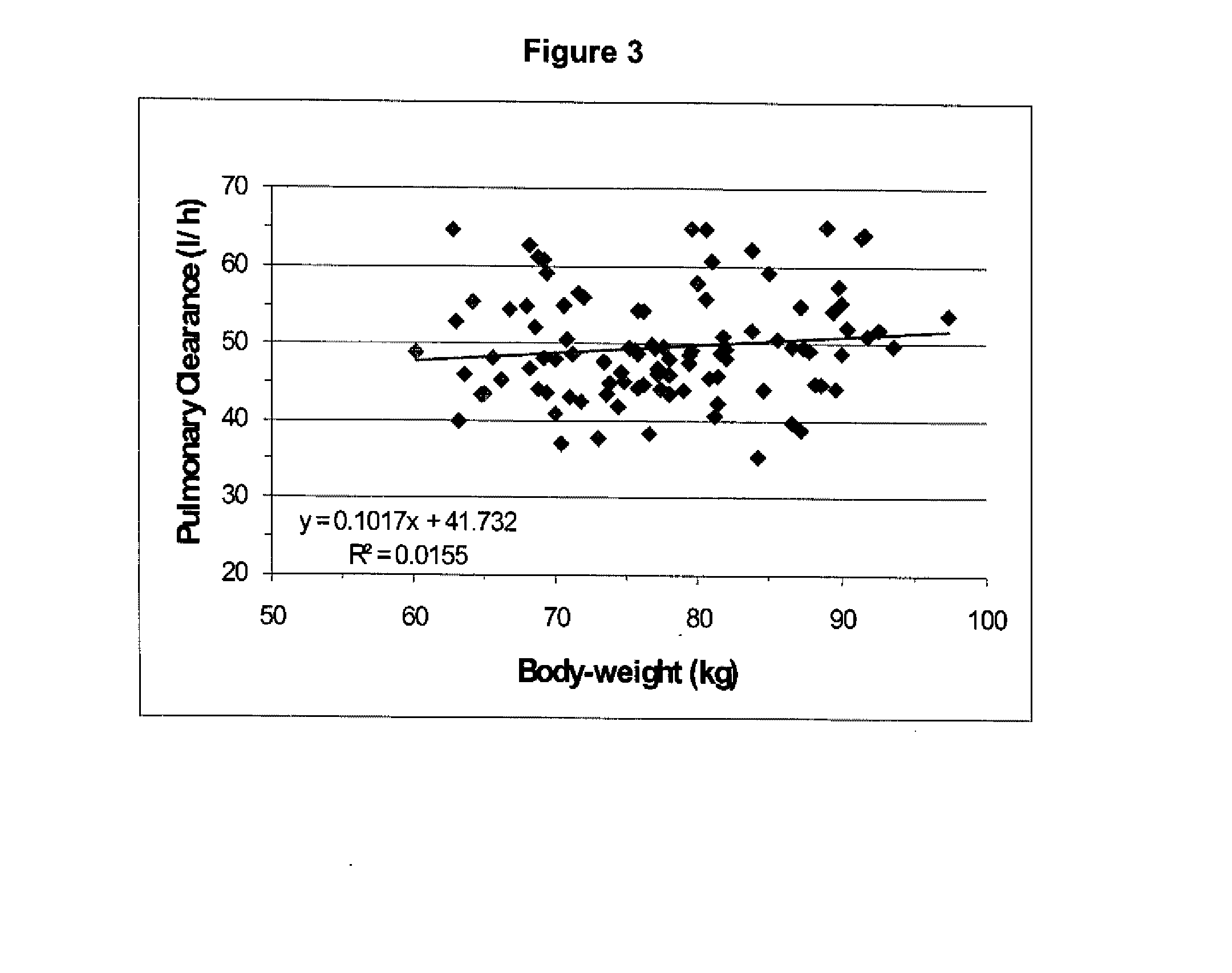
Dosing regimen for sedation with CNS 7056 (remimazolam)
ActiveUS20140080815A1Good sedative effectSafe and convenientBiocideNervous disorderDosing regimenRegimen
The invention relates to a dosing regimen for sedation with the fast-acting benzodiazepine CNS 7056 in combination with an opioid, in particular fentanyl, whereas CNS 7056 is given in a dose of 2 to 10 mg, preferably between 4 and 9 mg and most preferably between 5 and 8 mg.
Owner:PAION UK
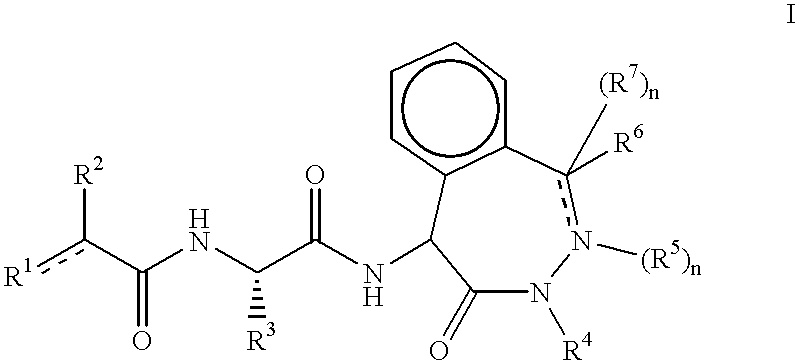
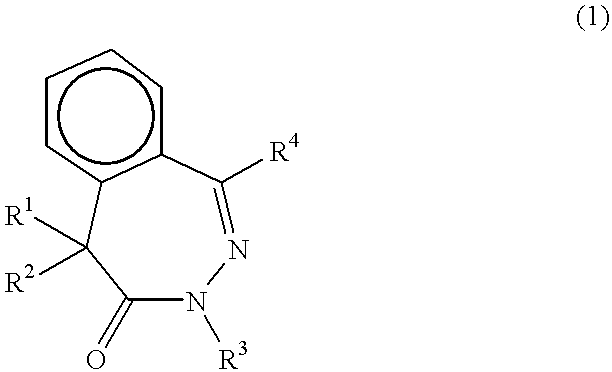
Benzodiazepinone beta -amyloid inhibitors: arylacetamidoalanyl derivatives
InactiveUS20020022621A1Alter one or more clinical indicia of disease activityBiocidePeptide/protein ingredientsAmyloidDisease cause
There is provided a series of arylacetamidoalanyl derivatives of benzodiazepinones of Formula I wherein R1 through R7 and n are defined herein, which are inhibitors of beta-amyloid peptide (beta-AP) production and are useful in the treatment of Alzheimer's Disease and other conditions characterized by aberrant extract cellular deposition of amyloid.
Owner:BRISTOL MYERS SQUIBB CO
Compositions and methods relating to novel compounds and targets thereof
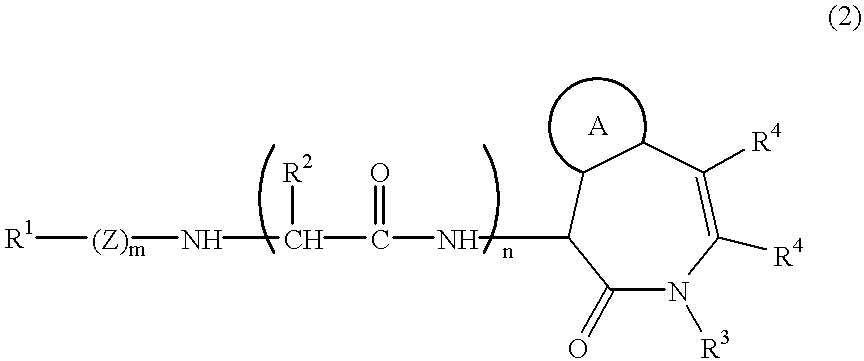
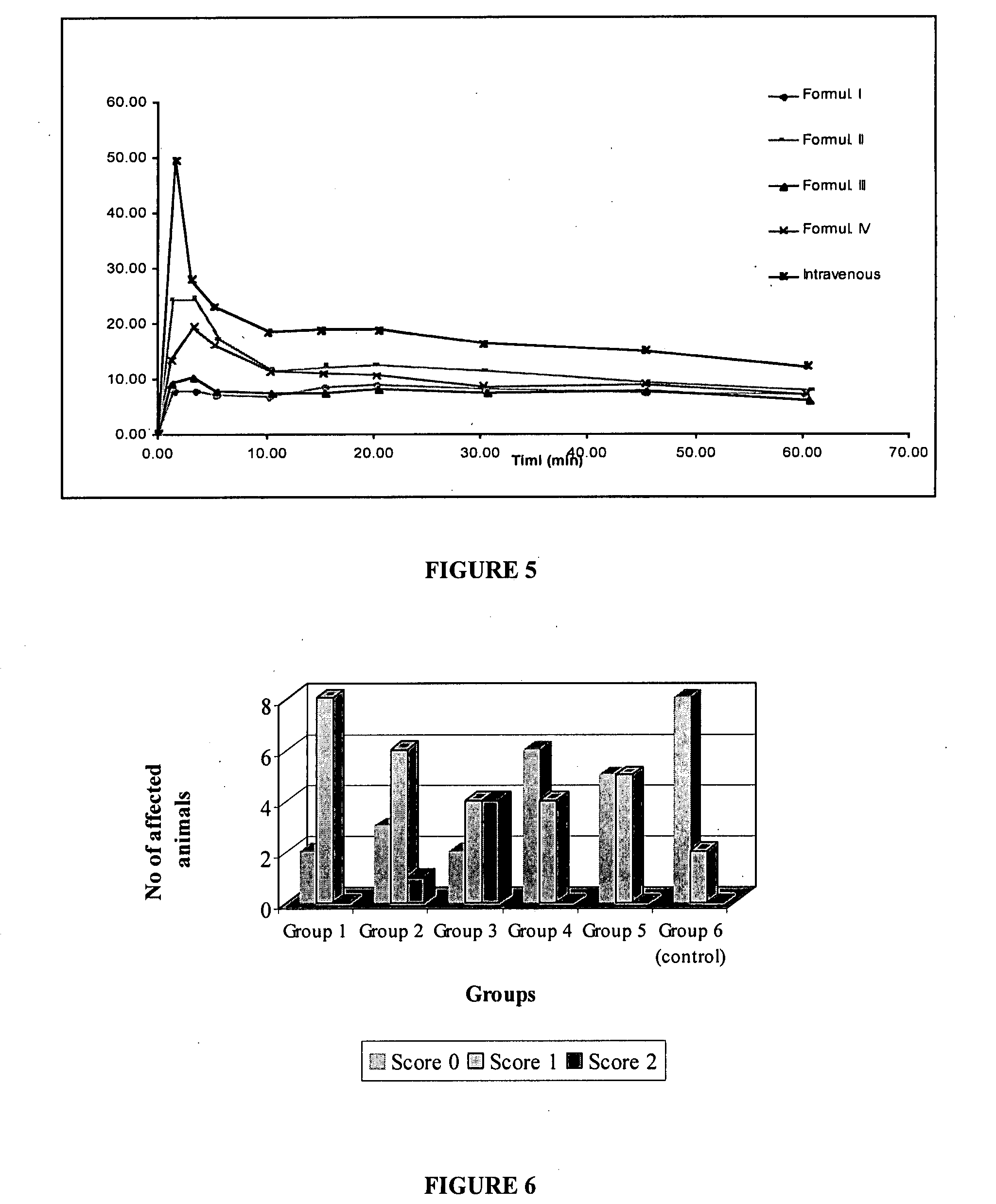
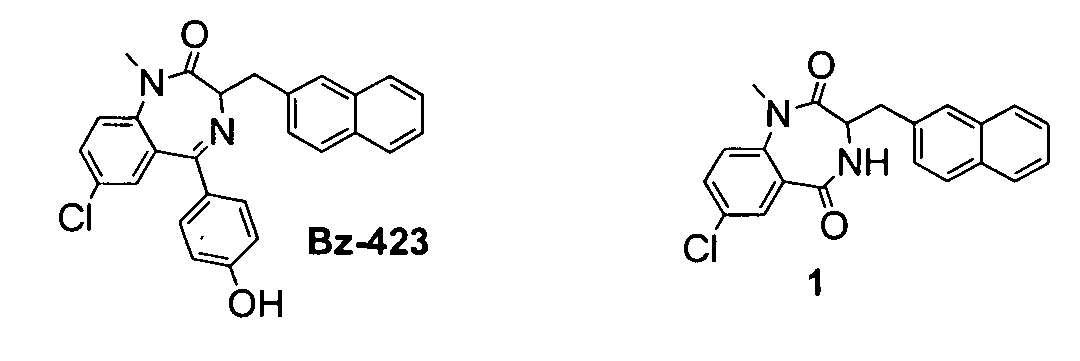
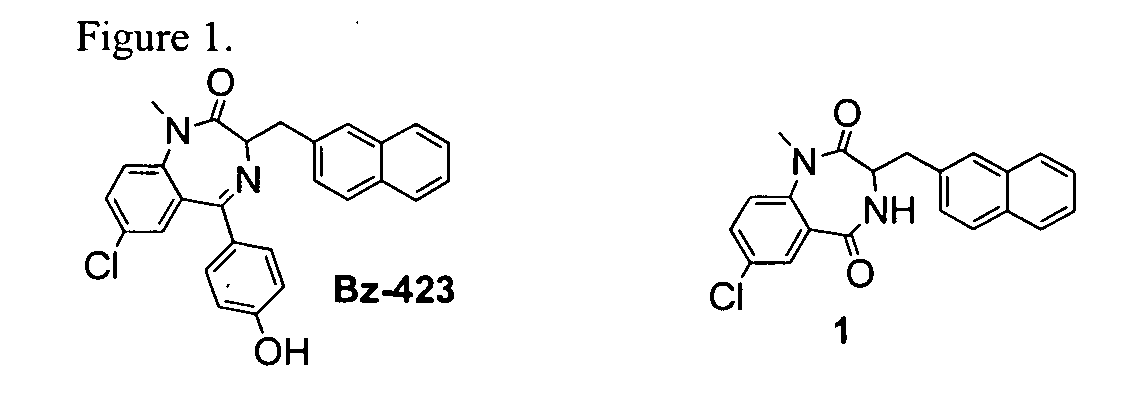
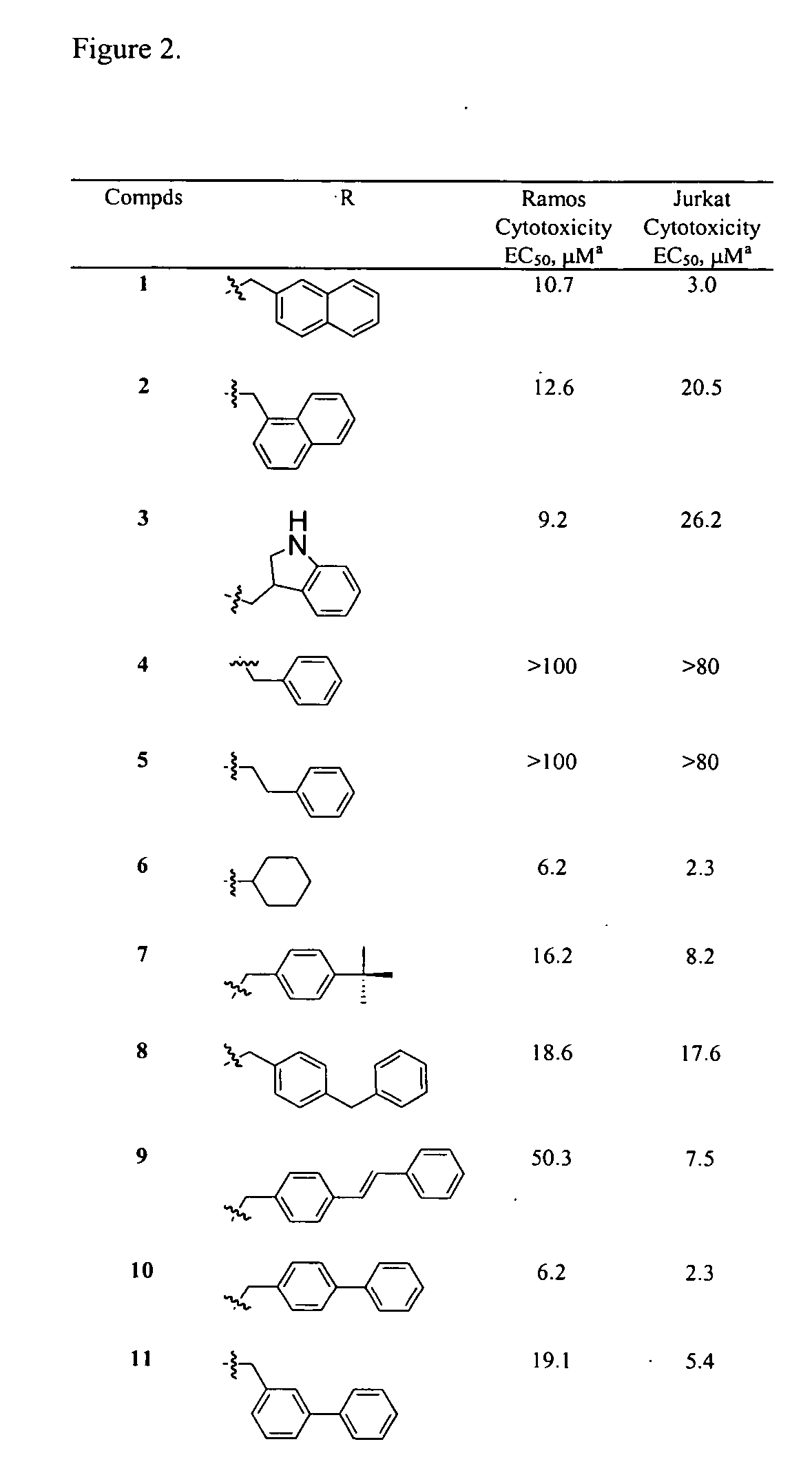
InactiveUS20060025388A1Raise active oxygen levelsReduce activationBiocideHydroxy compound active ingredientsBenzodiazepineAutoimmune responses
The present invention relates to novel chemical compounds, methods for their discovery, and their therapeutic use. In particular, the present invention provides benzodiazepine derivatives and methods of using benzodiazepine derivatives as therapeutic agents to treat a number of conditions associated with the faulty regulation of the processes of programmed cell death, autoimmunity, inflammation, and hyperproliferation, and the like.
