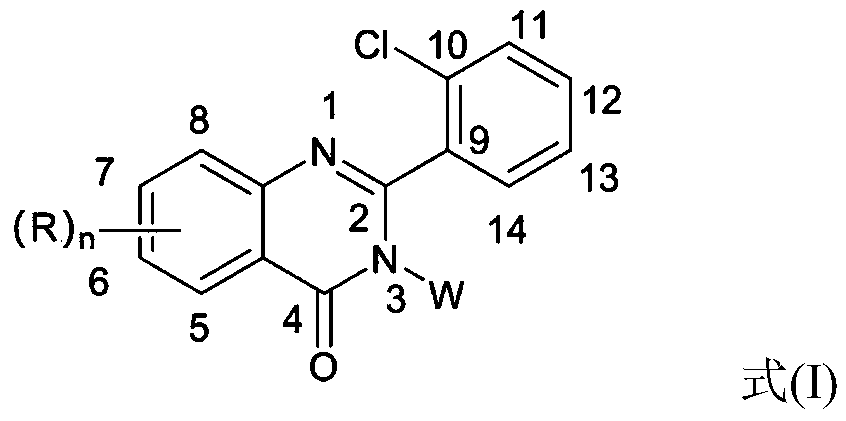
2-(2-chlorophenyl) quinazoline-4 (3H)-one derivative and preparation method and application thereof
A quinazoline and chlorophenyl technology, applied in the field of pharmaceutical research, can solve the problems of dizziness, cognitive and memory impairment, abuse, daytime drowsiness of drugs, etc. strong effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
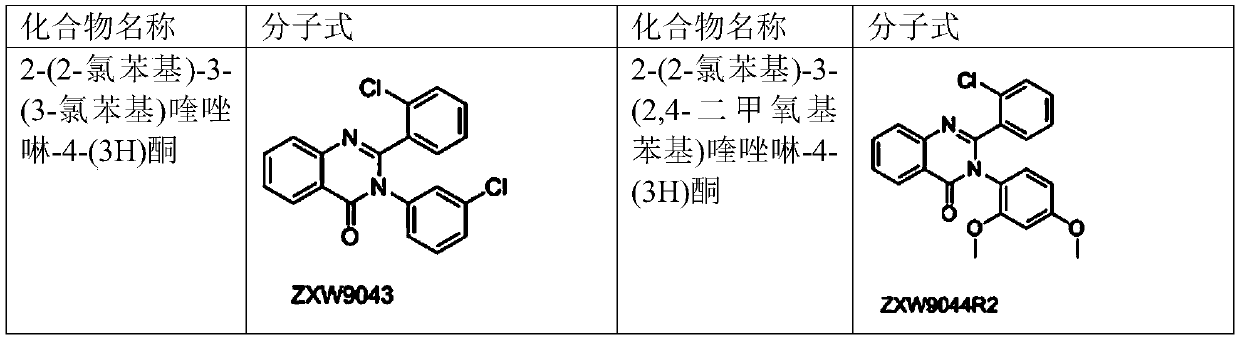
[0069] Example 1, 2-(2-Chlorophenyl)-3-(3-Chlorophenyl)quinazolin-4-(3H)one (Compound No.: ZXW9043)
[0070]
[0071] The first step, the preparation of 2-nitro-N-phenylbenzamide
[0072]
[0073] Weigh 2.0g (0.012mol) of o-nitrobenzoic acid and place it in a 100mL reaction flask, add 7.1g (0.06mol) of thionyl chloride, place the reaction flask in an oil bath, heat to reflux at 80°C, and after 2 hours Terminate the reaction, concentrate under reduced pressure to remove the solvent, add 20 mL of dichloromethane, concentrate again under reduced pressure to remove the solvent, and obtain a light yellow oil, add 20 mL of dichloromethane to form an acid chloride solution for use. Add 1.52g (0.012mol) of 3-chloroaniline to a 100mL reaction flask, add 1.42g (0.018mol) of pyridine, dissolve with 20mL of dichloromethane, add a dropping funnel to the reaction flask, vacuum nitrogen protection, put the reaction flask into In an ice bath, put the acid chloride solution in a droppin...
Embodiment 2
[0080] Example 2 2-(2-chlorophenyl)-3-(2,4-dimethoxyphenyl)quinazolin-4-(3H)one (compound number: ZXW9044R2)
[0081]
[0082] Using 2-amino-N-(2,4-dimethoxyphenyl)benzamide and 2-chlorobenzaldehyde as raw materials, according to the synthesis method described in Example 1, 150 mg of white solid was obtained, yield: 71.4%. 1 H NMR (400MHz, DMSO-d 6 )δ (ppm): 8.24-8.21 (dd, J 1 =8.1Hz,J 2 =1.1Hz,1H,ArH),7.94–7.90(m,1H,ArH),7.77(d,J=8.1Hz,1H,ArH),7.69-7.56(m,2H,ArH),7.38-7.10(m ,4H,ArH),6.93-6.82(m,2H,ArH),3.68(s,3H,OCH 3 ),3.61(s,3H,OCH 3 ); 13 C NMR (100MHz, CDCl 3 )δ (ppm): 162.3, 153.3, 148.9, 147.2, 134.9, 134.8, 132.0, 130.6, 130.3, 129.6, 127.8, 127.7, 127.3, 126.6, 121.4, 121.2, 121.2, 120.5, 112.1, 111.0; ESI, m / z) calculated value C 22 h 18 ClN 2 o 3 [(M+H) + ], 393.1000; measured value, 393.0999.
