Midazolam combination as well as preparation method and application thereof
A midazolam and composition technology, applied in the field of medicine and pharmacy, can solve problems such as unsatisfactory effect, and achieve the effects of good physical and chemical stability, reducing first-pass effect and high concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
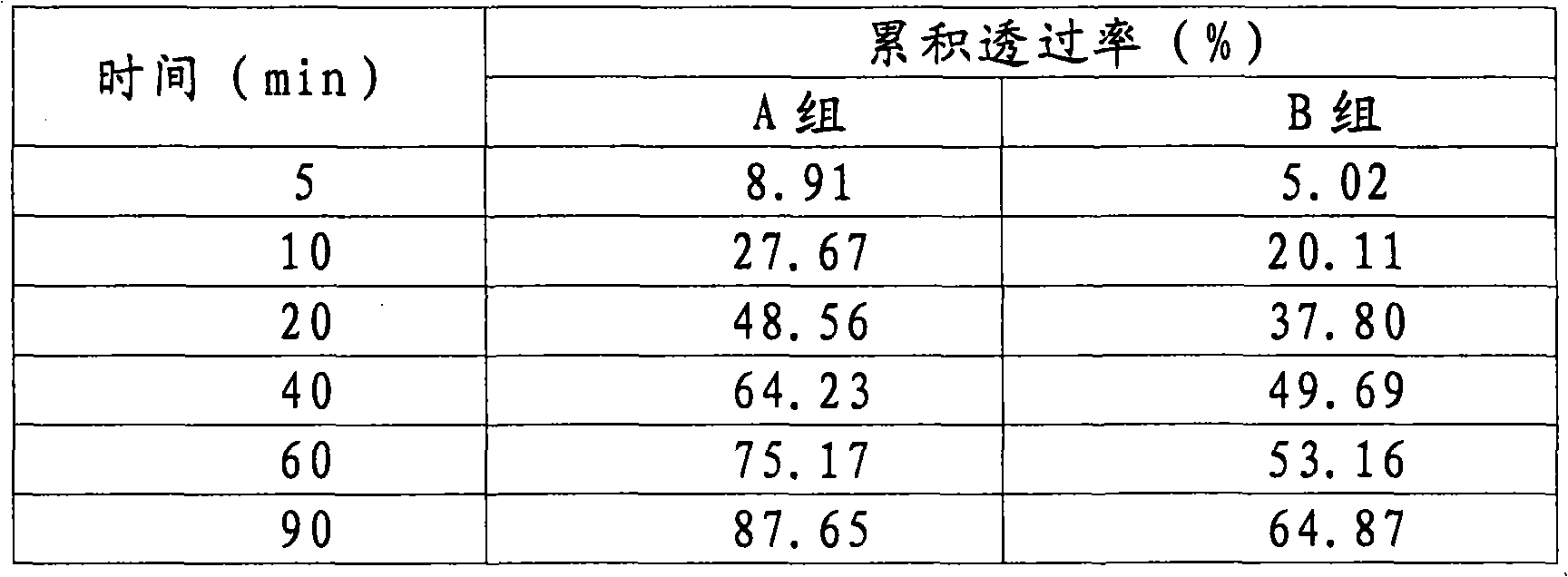
Image
Examples
Embodiment 1
[0043] Example 1: Preparation of midazolam composition sample 1
[0044] This product is a nasal spray (nasal spray) with a specification of 5mg / 100μL / spray (calculated as midazolam).
[0045] prescription:
[0046] Midazolam 5g
[0047] Propylene glycol: 40ml
[0048] Glycerin: 10ml
[0049] β-cyclodextrin 15g
[0050] Add water for injection to 100ml
[0051]
[0052] Preparation Process:
[0053] Accurately weigh 5g of midazolam and dissolve it in 30ml of propylene glycol. After fully dissolving, add 15ml of glycerin and an appropriate amount of β-CD aqueous solution, stir well or ultrasound to dissolve and mix, cool to room temperature and add water for injection to the full amount (add to 100ml). After the content determination is qualified, carry out sub-packaging. Thus, sample 1 of the midazolam composition was prepared. The pH of the preparation was measured with a pH meter to be 7.6.
[0054] Those skilled in the art can understand that although the...
Embodiment 2
[0055] Example 2: Preparation of midazolam composition sample 2
[0056] This product is a solution for the nasal cavity and is administered by dripping (nasal drops), with a specification of 50mg / ml (calculated as midazolam).
[0057] prescription:
[0058] Midazolam 5g
[0059] Propylene glycol: 60ml
[0060] Glycerin: 5ml
[0061] Hydroxypropyl-βcyclodextrin 21g
[0062] Add water for injection to 100ml
[0063]
[0064] Preparation Process:
[0065] Accurately weigh 5g of midazolam and dissolve it in 60ml of propylene glycol. After fully dissolving, add 5ml of glycerin and an appropriate amount of Hp-β-CD aqueous solution, stir well or ultrasound to dissolve and mix, cool to room temperature and add water for injection to the full amount ( Add to 100ml). After the intermediate content is qualified, it is divided into packaging. Thus, sample 2 of the midazolam composition was prepared. The pH of the preparation was determined to be 7.8.
[0066] Those ...
Embodiment 3
[0067] Example 3: Preparation of midazolam composition sample 3
[0068] This product is a solution that is administered through the nasal route, and the concentration of the solution is 50mg / ml (calculated as midazolam).
[0069] prescription:
[0070] Midazolam maleate 6.8g
[0071] Propylene glycol: 50ml
[0072] β-cyclodextrin 5g
[0073] Add water for injection to 100ml
[0074]
[0075] Note: In the above prescription, the main medicine used is midazolam maleate, 6.8g midazolam maleate, which is equivalent to 5g midazolam. Since different salt types are involved, in order to facilitate the measurement and comparison of the concentration of different solutions, the free midazolam is uniformly converted to express the drug concentration. In other words, in this prescription, the concentration of the solution is still 50mg / ml (in midazolam).
[0076] Preparation Process:
[0077] Accurately weigh 6.8g of midazolam maleate and dissolve it in 50ml of propy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com