Owner:RGT UNIV OF MICHIGAN
Pyrrolobenzodiazepines
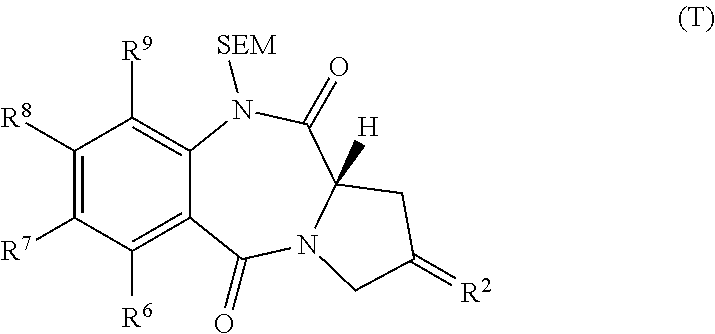
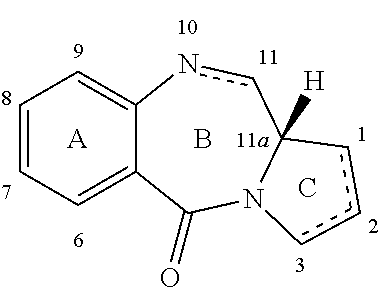
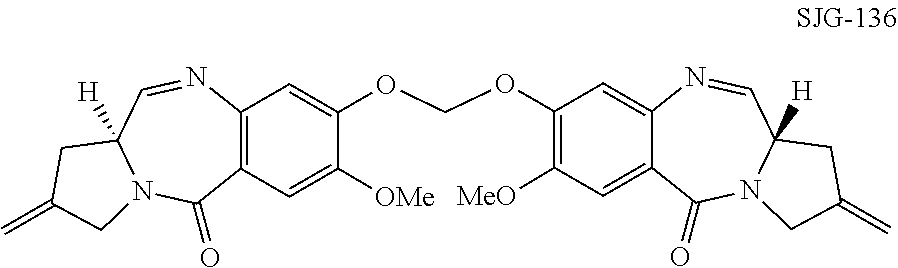
The invention relates to certain pyrrolobenzodiazepines (PBDs), and in particular pyrrolobenzodiazepine dimers bearing C2 substitutions, including compounds of formula (T): wherein: R2 is CHR2A, and R2A is independently selected from H, R, CO2R, COR, CHO, CO2H, and halo; R6 and R9 are independently selected from H, R, OH, OR, SH, SR, NH2, NHR, NRR′, NO2, Me3Sn and halo; R7 is independently selected from H, R, OH, OR, SH, SR, NH2, NHR, NRR′, NO2, Me3Sn and halo; R8 is independently selected from H, R, OH, OR, SH, SR, NH2, NHR, NRR′, NO2, Me3Sn and halo; R is independently selected from optionally substituted C1-12 alkyl, C3-20 heterocyclyl and C5-20 aryl groups; or the compound is a dimer with each monomer being of formula (M), where the R7 groups or R8 groups of each monomer form together a dimer bridge having the formula —X—R″—X— linking the monomers; wherein R″ is a C3-12 alkylene group, which chain may be interrupted by one or more heteroatoms, e.g. O, S, N(H), and / or aromatic rings, e.g. benzene or pyridine; and each X is independently selected from O, S, or N(H); or any pair of adjacent groups from R6 to R9 together form a group —O—(CH2)p—O—, where p is 1 or 2, and salts and solvates thereof, and their use as intermediates for the preparation of other PBD compounds.
Owner:MEDIMMUNE LTD
Combinations of GABA modulators and anticonvulsants, and atypical antipsychotics
InactiveUS20050004106A1Reduce the amount requiredGood effectBiocideNervous disorderBenzodiazepineAtypical antipsychotic
This invention relates to combinations of an atypical antipsychotic, and a GABA modulator, a benzodiazepine, and / or an anticonvulsant drug, kits containing such combinations, pharmaceutical compositions comprising such combinations, and methods of using such combinations to treat patients suffering from treatment-resistant anxiety disorders, psychotic disorders or conditions, or mood disorders or conditions.
Owner:PFIZER INC
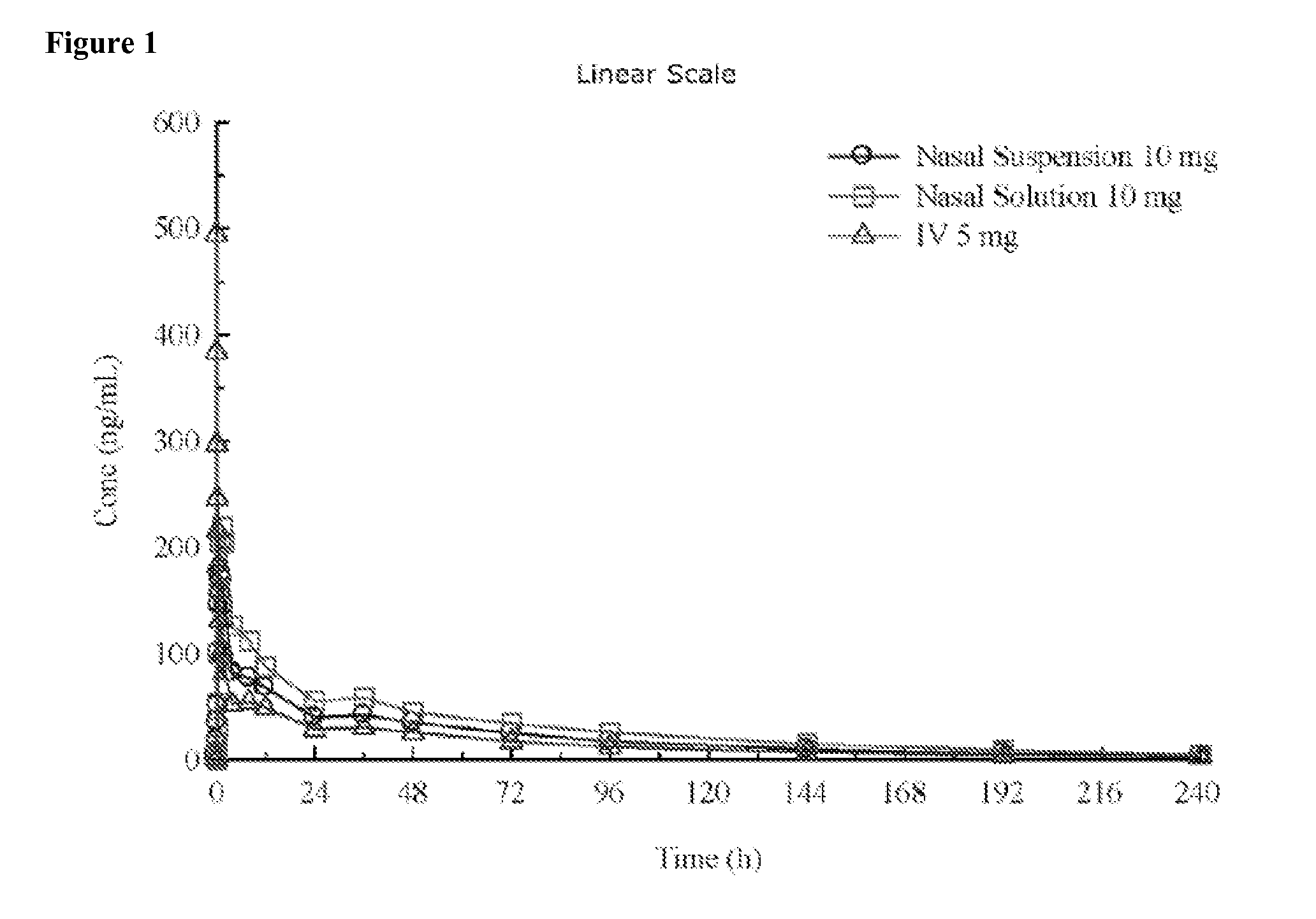
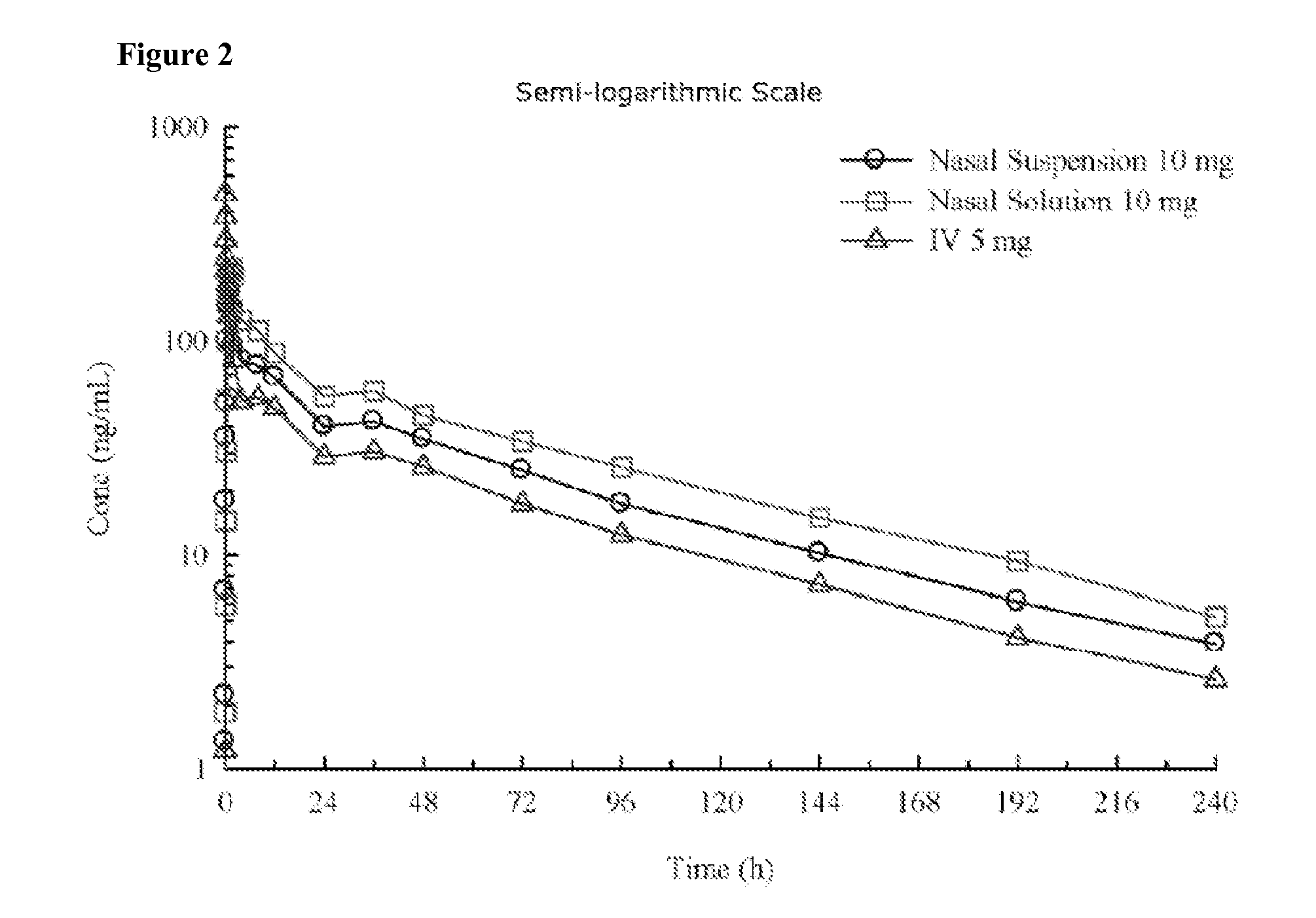
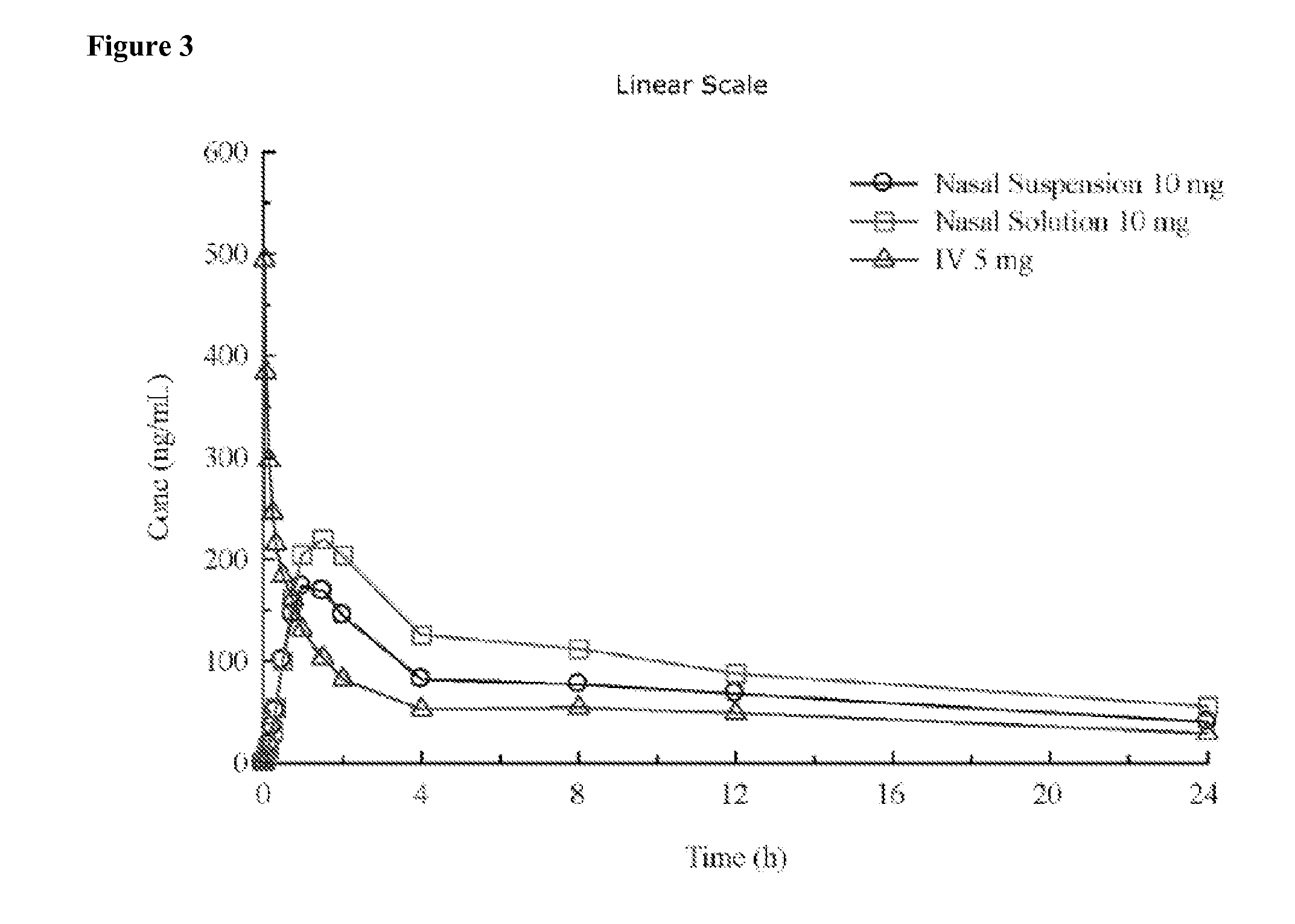
Administration of benzodiazepine compositions
InactiveUS20090258865A1Improve appearance and odor and tasteAvoid degradationBiocideNervous disorderDrugBenzodiazepine
The invention relates to pharmaceutical compositions comprising one or more benzodiazepine drugs for nasal administration, methods for producing and for using such compositions.
