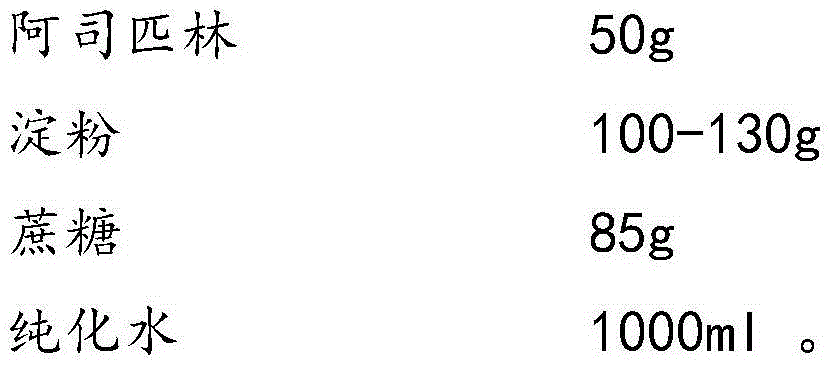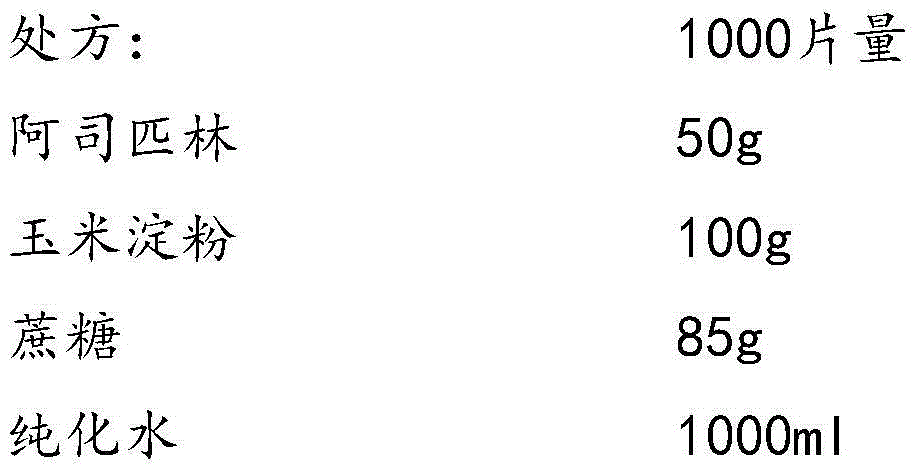Patents
Literature
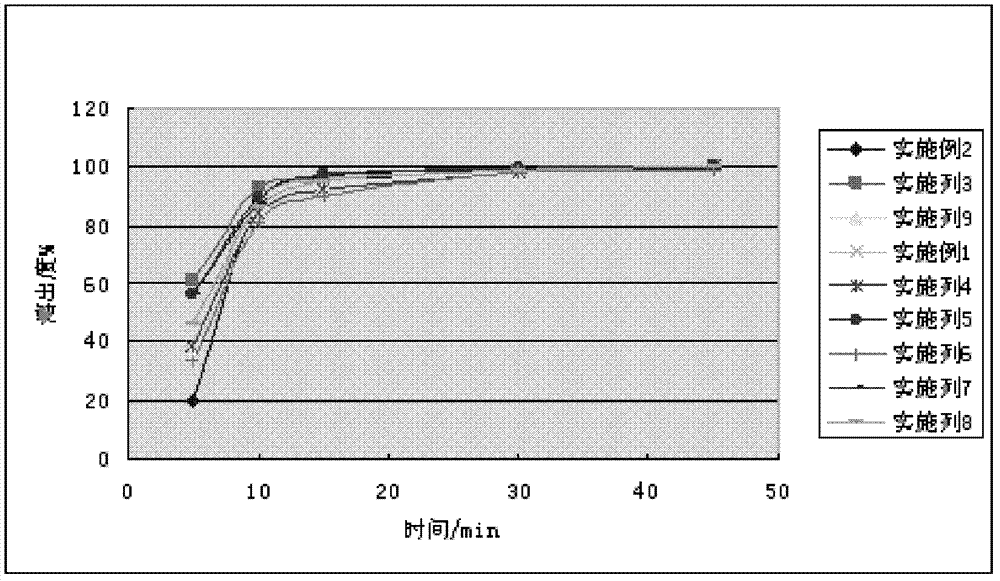
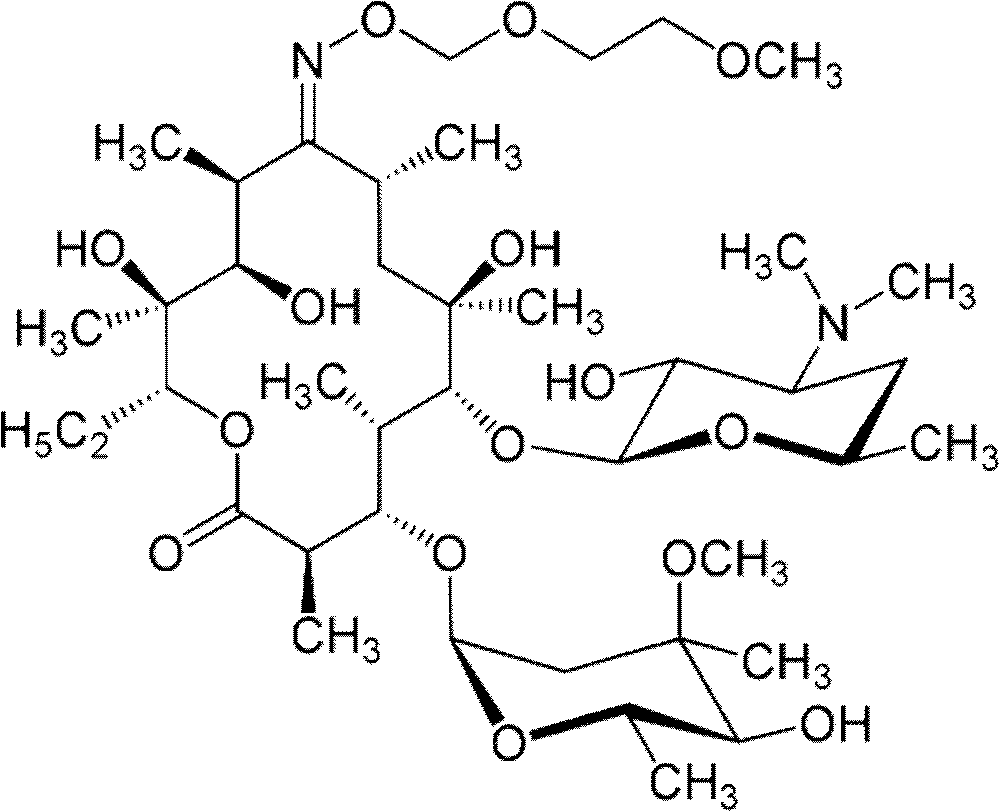
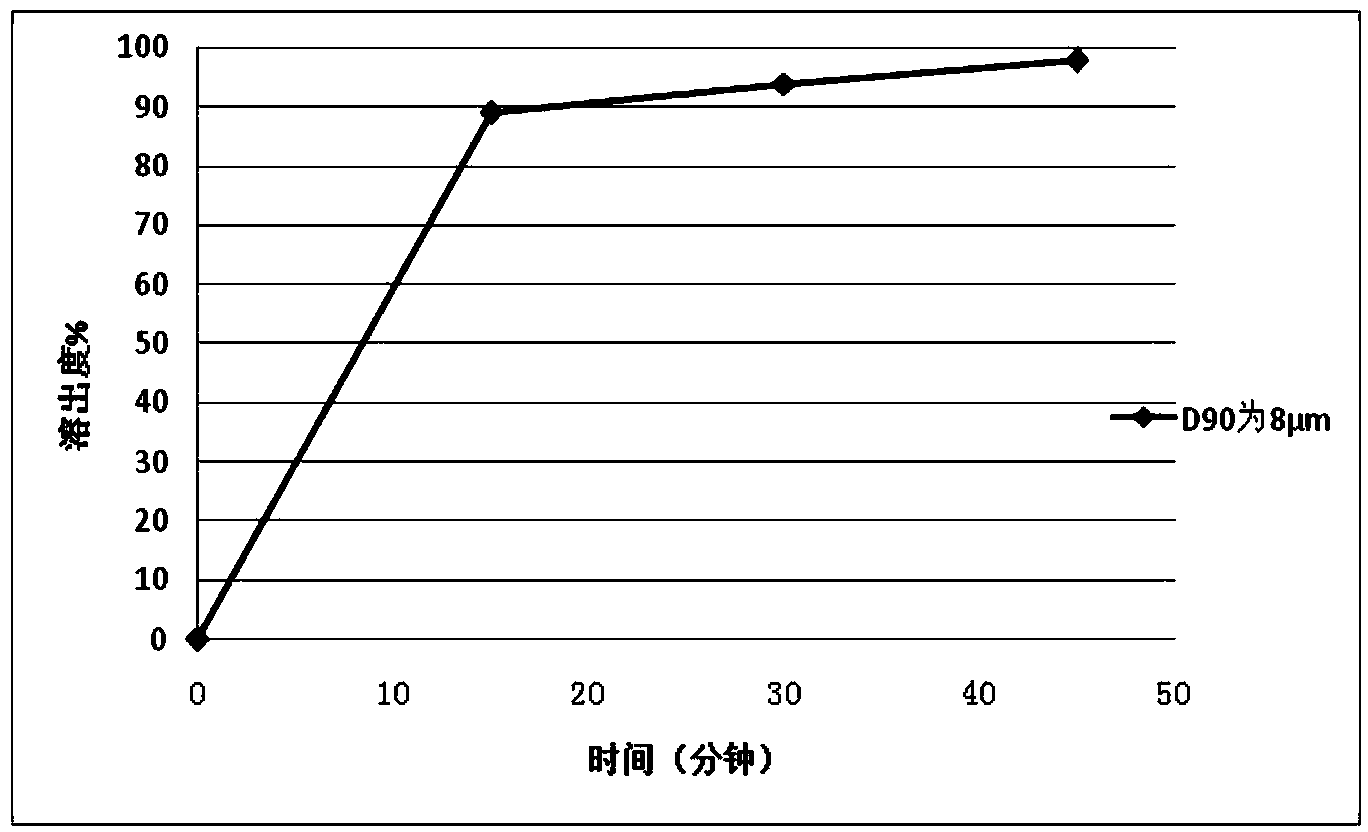
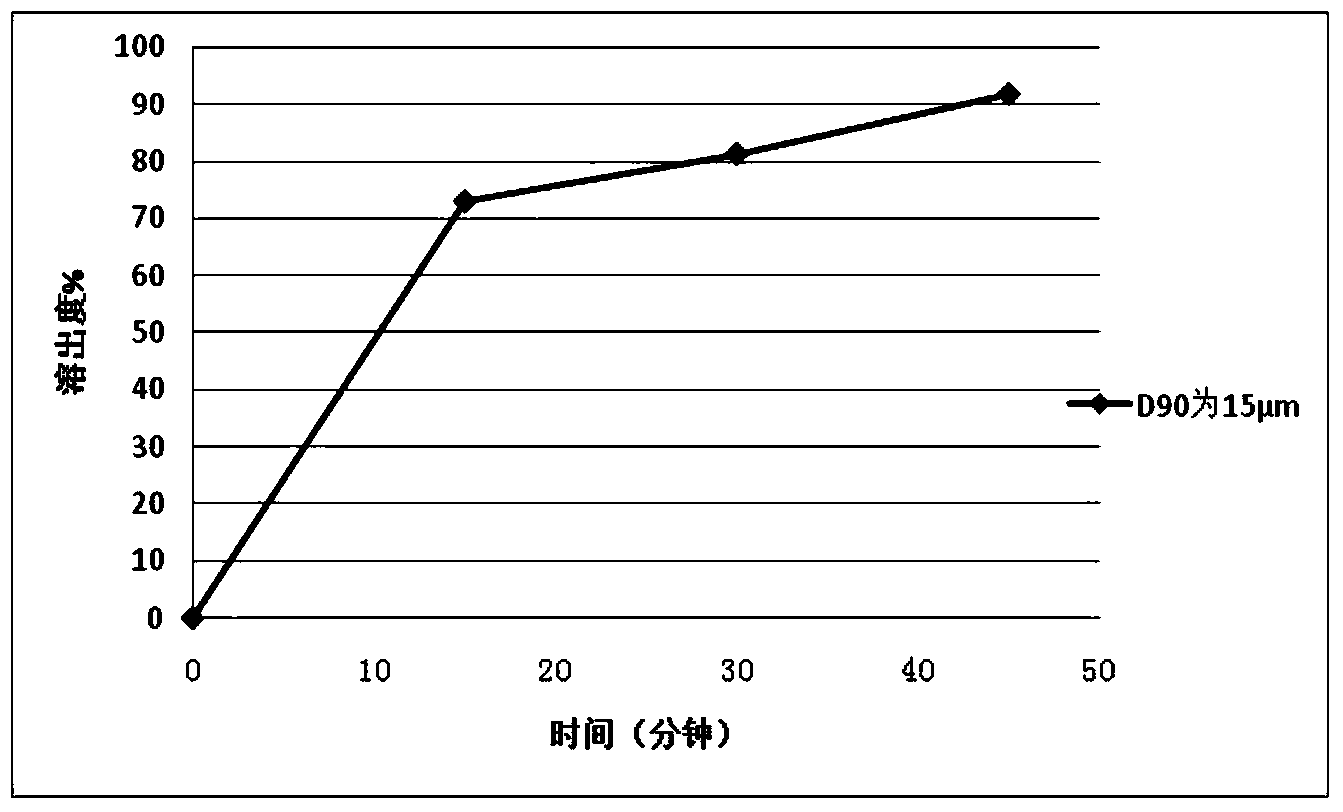
166results about How to "Improve the disintegration effect" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Solid Preparation
InactiveUS20080031942A1Improve the disintegration effectHigh dissolution ratePowder deliveryBiocidePharmaceutical drugMedicinal chemistry
A medical drug, in particular, solid preparations containing a medicinal ingredient with high tendency toward gelation, characterized by simultaneously containing a surface modifier and an acid or base. This characteristic realizes improvement to the disintegration easiness, production efficiency and stability of the solid preparations containing the above medicinal ingredient.