Embodiment 3
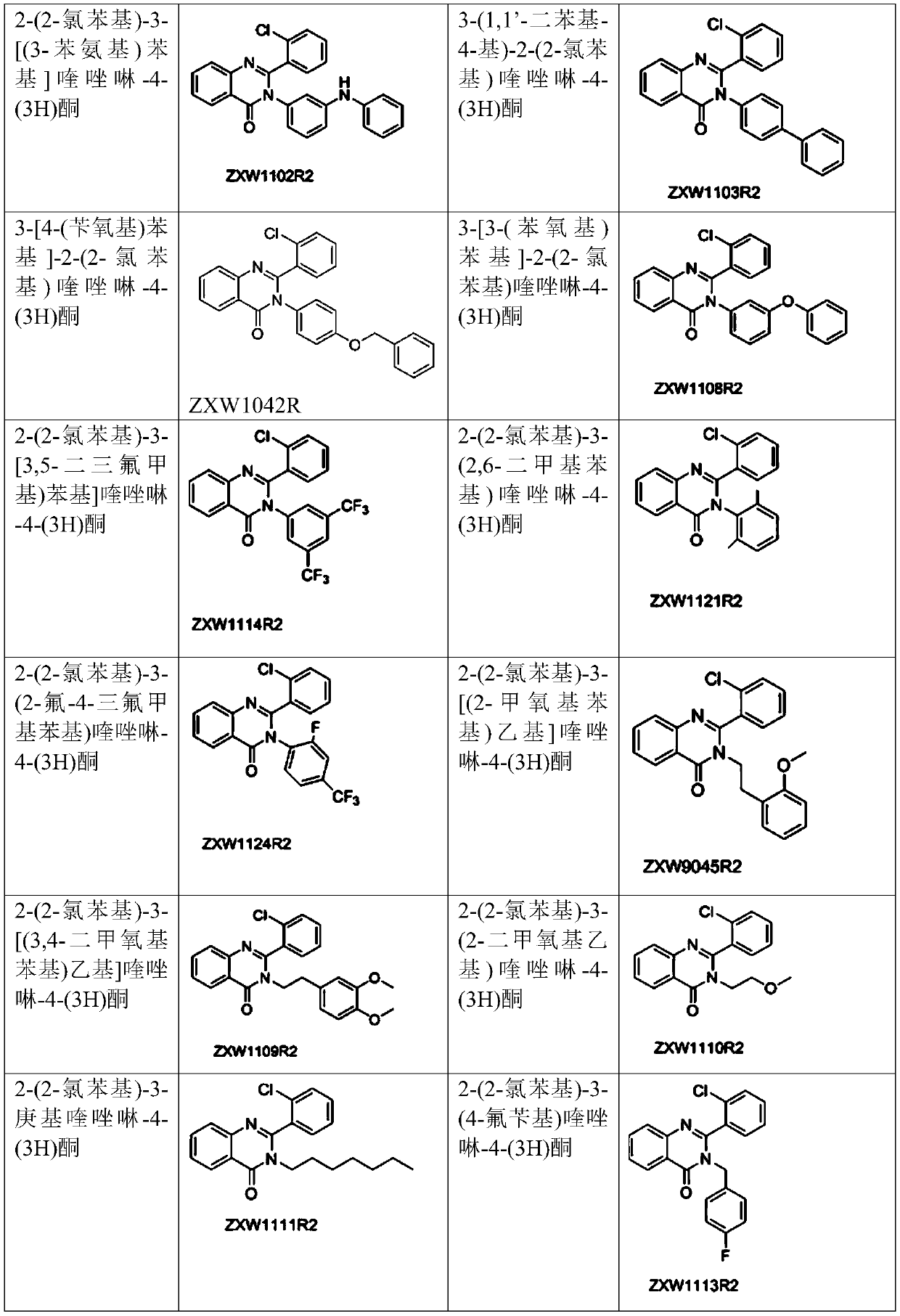
[0083] Example 3 2-(2-chlorophenyl)-3-[(3-phenylamino)phenyl]quinazolin-4-(3H)one (compound number: ZXW1102R2)
[0084]
[0085] Using 2-amino-N-(3-(phenylamino)phenyl)benzamide and 2-chlorobenzaldehyde as raw materials, according to the synthesis method described in Example 1, 280 mg of white solid was obtained, yield: 65.9%. 1 H NMR (400MHz, DMSO-d 6 )δ(ppm):8.23(d,J 1 =7.8Hz,1H,ArH),7.95-7.89(m,2H,ArH),7.76(d,J=8.1Hz,1H,ArH),7.65-7.61(m,1H,ArH),7.43-7.31(m ,3H,ArH),6.70-6.67(m,4H,ArH),7.09-6.92(m,4H,ArH),6.70-6.67(m,1H,ArH); 13 C NMR (100MHz, DMSO-d 6 )δ (ppm): 161.9, 153.6, 147.7, 142.4, 141.4, 135.0, 134.8, 131.4, 130.2, 129.7, 129.4, 129.3, 127.9, 127.8, 127.0, 126.8, 122.5, 122.1, 120.3, 119.3; ESI, m / z) calculated value C 26 h 19 ClN 3 O[(M+H) + ], 424.1211; measured value, 424.1211.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com