Owner:NEURELIS INC
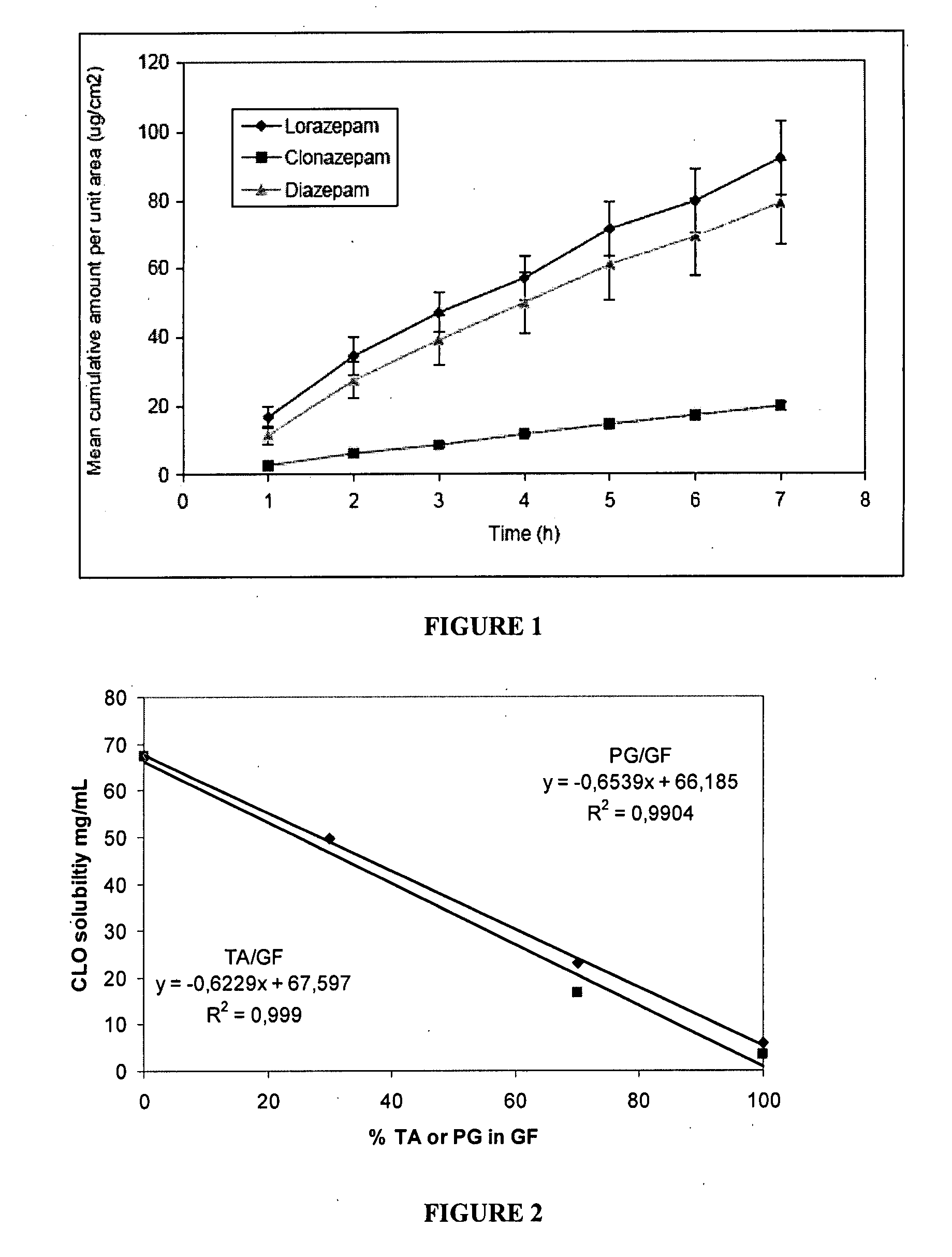
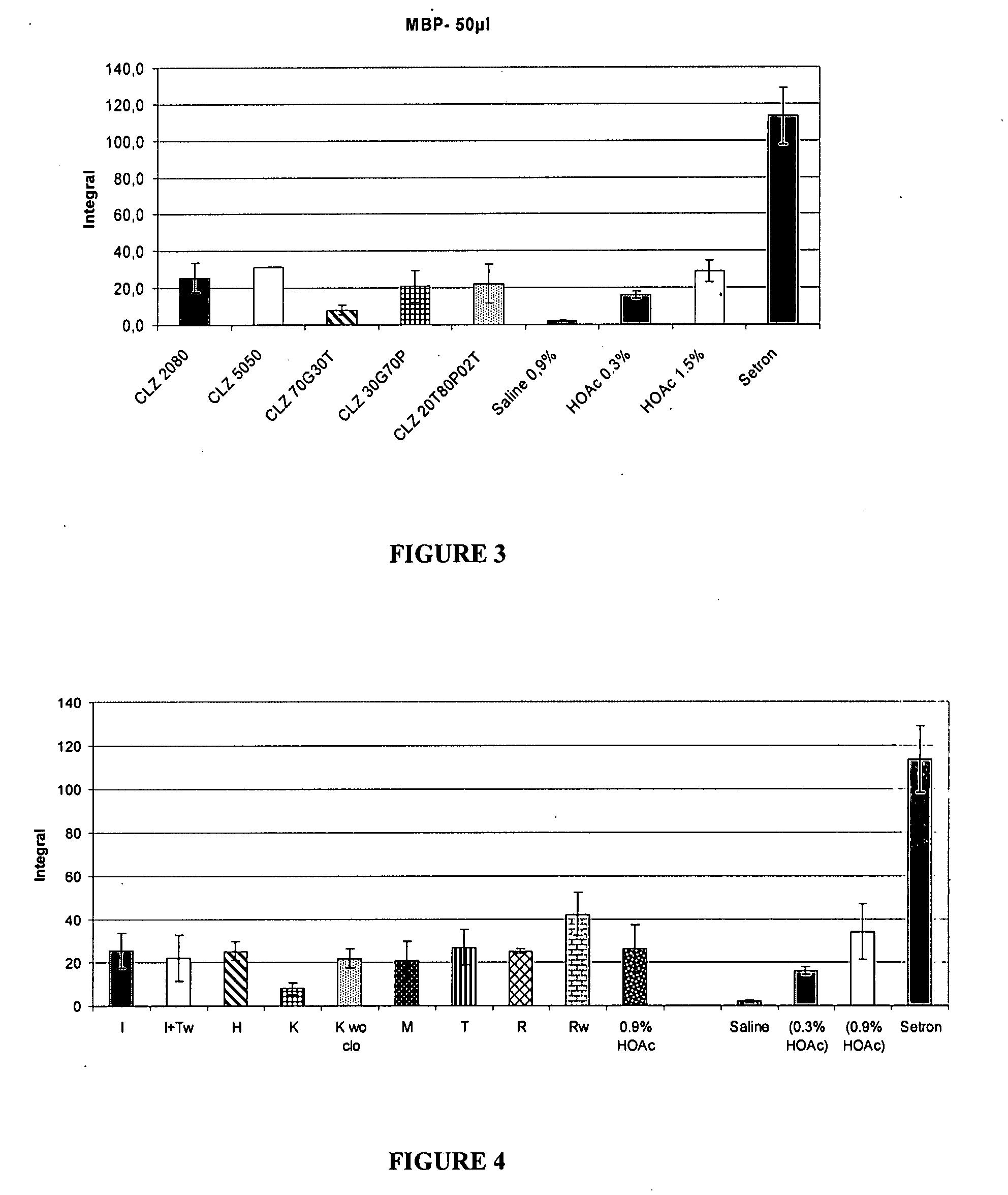
Pharmaceutical compositions of benzodiazepines and method of use thereof
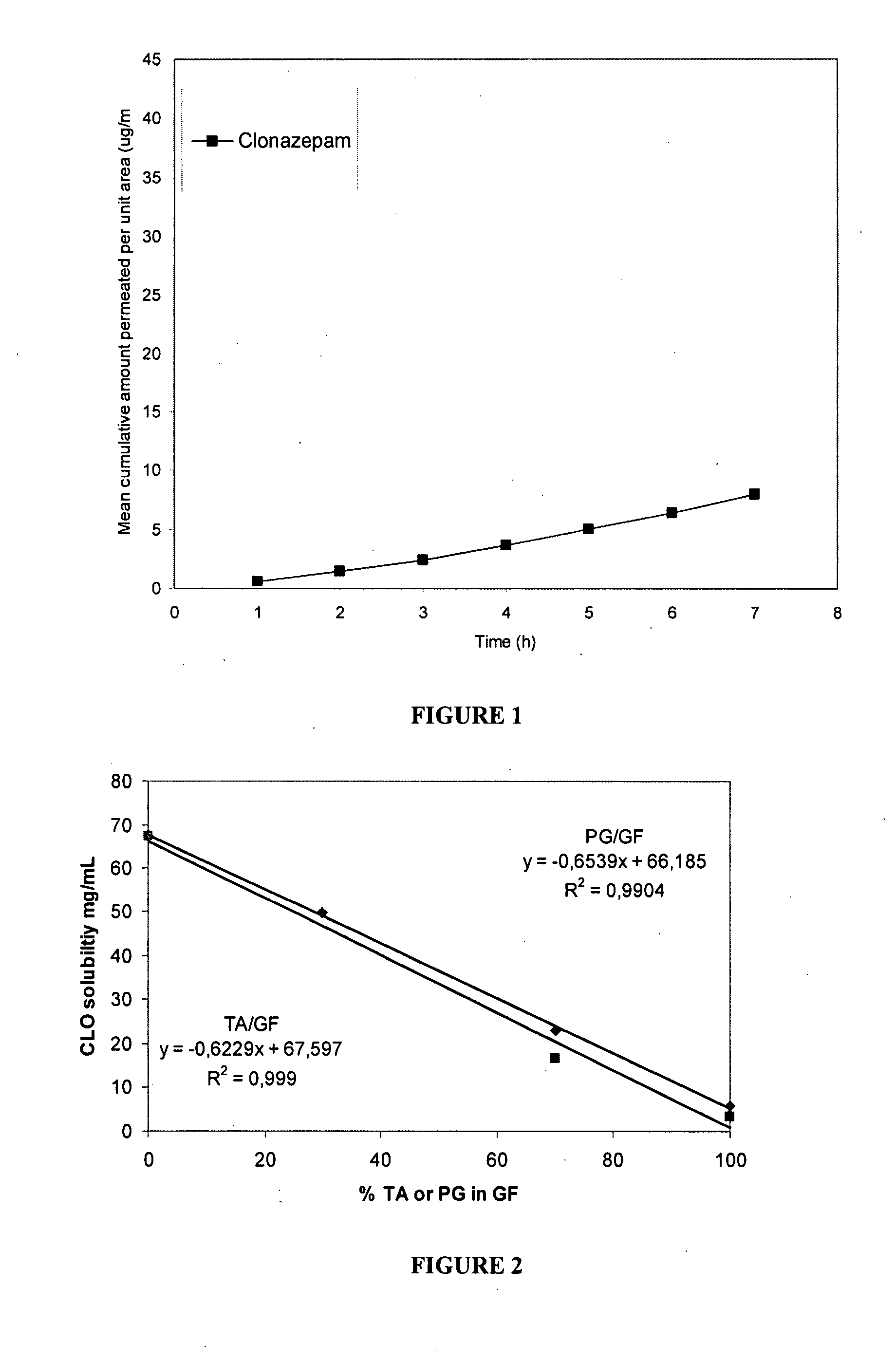
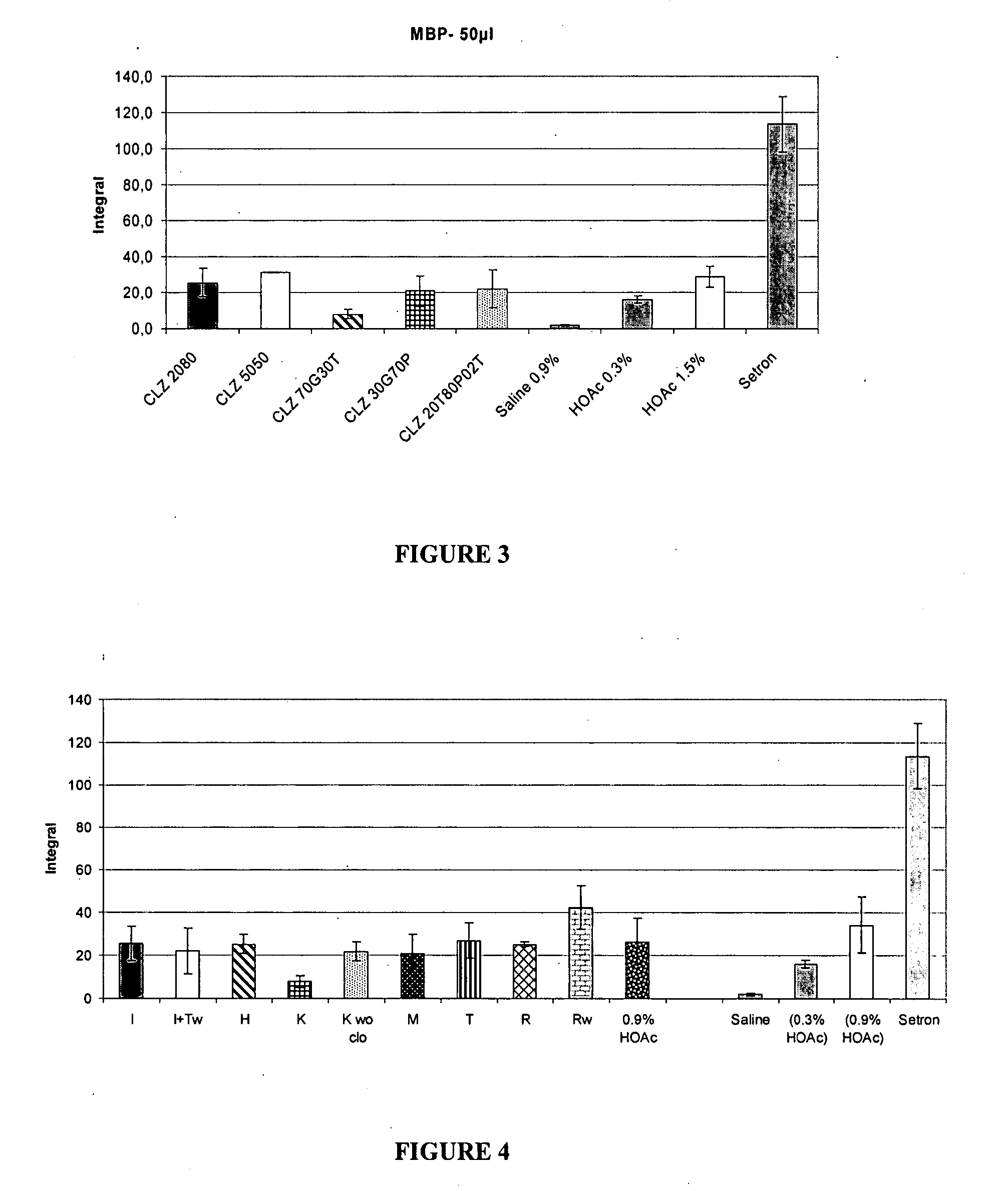
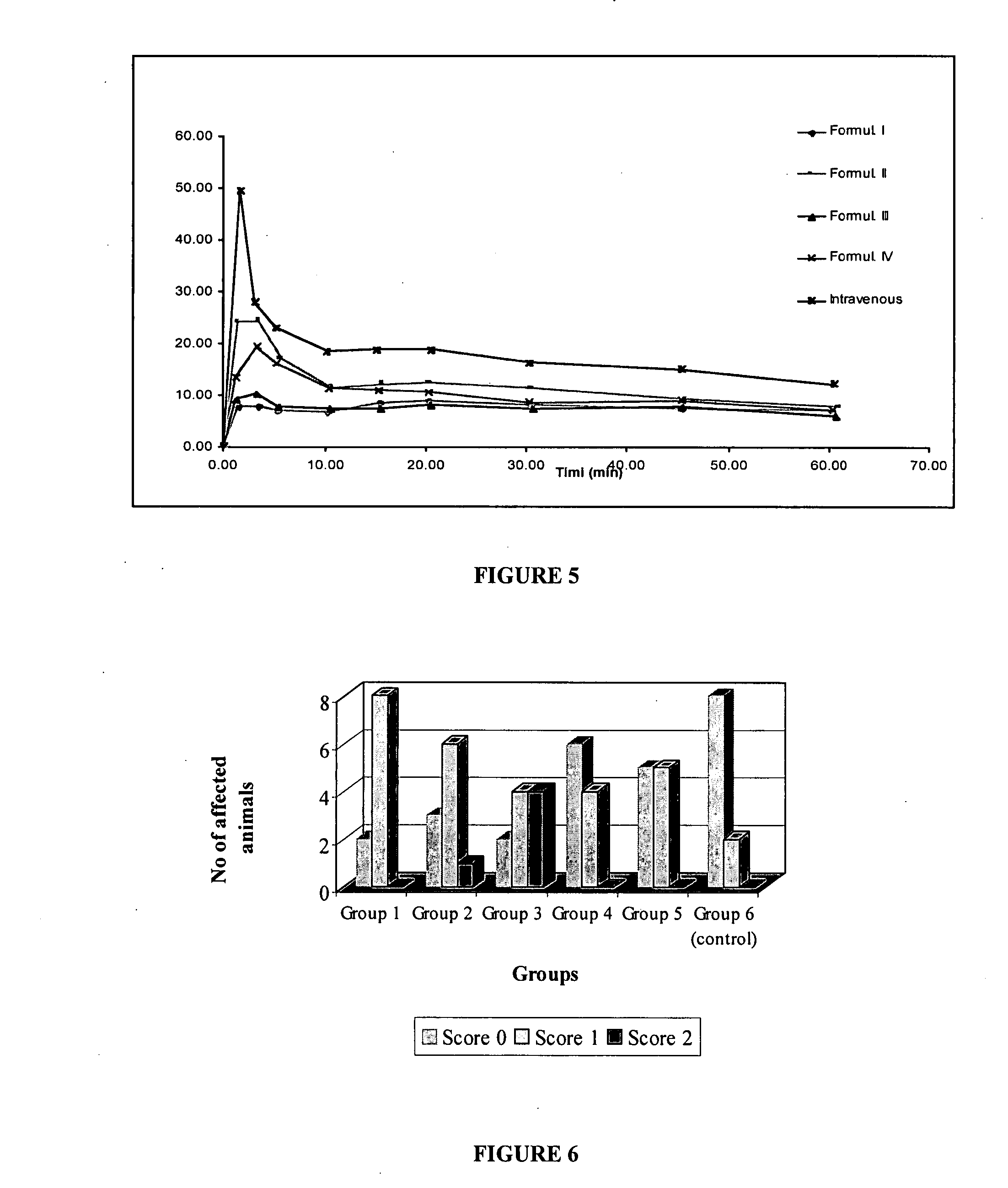
The present invention includes benzodiazepine compositions formulated for intranasal administration, comprising a binary solvent system comprising a first solvent in which the benzodiazepine is soluble, the first solvent capable of penetrating nasal mucosal tissue, and a second solvent in which the benzodiazepine in less soluble. The compositions of the present invention may be used to treat a variety of disorders including, but not limited to, panic attacks, muscle spasms, anxiety, and seizures. In one aspect, the present invention relates to a fast-acting, clonazepam composition for transnasal administration that can be used for the treatment of seizure clusters.
Owner:JAZZ PHARMA
C8—linked pyrrolo[2,1-c][1,4]benzodiazepine-acridone/acridine hybrids
