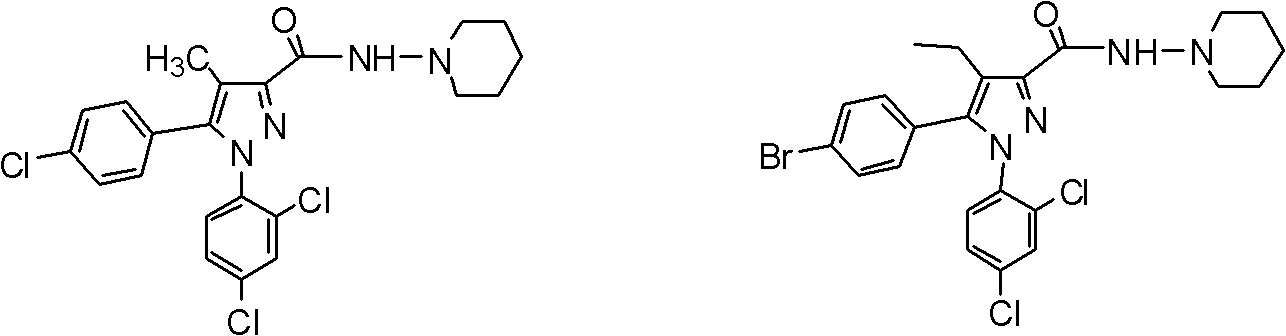
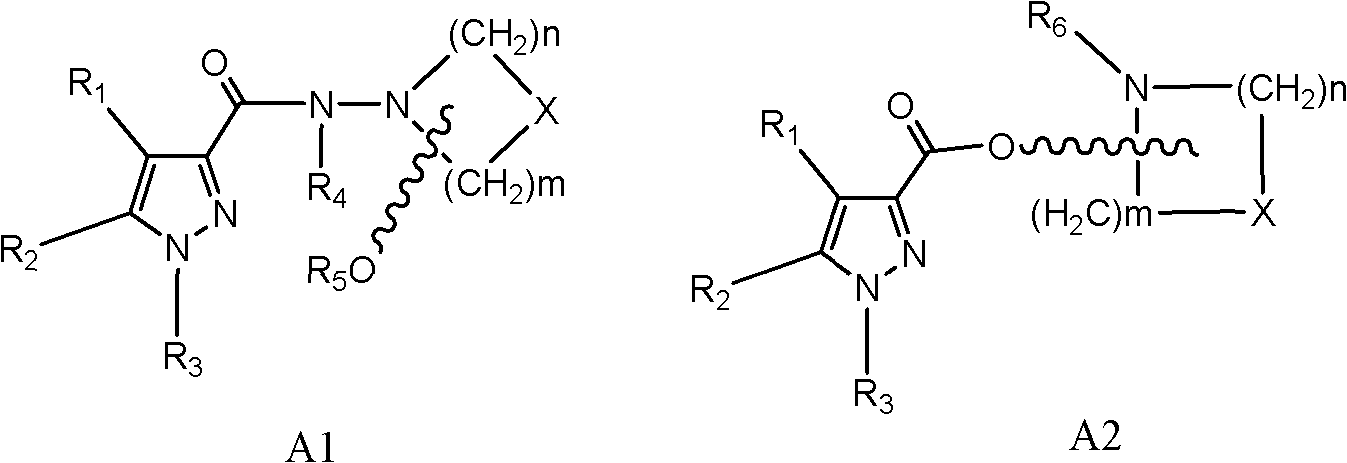
Chiral CB1 (cannabinoid) receptor inhibitor, and preparation method and medical application thereof
A receptor inhibitor, C1-C16 technology, used in organic chemistry, pharmaceutical formulations, organic active ingredients, etc., can solve problems such as no further reports, and achieve the effect of overcoming toxicity and side effects, low toxicity, and reduced toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
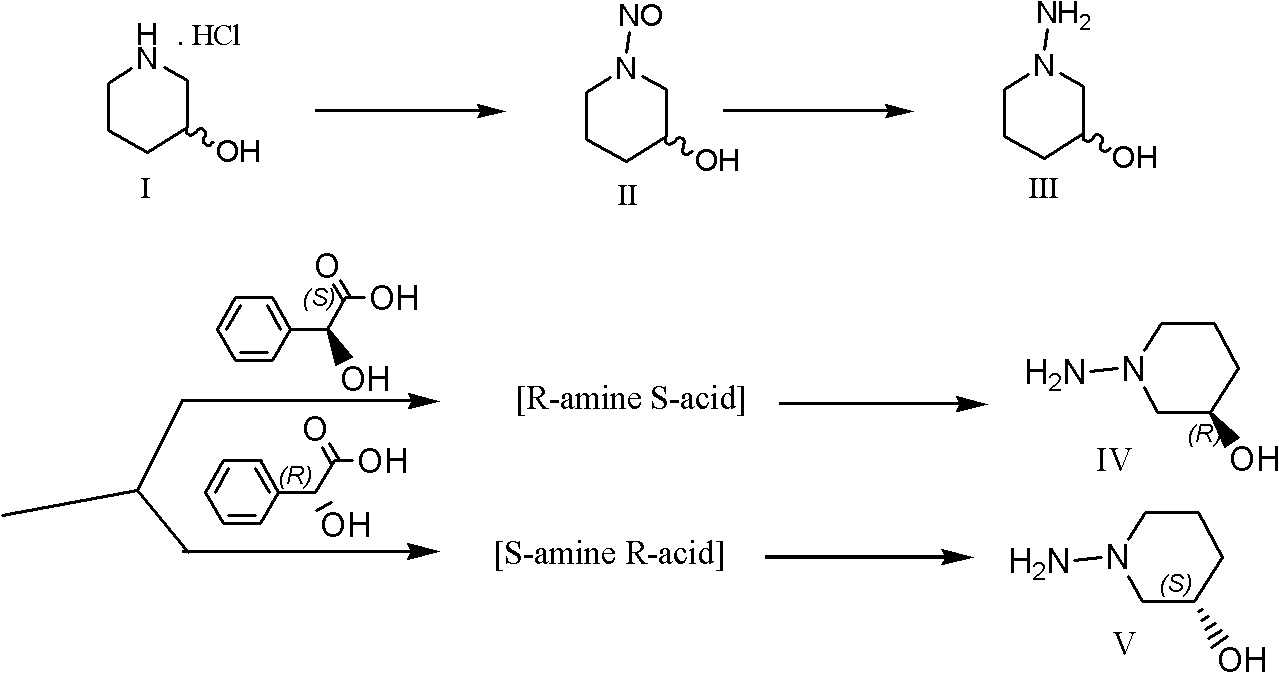
[0080] Example 1: (R / S)-1-nitroso-3-hydroxy-piperidine (II)
[0081] 3-hydroxypiperidine hydrochloride 40.03g (0.291mol), NaNO 2 , 40.2g (0.583mol) were dissolved in 200ml and 100ml of water, mixed, cooled to 0 ℃. Slowly add HOAc26.2g (0.437mol) dropwise therein at 0 to 5°C, and complete the addition within 60 minutes; then stir at 0°C for 4 hours and the reaction is complete. TLC: 0.80 (product nitroso); 0.28 (raw material) (ethanol:ammonia=10:1). Take Na 2 CO 3 30 g to neutralize residual acetic acid; use CH 2 Cl 2 Extract 6 times, 150ml each time; combine the organic phase; use Na 2 SO 4 Drying; Filtration; Concentration, 36.7g of yellow liquid was obtained; Yield 96.9%. directly used in the next reduction reaction. 1 H-NMR (CDCl 3 ): δ, 1.67-2.00 (m, 4H); 3.40-4.30 (m, 6H, containing OH).
Embodiment 2
[0082] Example 2: (R / S)-1-amino-3-hydroxy-piperidine (III)
[0083] Add 16.16g (0.426mol) of lithium aluminum hydride to 450ml of anhydrous THF that has been dried and distilled over sodium metal, and stir at about 40°C for 15 minutes; add dropwise 18.44g (0.142mol, 1 eq) in 100 ml dry THF. During the dropwise addition, the temperature