The present invention provides novel pyrrolo-[2,1-c][1,4]benzodiazepine hybrids useful as anti-tumour agents and a process for the preparation thereof.
Owner:COUNCIL OF SCI & IND RES
Novel 1,4-benzodiazepine-2,5-diones with therapeutic properties
ActiveUS20070111994A1Rapid and easy identificationReduce the amount requiredBiocideAntipyreticDiketoneBenzene
The present invention relates to novel chemical compounds, methods for their discovery, and their therapeutic use. In particular, the present invention provides novel 1,4-benzodiazepine-2,5-dione compounds, and methods of using novel 1,4-benzodiazepine-2,5-dione compounds as therapeutic agents to treat a number of conditions associated with the faulty regulation of the processes of programmed cell death, autoimmunity, inflammation, hyperproliferation, and the like.
Owner:RGT UNIV OF MICHIGAN
2-Substituted imidazo[1,2-A]pyridine derivatives
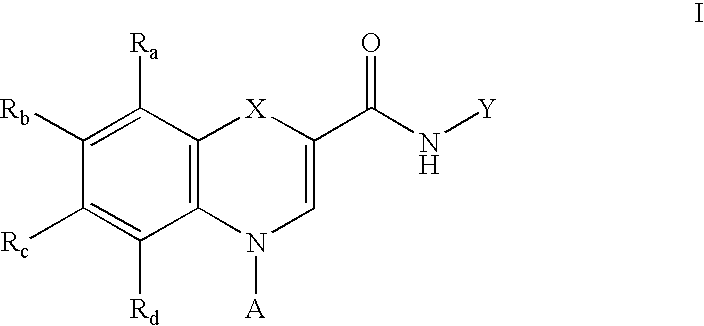
Disclosed are compounds of the formula: and the pharmaceutically acceptable salts, prodrugs and solvates thereof, wherein X, A, B, C, D and W are defined herein, which compounds bind with high selectivity and high affinity to the benzodiazepine site of the GABAA receptors and are therefore useful in the treatment of certain central nervous system (CNS) diseases and as probes for the localization of GABAA receptors in tissue samples.
Owner:NEUROGEN
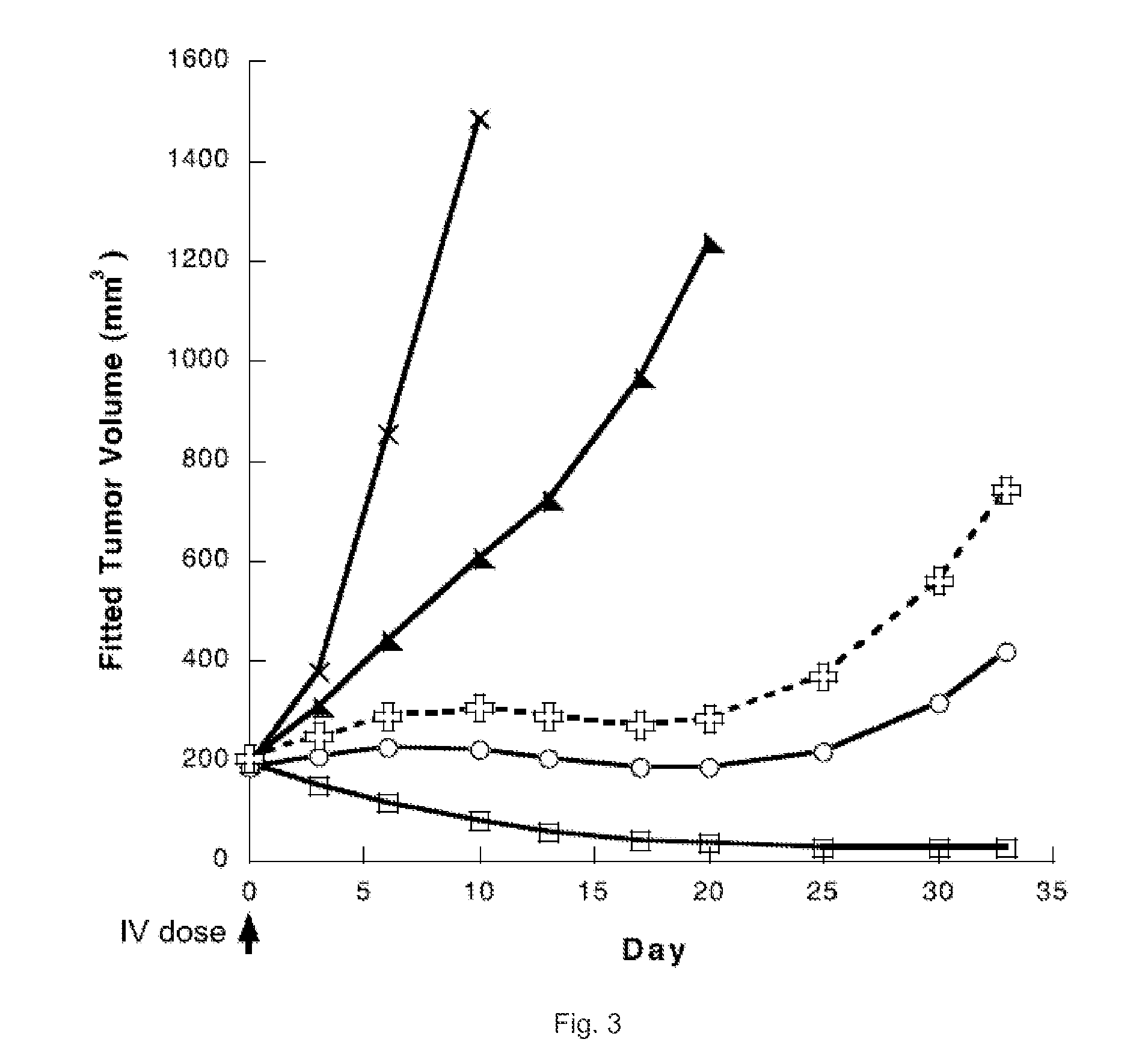
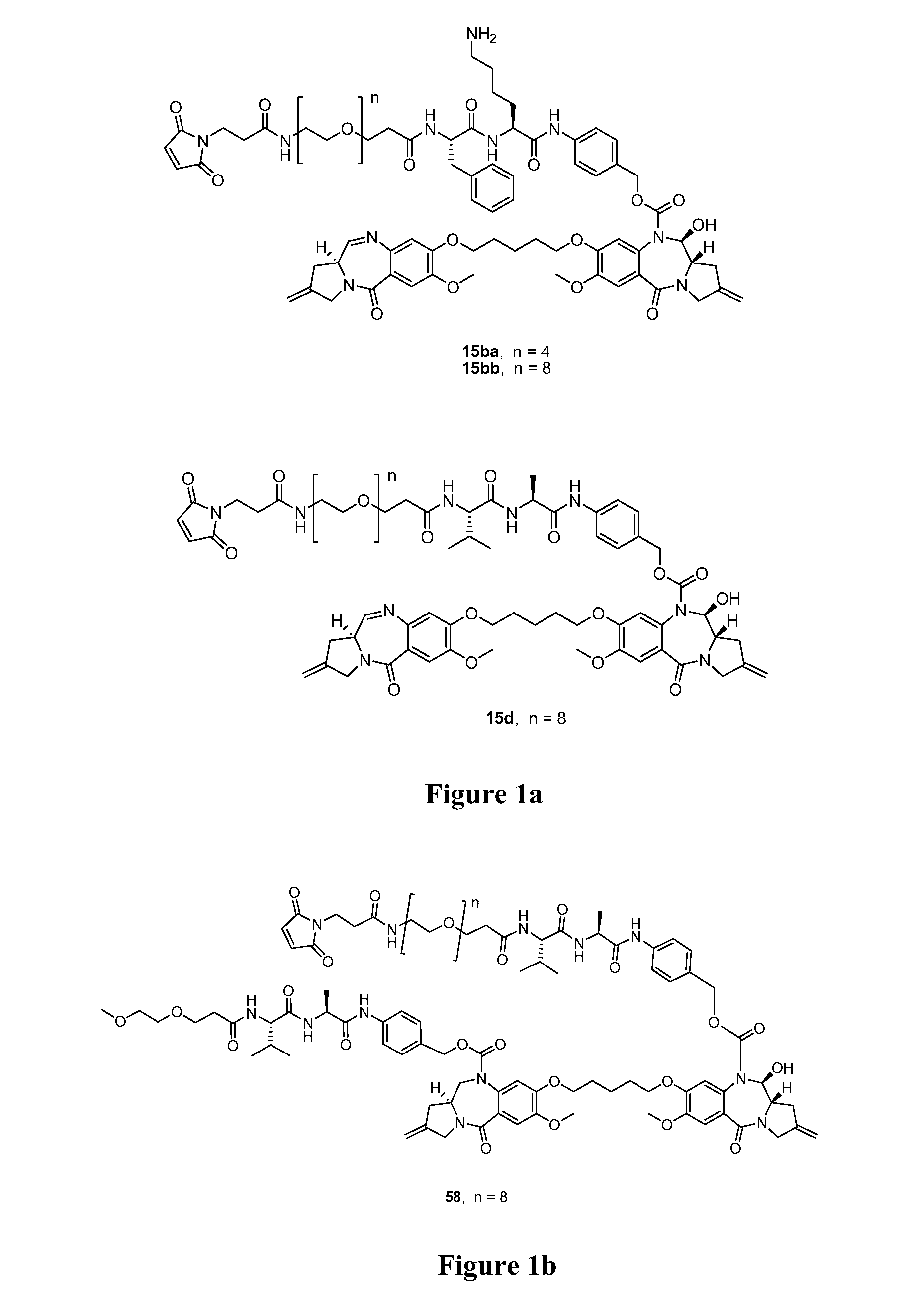
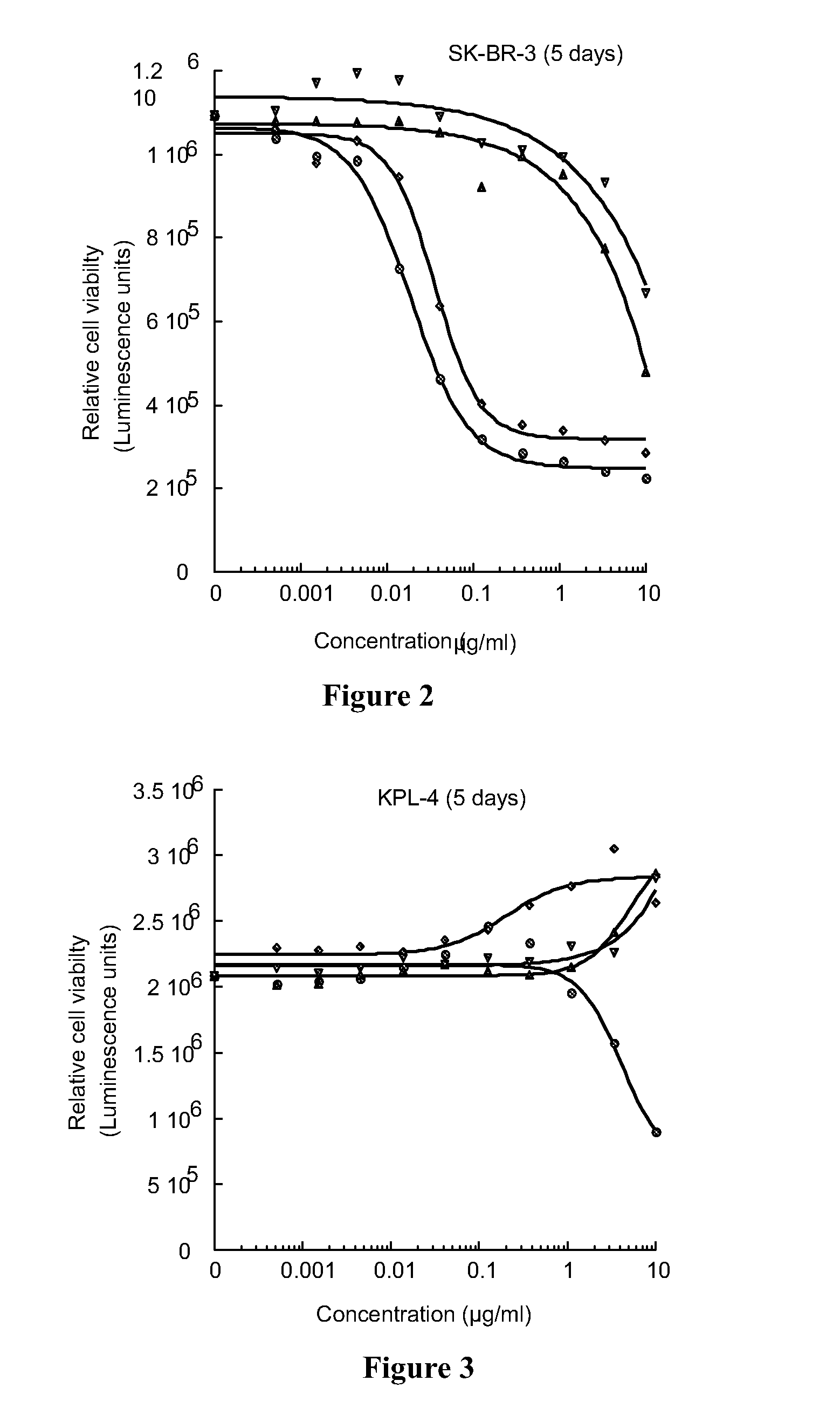
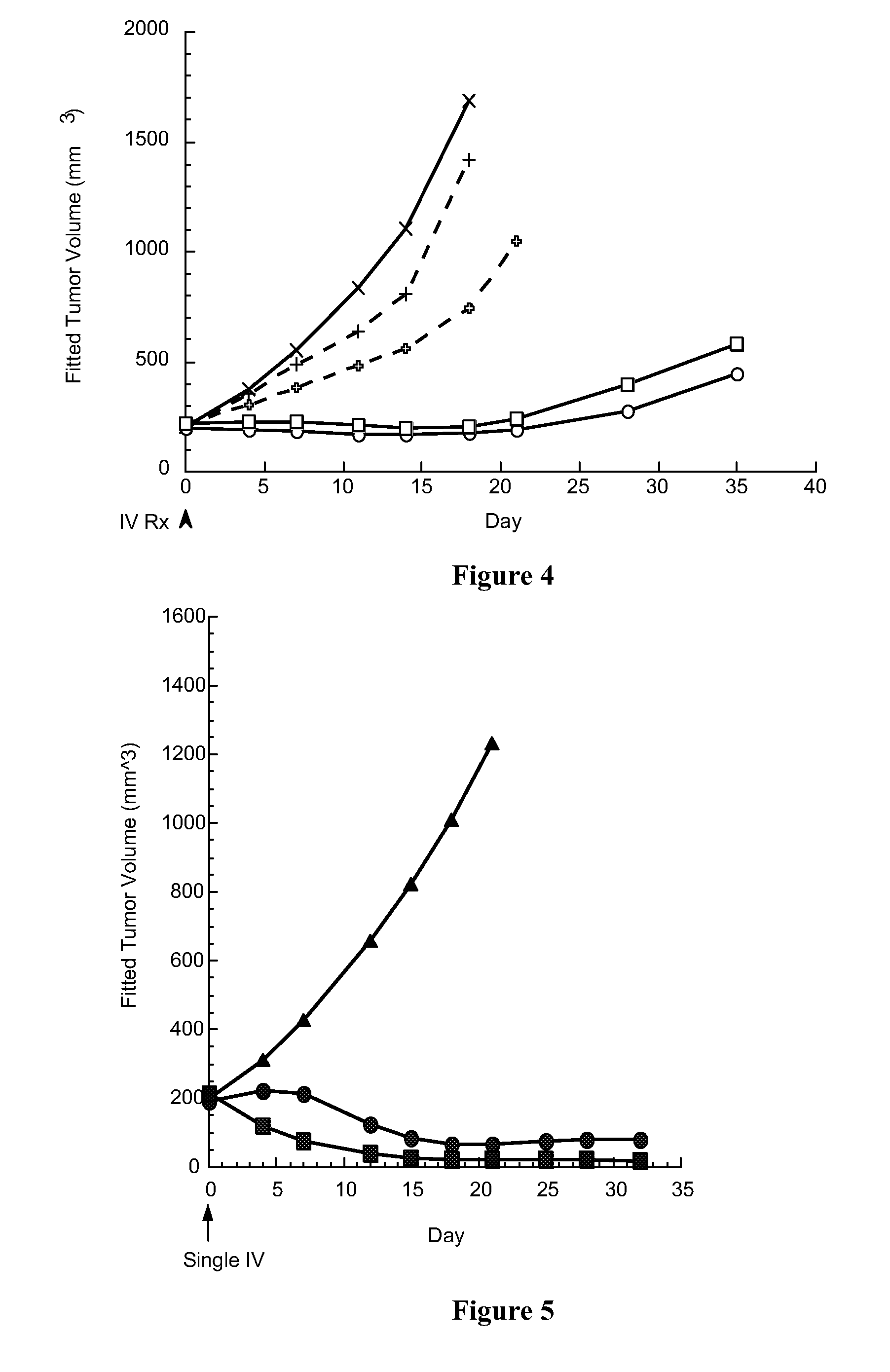
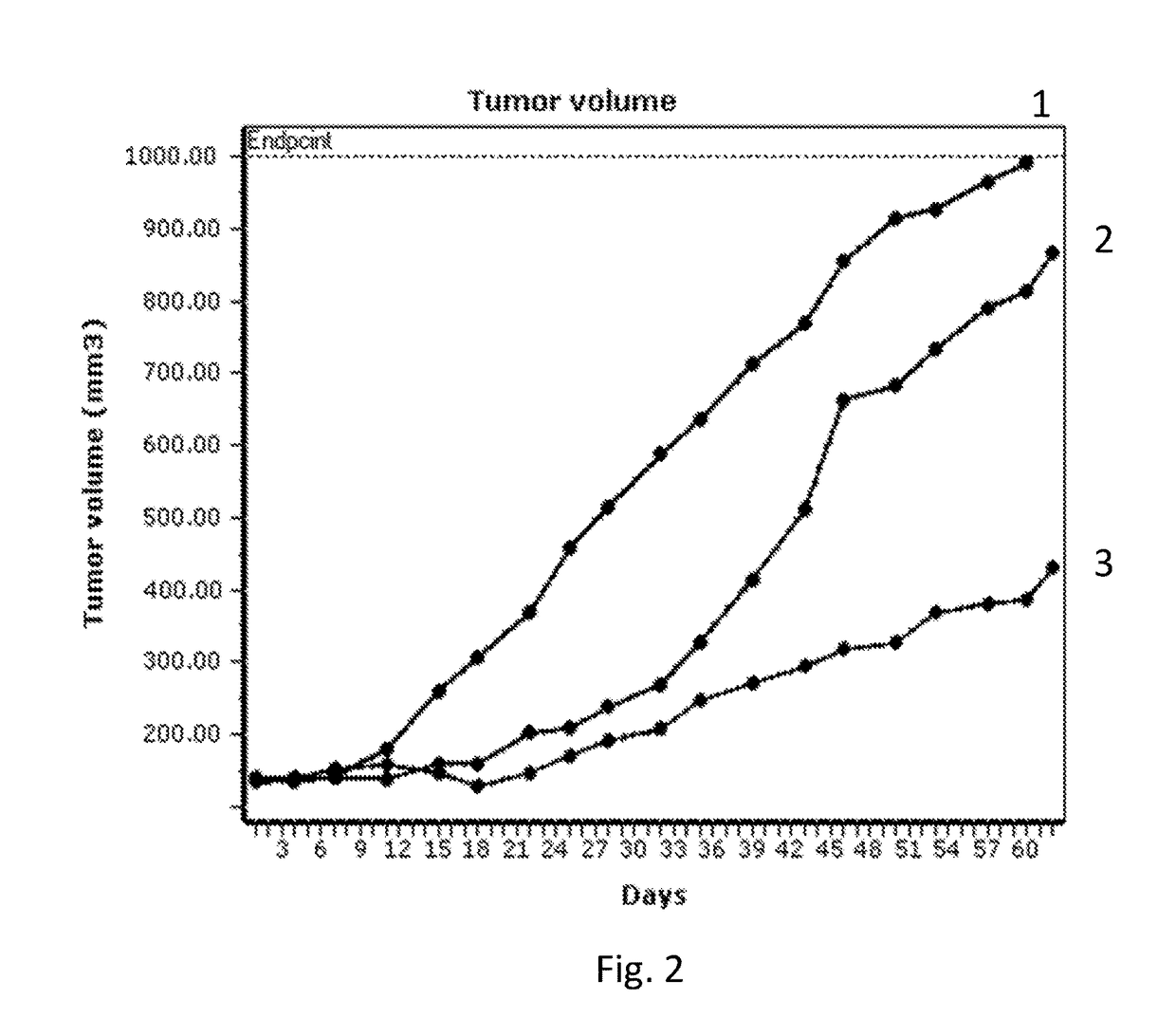
Cytotoxic benzodiazepine derivatives
ActiveUS20160106863A1Hinder abnormal cell growthTreating and lessening severityAntibacterial agentsNervous disorderBenzodiazineBenzodiazepine Compounds
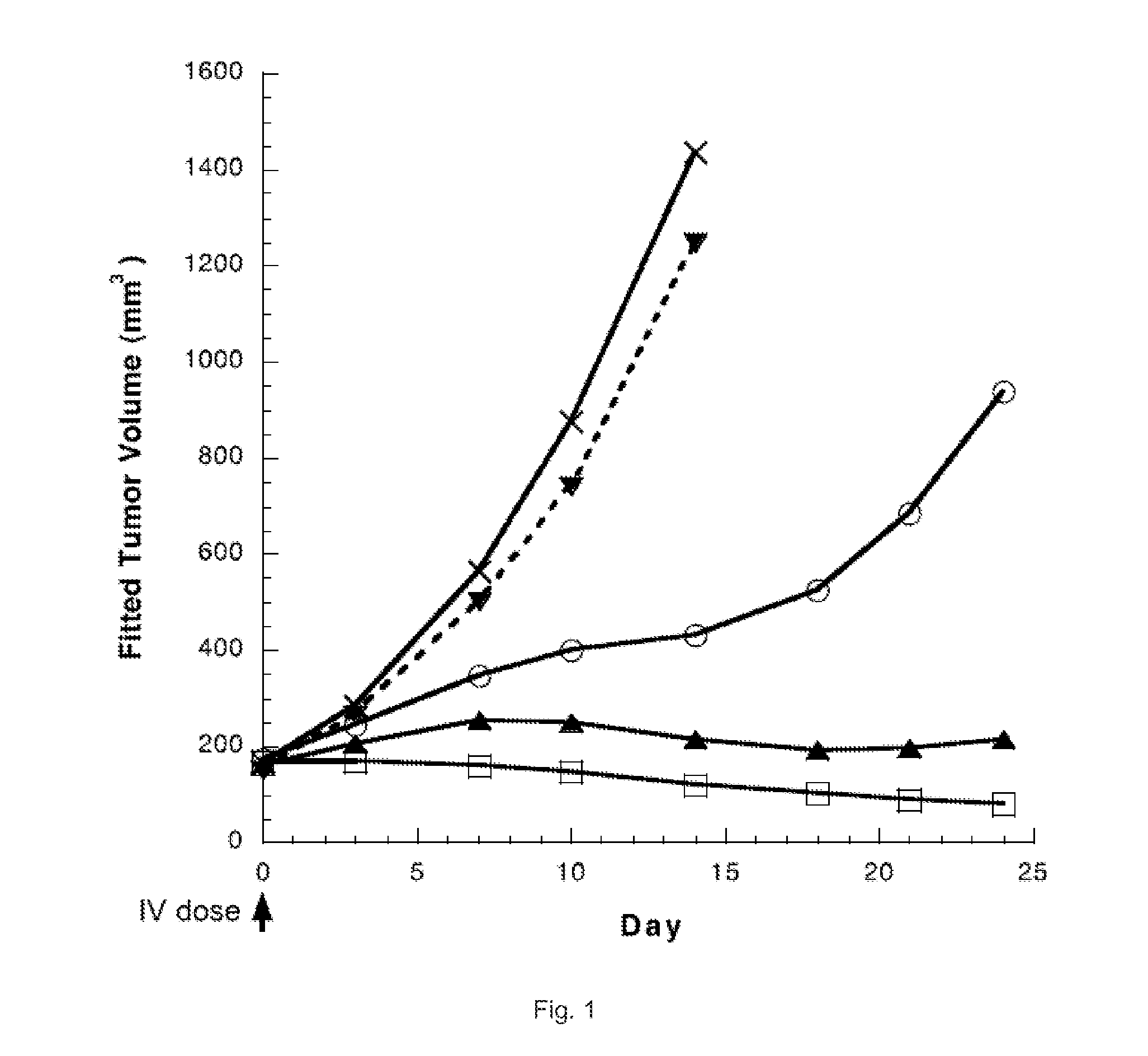
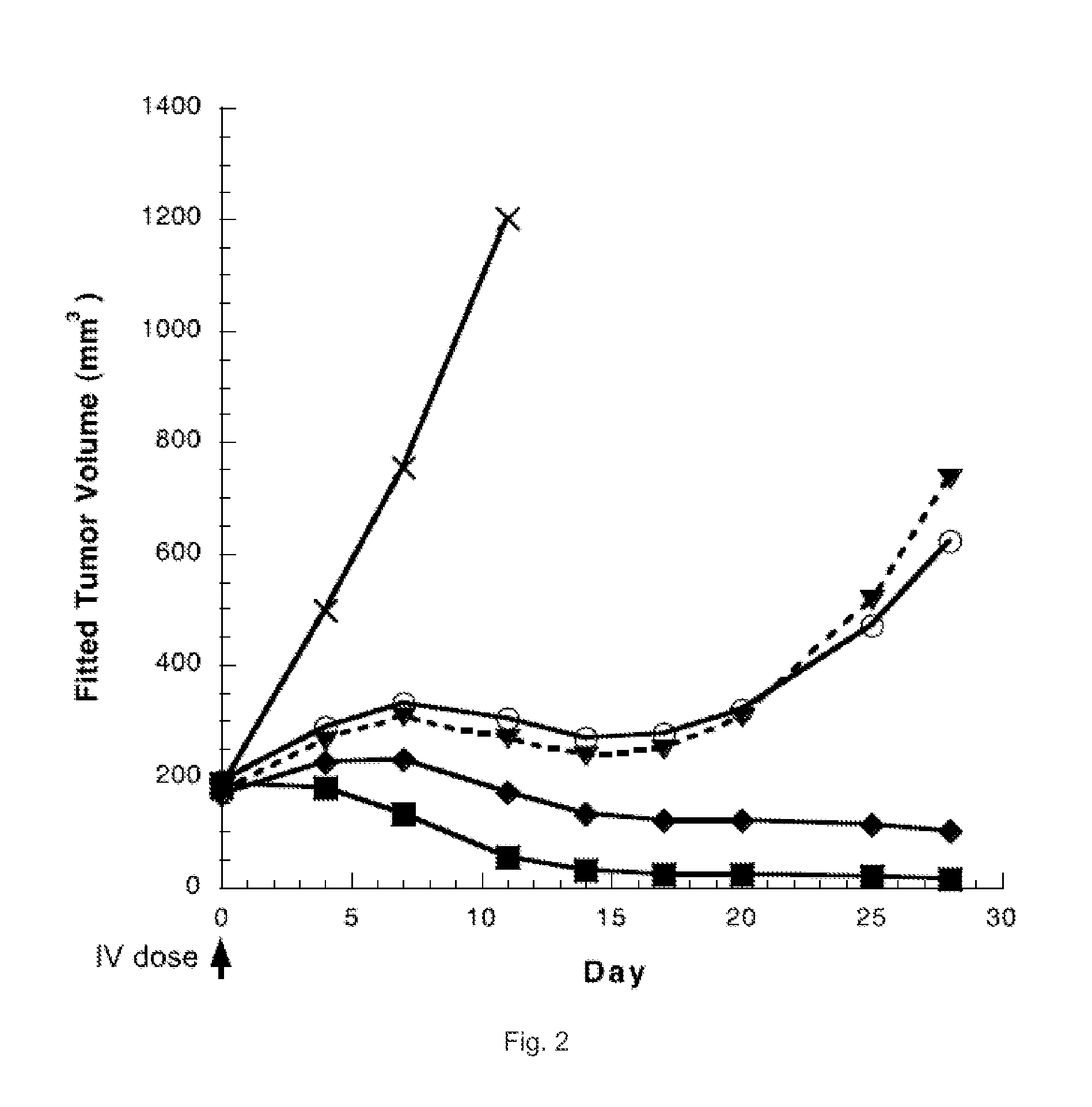
The invention relates to novel benzodiazepine derivatives with antiproliferative activity and more specifically to novel benzodiazepine compounds of formula (I)-(VII). The invention also provides conjugates of the benzodiazepine compounds linked to a cell-binding agent. The invention further provides compositions and methods useful for inhibiting abnormal cell growth or treating a proliferative disorder in a mammal using the compounds or conjugates of the invention.