was controlled at 40-45° C., and the dropwise addition took 45 minutes. Heat to reflux for 8 hours; cool to room temperature, add a mixture of 60ml of undried THF and 60ml of water (1:1); stir at room temperature at 30°C for 0.5 hours. Filter; wash the filter cake with THF (2×50ml); put the filter cake into 150ml THF and heat to reflux for 10 minutes, then filter again; repeat this operation twice. Combine all filtrates; use anhydrous Na 2 SO 4 After drying and evaporation, 14.7 g of light yellow liquid was obtained (the color is lighter than that of the raw material nitro compound); the yield of crude product is 89.1%. After drying in vacu...
Embodiment 3
[0085] Example 3: (R)-1-amino-3-hydroxypiperidine (IV)
[0086] A) Resolution of (R / S)-1-amino-3-hydroxy-piperidine:
[0087] Take 13 g (0.112 mol) of the above-mentioned racemic 1-amino-3-hydroxy-piperidine, dissolve it in 120 ml of isopropanol; add 17.0 g (0.112 mol) of S-mandelic acid to it in 150 ml of isopropanol at room temperature The solution. Crystals precipitated after about 1 hour; filtered after being placed in a refrigerator at 0°C for 4 hours; the filter cake was washed 4 times with cold ethyl acetate, 50ml each time; vacuum-dried to obtain 16.3g of white crystals; [α] D : +65.23° (C, 20.05; H 2 0, 25°C).
[0088] Dissolve the crude product in a mixture of 200ml of isopropanol, 30ml of methanol and 2ml of water; heat to reflux for 6 hours; cool to room temperature; place in a refrigerator at 0°C and filter after 6 hours; the filter cake is washed with cold ethyl acetate 4 times, 50ml each time; vacuum dried to obtain 11.28g of white crystals; [α] D : +65.84°...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com