Owner:IMMUNOGEN INC
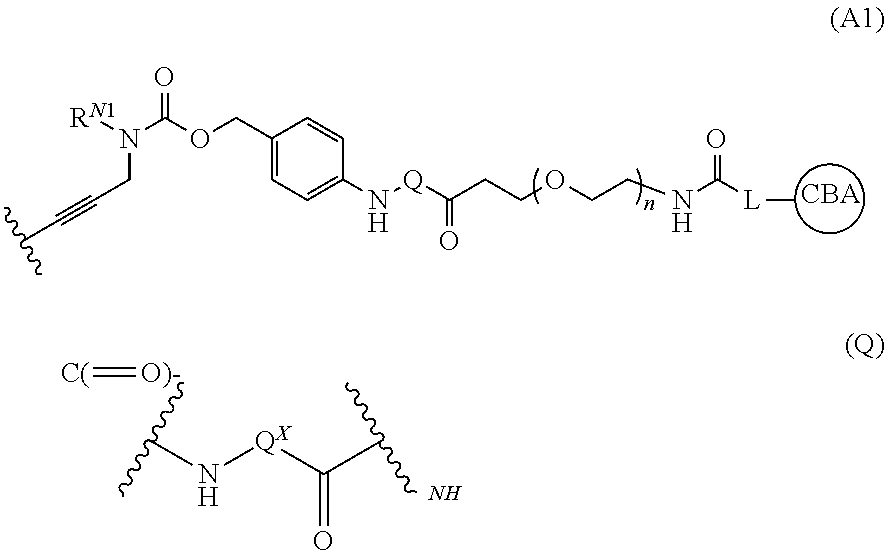
Pyrrolobenzodiazepines and conjugates thereof
ActiveUS20170239365A1Organic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsDipeptidePyrrolobenzodiazepine
A conjugate comprising a PBD dimer linked from the tether joining the two PBD units, wherein the linker is of formula (A1): (Formula (A1)) wherein RN1 is selected from H and C1-4 alkyl; L is a linker connected to a cell binding agent; CBA is the cell binding agent; n is an integer selected in the range of 0 to 48; Q is: (Formula (Q)), where Qx is such that Q is an amino-acid residue, a dipeptide residue or a tripeptide residue.
Owner:MEDIMMUNE LTD
Administration of benzodiazepine compositions
ActiveUS20130065886A1Prevent degradationGood lookingBiocideNervous disorderBenzodiazepineNasal administration
The invention relates to pharmaceutical compositions comprising one or more benzodiazepine drugs for nasal administration, methods for producing and for using such compositions.
Owner:NEURELIS INC
Substituted 4H-1,4-benzothiazine-2-carboxamide: GABA brain receptor ligands
Disclosed are 4H-1,4-Benzothiazine-2-carboxamides. These compounds are highly selective agonists, antagonists or inverse agonists for GABAa brain receptors or prodrugs of agonists, antagonists or inverse agonists for GABAa brain receptors. These compounds are useful in the diagnosis and treatment of anxiety, depression, sleep, cognitive and seizure disorders, and overdose with benzodiazepine drugs and for enhancement of alertness. Pharmaceutical compositions, including packaged pharmaceutical compositions, are further provided. Compounds of the invention are also useful as probes for the localization of GABAA receptors in tissue samples.
Owner:NEUROGEN
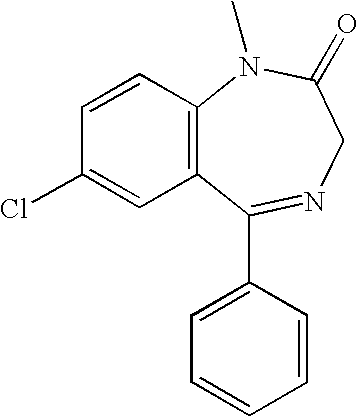
Pharmaceutical compositions of clonazepam and method of use thereof
The present invention includes benzodiazepine compositions formulated for intranasal administration, comprising a binary solvent system comprising a first solvent in which the benzodiazepine is soluble, the first solvent capable of penetrating nasal mucosal tissue, and a second solvent in which the benzodiazepine in less soluble. The compositions of the present invention may be used to treat a variety of disorders including, but not limited to, panic attacks, muscle spasms, anxiety, and seizures. In one aspect, the present invention relates to a fast-acting, clonazepam composition for transnasal administration that can be used for the treatment of seizure clusters.
Owner:JAZZ PHARMA INC
Processes for the preparation of benzodiazepine derivatives
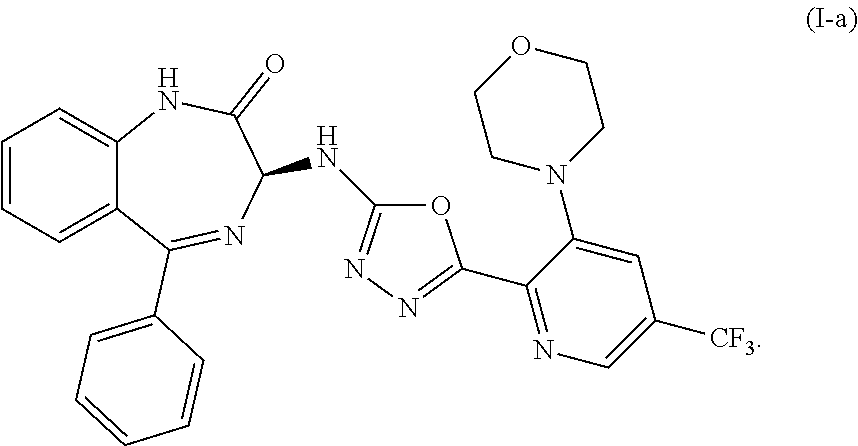
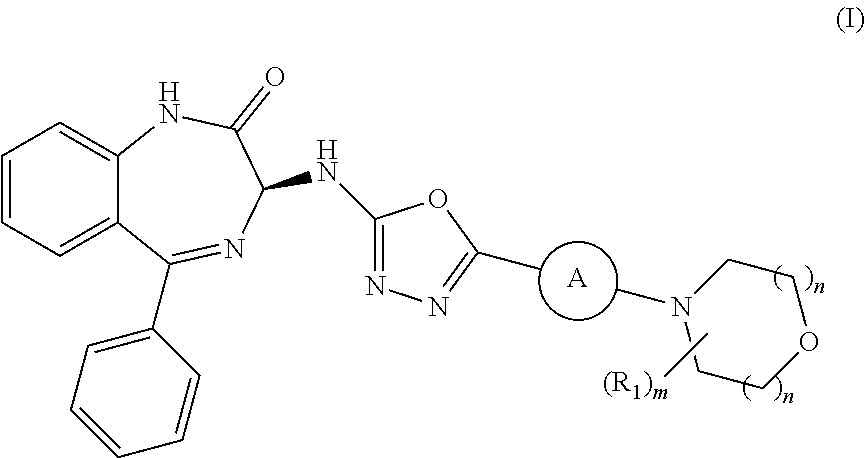
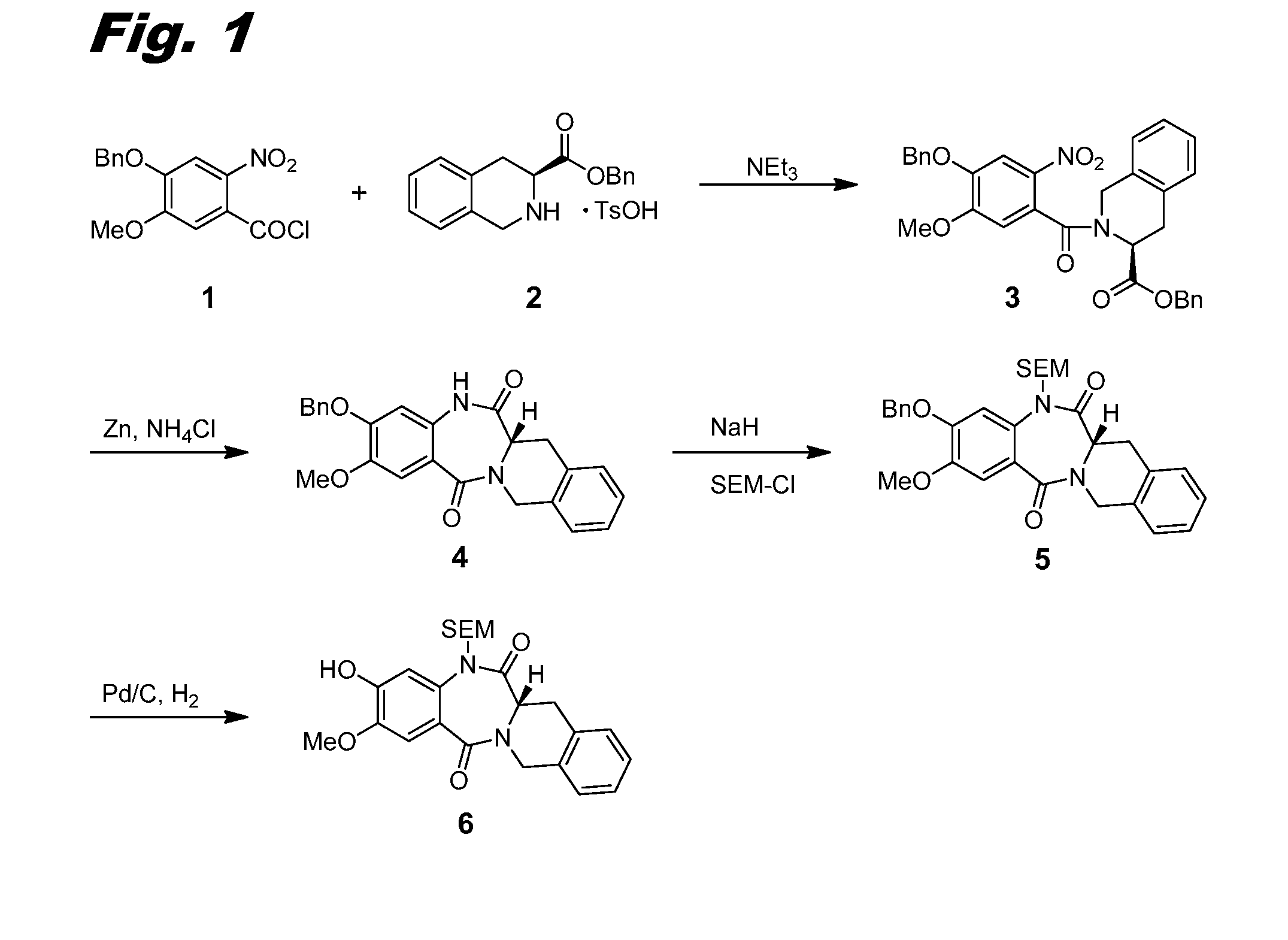
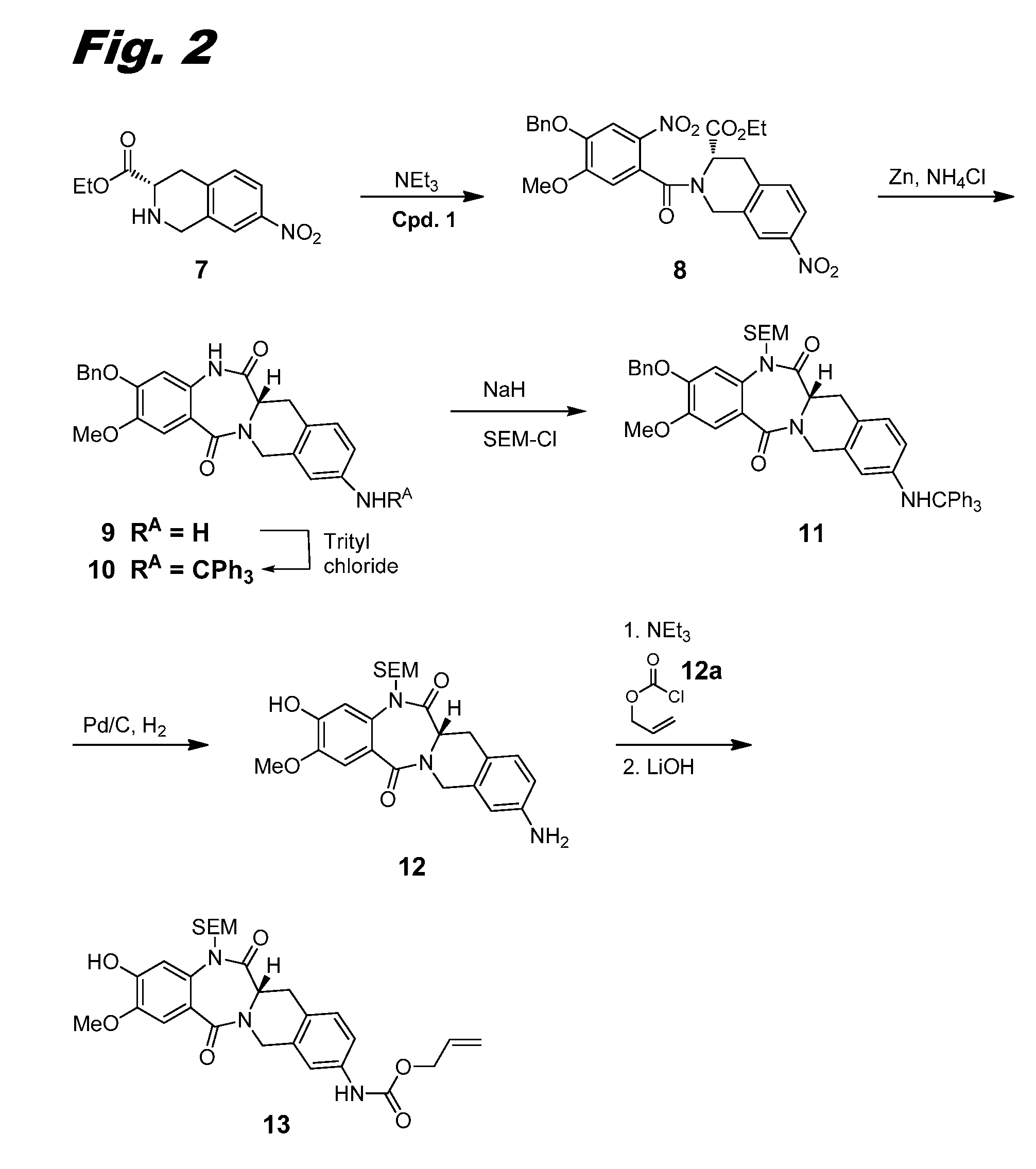
ActiveUS10358441B2Improve production yieldReduce processing stepsOrganic active ingredientsOrganic chemistryBenzodiazepineBenzodiazine
The present invention relates to processes and intermediates useful in the preparation of biologically active molecules, especially in the synthesis of Respiratory Syncytial Virus (RSV) inhibitors. The present invention also relates to processes and intermediates for the preparation of compounds of formula (I):In particular, the present invention also relates to processes and intermediates for the preparation of compound I-a:
Owner:ENANTA PHARM INC
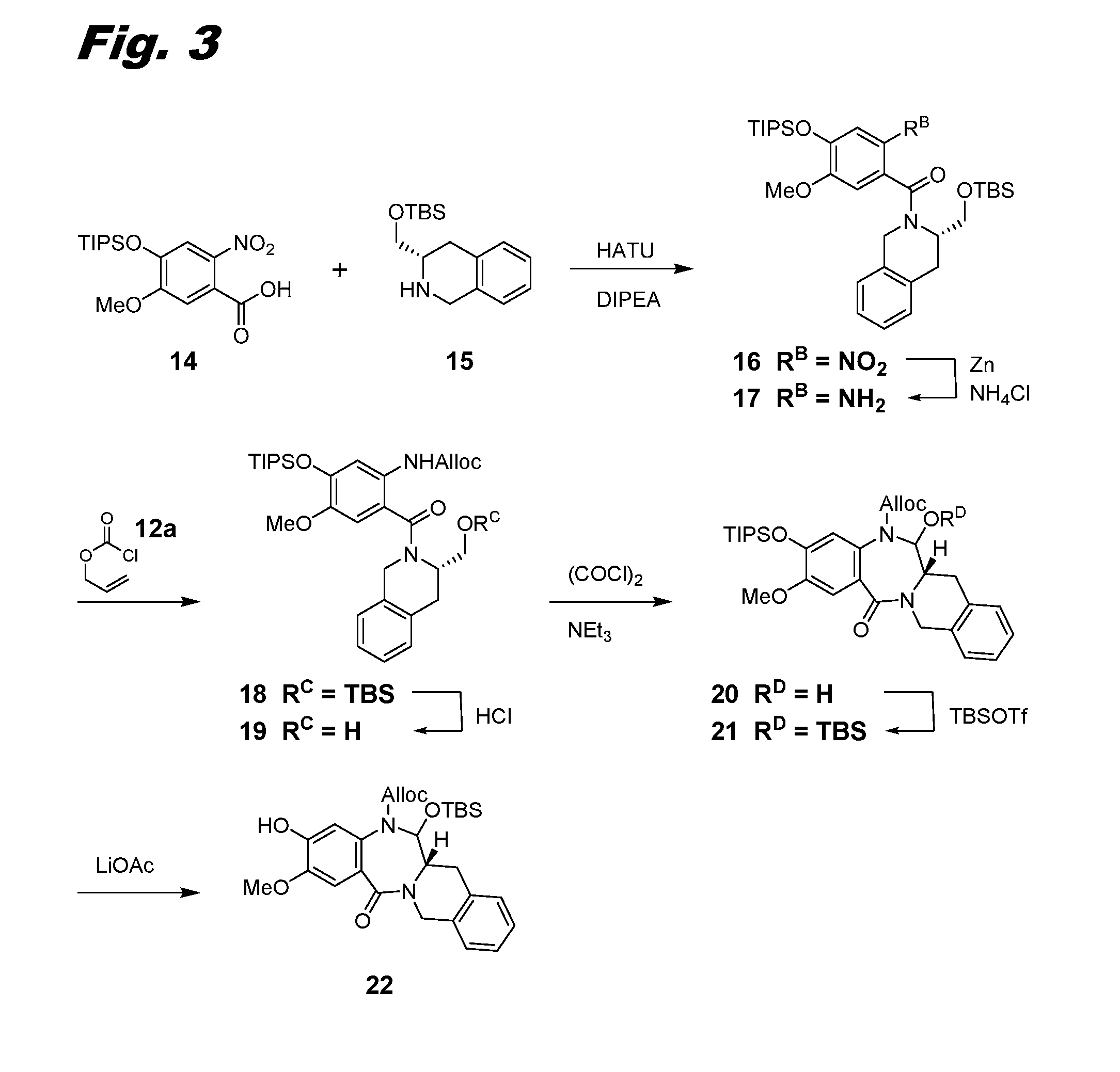
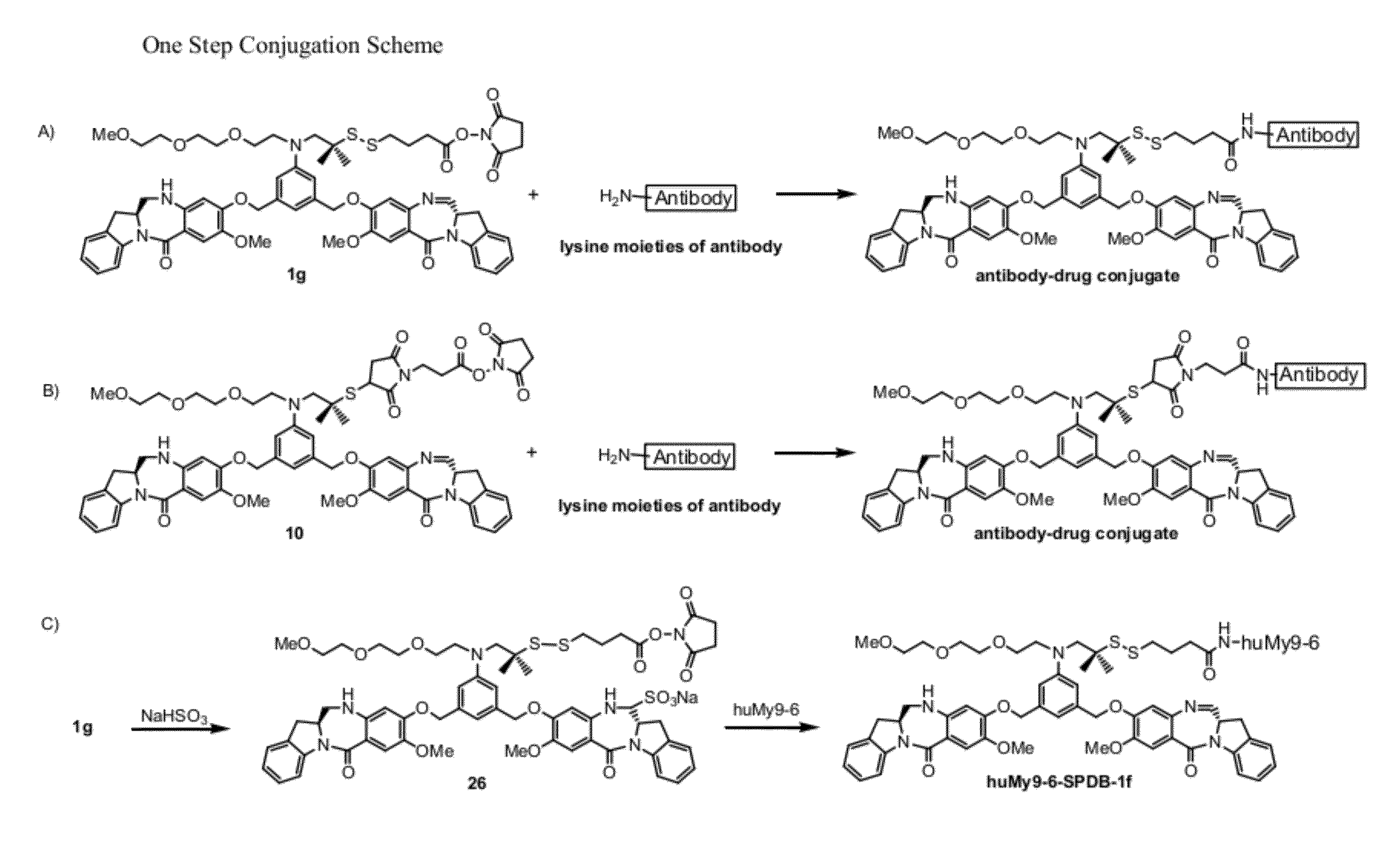
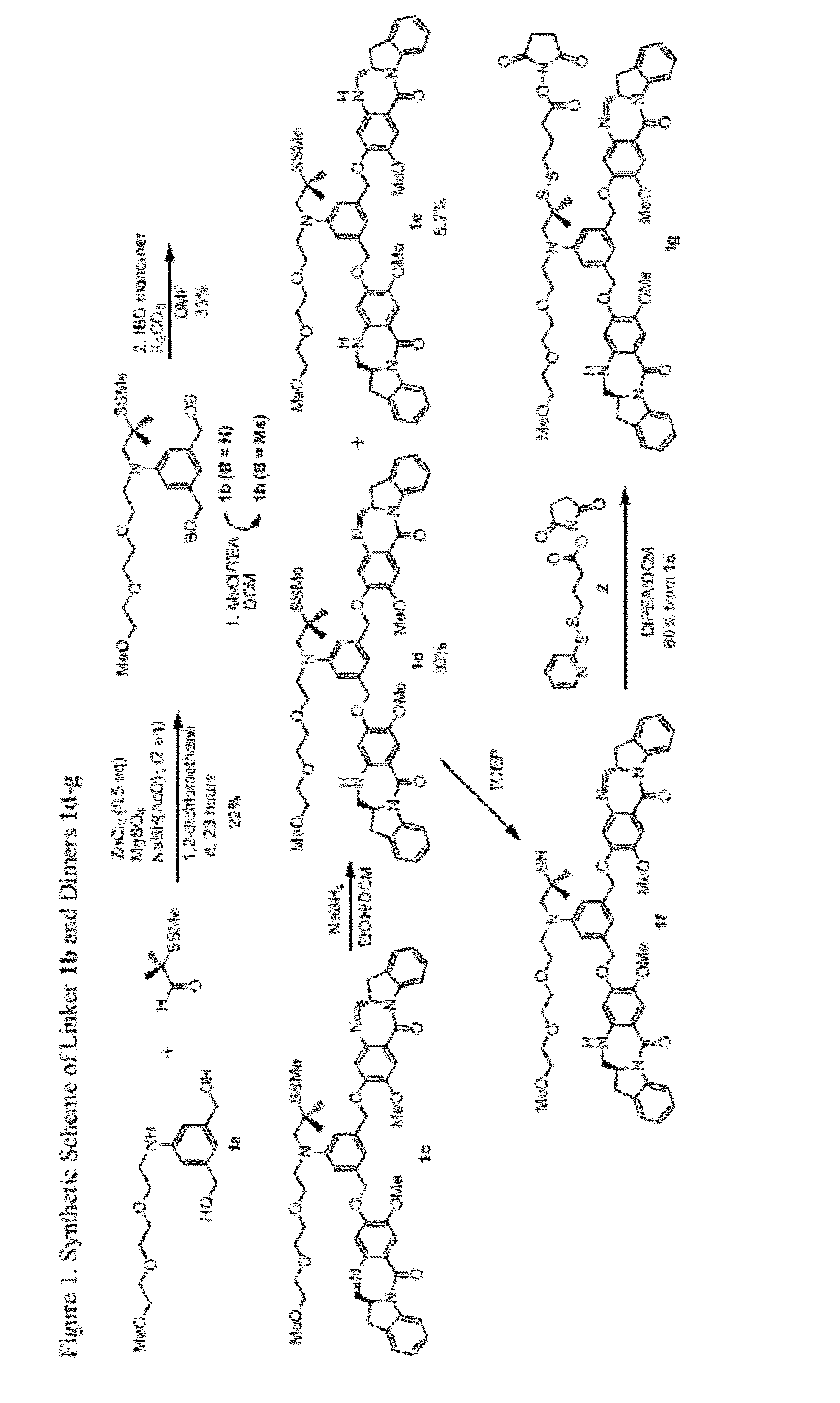
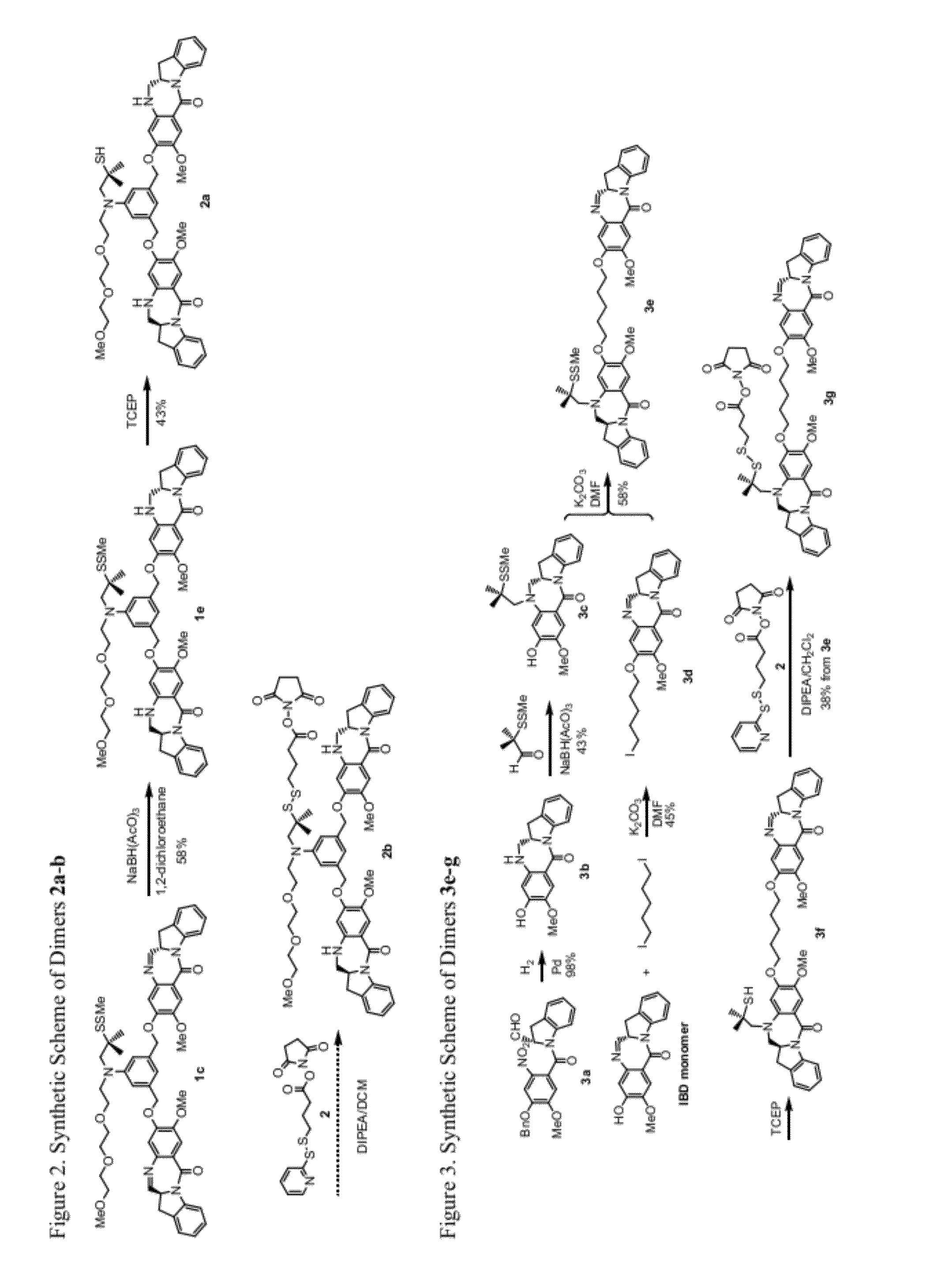
Heteroarylene-bridged benzodiazepine dimers, conjugates thereof, and methods of making and using
ActiveUS20160199510A1Imine bond can be reducedReduce bondingDigestive systemImmunoglobulinsBenzodiazepineDimer
Benzodiazepine dimers having a structure represented bywherein X comprises a heteroaromatic moiety and is as further defined in the application; R1 isand the other variables in formulae (I), (Ia), and (Ib) are as defined in the application. Such dimers are useful as anti-cancer agents, especially when used in an antibody-drug conjugate (ADC).
Owner:BRISTOL MYERS SQUIBB CO
Cytotoxic benzodiazepine derivatives
Owner:IMMUNOGEN INC
Cytotoxic benzodiazepine derivatives
The invention relates to novel benzodiazepine derivatives with antiproliferative activity and more specifically to novel benzodiazepine compounds of formula (I)-(VII). The invention also provides conjugates of the benzodiazepine compounds linked to a cell-binding agent. The invention further provides compositions and methods useful for inhibiting abnormal cell growth or treating a proliferative disorder in a mammal using the compounds or conjugates of the invention.
Owner:IMMUNOGEN INC
Pharmaceutical compositions of benzodiazepines and method of use thereof
The present invention includes benzodiazepine compositions formulated for intranasal administration, comprising a binary solvent system comprising a first solvent in which the benzodiazepine is soluble, the first solvent capable of penetrating nasal mucosal tissue, and a second solvent in which the benzodiazepine in less soluble. The compositions of the present invention may be used to treat a variety of disorders including, but not limited to, panic attacks, muscle spasms, anxiety, and seizures. In one aspect, the present invention relates to a fast-acting, clonazepam composition for transnasal administration that can be used for the treatment of seizure clusters.
Owner:JAZZ PHARMA INC
Benzodiazepine dimers, conjugates thereof, and methods of making and using
ActiveUS20160200742A1Imine bond can be reducedReduce bondingImmunoglobulins against cell receptors/antigens/surface-determinantsPharmaceutical non-active ingredientsBenzodiazepineDimer
Benzodiazepine dimers having a structure represented bywherein R1 iswherein the variables in formulae (I), (Ia), and (Ib) are as defined in the application. Such dimers are useful as anti-cancer agents, especially when used in an antibody-drug conjugate (ADC).
Owner:BRISTOL MYERS SQUIBB CO
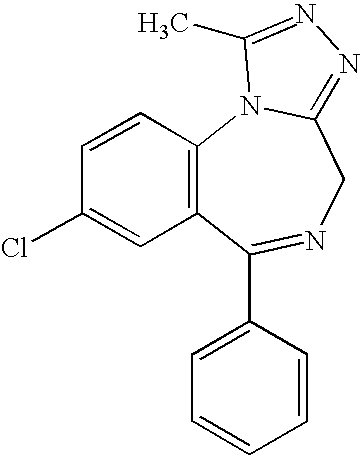
Substituted imidazo[1,5-a][1,2,4]triazolo[1,5-d][1,4]benzodiazepine derivatives
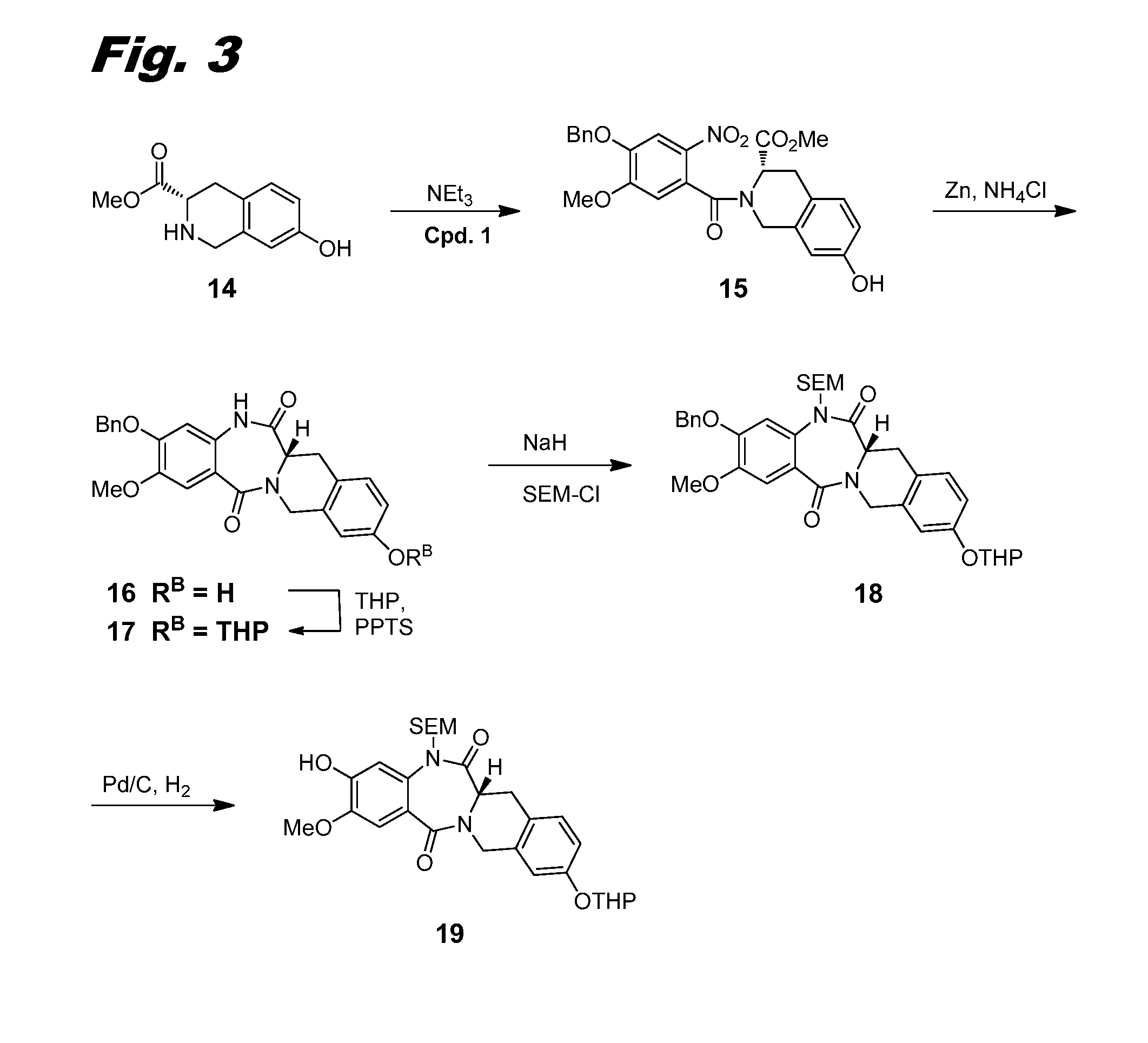
The present invention is concerned with a method of treating a disease selected from the group consisting of cognitive disorders, anxiety, Alzheimer's disease, and schizophrenia comprising administering a therapeutically effective amount of a substituted imidazo[1,5-a][1,2,4]triazolo[1,5-d][1,4]benzodiazepine derivatives of the following formula wherein R1 is halogen, lower alkyl, lower alkynyl, cycloalkyl, lower alkoxy, OCF3, —NHR, —NHC(O)R or —NHSO2R; R2 is hydrogen, methyl or aryl which is unsubstituted or substituted by one or two substituents selected from the group consisting of halogen and lower alkoxy; R3 is hydrogen, lower alkyl, lower alkenyl, cycloalkyl, lower alkoxy, —O(CH2)n+1—O-lower alkyl, —(CH2)n-aryl which is optionally substituted by lower alkyl or halogen, heteroaryl, —NHR, —N(R)2, wherein each R can be the same or different, —NHCH2C≡CH, or pyrrolidin-1-one; R is hydrogen, lower alkyl, lower alkyl substituted by halogen, heteroaryl, —(CH2)nO-lower alkyl, —NH-lower alkyl, cycloalkyl or aryl, and n is 0, 1, 2 or 3; and with their pharmaceutically acceptable acid addition salts. The invention also provides novel compounds of formula I-A and pharmaceutical compositions containing them. The most preferred indication is Alzheimer's disease.
Owner:F HOFFMANN LA ROCHE & CO AG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Non-cross-linking pyrrolo[2,1-c][1,4]benzodiazepines and process thereof Non-cross-linking pyrrolo[2,1-c][1,4]benzodiazepines and process thereof](https://images-eureka.patsnap.com/patent_img/a1449a37-a515-47d2-a66e-d0d6055aa076/US06884799-20050426-D00001.png)
![Non-cross-linking pyrrolo[2,1-c][1,4]benzodiazepines and process thereof Non-cross-linking pyrrolo[2,1-c][1,4]benzodiazepines and process thereof](https://images-eureka.patsnap.com/patent_img/a1449a37-a515-47d2-a66e-d0d6055aa076/US06884799-20050426-C00001.png)
![Non-cross-linking pyrrolo[2,1-c][1,4]benzodiazepines and process thereof Non-cross-linking pyrrolo[2,1-c][1,4]benzodiazepines and process thereof](https://images-eureka.patsnap.com/patent_img/a1449a37-a515-47d2-a66e-d0d6055aa076/US06884799-20050426-C00002.png)
![11-hydroxy-5h-pyrrolo[2,1-c][1,4] benzodiazepin-5-one derivatives as key intermediates for the preparation of c2 substituted pyrrolobenzodiazepines 11-hydroxy-5h-pyrrolo[2,1-c][1,4] benzodiazepin-5-one derivatives as key intermediates for the preparation of c2 substituted pyrrolobenzodiazepines](https://images-eureka.patsnap.com/patent_img/dfe0bdc2-9bfe-4984-a8af-93415b268a91/US07741319-20100622-C00001.png)
![11-hydroxy-5h-pyrrolo[2,1-c][1,4] benzodiazepin-5-one derivatives as key intermediates for the preparation of c2 substituted pyrrolobenzodiazepines 11-hydroxy-5h-pyrrolo[2,1-c][1,4] benzodiazepin-5-one derivatives as key intermediates for the preparation of c2 substituted pyrrolobenzodiazepines](https://images-eureka.patsnap.com/patent_img/dfe0bdc2-9bfe-4984-a8af-93415b268a91/US07741319-20100622-C00002.png)
![11-hydroxy-5h-pyrrolo[2,1-c][1,4] benzodiazepin-5-one derivatives as key intermediates for the preparation of c2 substituted pyrrolobenzodiazepines 11-hydroxy-5h-pyrrolo[2,1-c][1,4] benzodiazepin-5-one derivatives as key intermediates for the preparation of c2 substituted pyrrolobenzodiazepines](https://images-eureka.patsnap.com/patent_img/dfe0bdc2-9bfe-4984-a8af-93415b268a91/US07741319-20100622-C00003.png)
![C2-fluoro pyrrolo [2,1-c][1,4]benzodiazepine dimers C2-fluoro pyrrolo [2,1-c][1,4]benzodiazepine dimers](https://images-eureka.patsnap.com/patent_img/f2149433-8c4c-4cc9-a8a5-f3c8e67361b7/US07189710-20070313-C00001.png)
![C2-fluoro pyrrolo [2,1-c][1,4]benzodiazepine dimers C2-fluoro pyrrolo [2,1-c][1,4]benzodiazepine dimers](https://images-eureka.patsnap.com/patent_img/f2149433-8c4c-4cc9-a8a5-f3c8e67361b7/US07189710-20070313-C00002.png)
![C2-fluoro pyrrolo [2,1-c][1,4]benzodiazepine dimers C2-fluoro pyrrolo [2,1-c][1,4]benzodiazepine dimers](https://images-eureka.patsnap.com/patent_img/f2149433-8c4c-4cc9-a8a5-f3c8e67361b7/US07189710-20070313-C00003.png)
![C8—linked pyrrolo[2,1-c][1,4]benzodiazepine-acridone/acridine hybrids C8—linked pyrrolo[2,1-c][1,4]benzodiazepine-acridone/acridine hybrids](https://images-eureka.patsnap.com/patent_img/9137b741-81bb-4108-b21a-567f2cb25f70/US07056913-20060606-D00001.png)
![C8—linked pyrrolo[2,1-c][1,4]benzodiazepine-acridone/acridine hybrids C8—linked pyrrolo[2,1-c][1,4]benzodiazepine-acridone/acridine hybrids](https://images-eureka.patsnap.com/patent_img/9137b741-81bb-4108-b21a-567f2cb25f70/US07056913-20060606-C00001.png)
![C8—linked pyrrolo[2,1-c][1,4]benzodiazepine-acridone/acridine hybrids C8—linked pyrrolo[2,1-c][1,4]benzodiazepine-acridone/acridine hybrids](https://images-eureka.patsnap.com/patent_img/9137b741-81bb-4108-b21a-567f2cb25f70/US07056913-20060606-C00002.png)
![2-Substituted imidazo[1,2-A]pyridine derivatives 2-Substituted imidazo[1,2-A]pyridine derivatives](https://images-eureka.patsnap.com/patent_img/a616360a-75ae-4ab3-89a9-9271927c371d/US20020032200A1-20020314-C00001.png)
![2-Substituted imidazo[1,2-A]pyridine derivatives 2-Substituted imidazo[1,2-A]pyridine derivatives](https://images-eureka.patsnap.com/patent_img/a616360a-75ae-4ab3-89a9-9271927c371d/US20020032200A1-20020314-C00002.png)
![2-Substituted imidazo[1,2-A]pyridine derivatives 2-Substituted imidazo[1,2-A]pyridine derivatives](https://images-eureka.patsnap.com/patent_img/a616360a-75ae-4ab3-89a9-9271927c371d/US20020032200A1-20020314-C00003.png)
![Substituted imidazo[1,5-a][1,2,4]triazolo[1,5-d][1,4]benzodiazepine derivatives Substituted imidazo[1,5-a][1,2,4]triazolo[1,5-d][1,4]benzodiazepine derivatives](https://images-eureka.patsnap.com/patent_img/2f884707-4d59-4c0e-8635-fd8a530fb6bb/US20060079507A1-20060413-C00001.png)
![Substituted imidazo[1,5-a][1,2,4]triazolo[1,5-d][1,4]benzodiazepine derivatives Substituted imidazo[1,5-a][1,2,4]triazolo[1,5-d][1,4]benzodiazepine derivatives](https://images-eureka.patsnap.com/patent_img/2f884707-4d59-4c0e-8635-fd8a530fb6bb/US20060079507A1-20060413-C00002.png)
![Substituted imidazo[1,5-a][1,2,4]triazolo[1,5-d][1,4]benzodiazepine derivatives Substituted imidazo[1,5-a][1,2,4]triazolo[1,5-d][1,4]benzodiazepine derivatives](https://images-eureka.patsnap.com/patent_img/2f884707-4d59-4c0e-8635-fd8a530fb6bb/US20060079507A1-20060413-C00003.png)