Owner:TAKEDA PHARMA CO LTD
Film-shaped, dissolvable preparations for active substance release and method for the production thereof
InactiveUS20050175675A1Increase liquid absorptionFast formingPill deliveryPharmaceutical non-active ingredientsWater solubleProduct gas
A film-shaped preparation disintegratable in aqueous media for administration of substances to the human or animal body. The preparation contains at least one water-soluble polymer. The preparation contains one or more components that produce a gas upon action of moisture, being in the presence of an aqueous medium or by a temperature change.
Owner:LTS LOHMANN THERAPIE-SYST AG
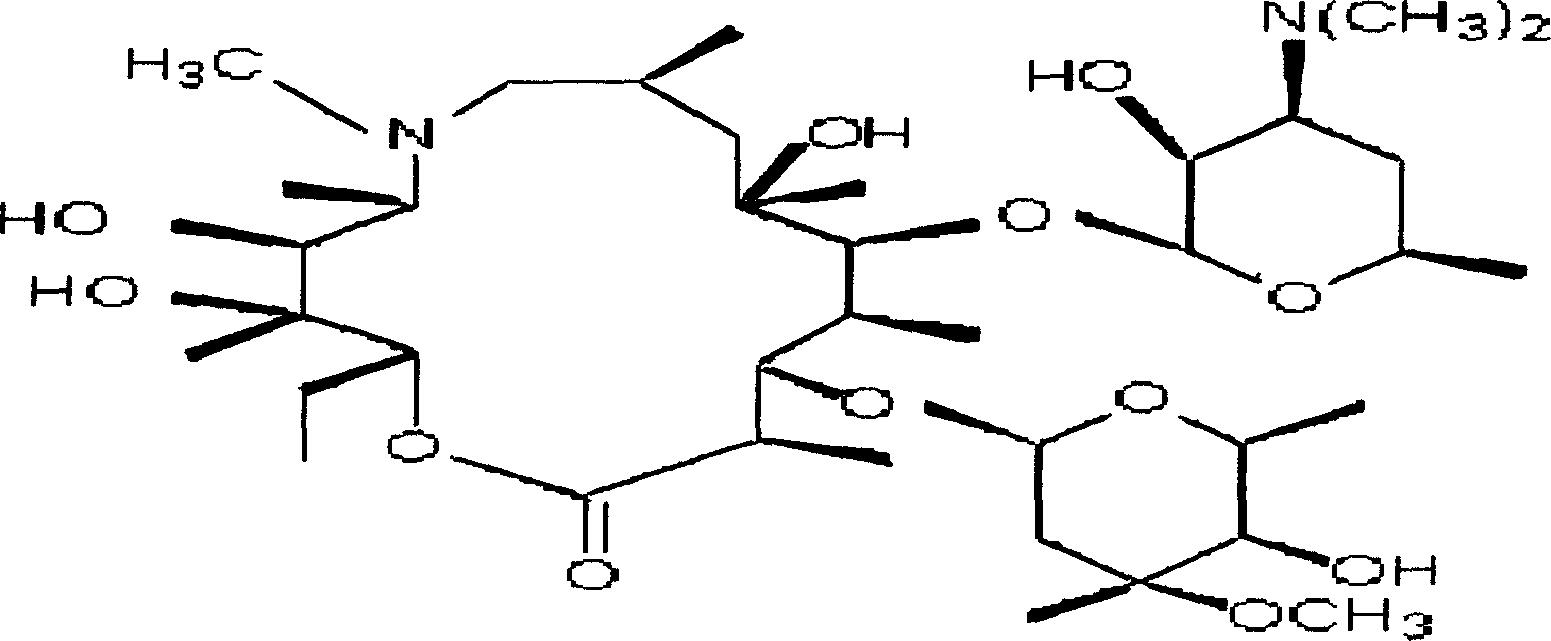
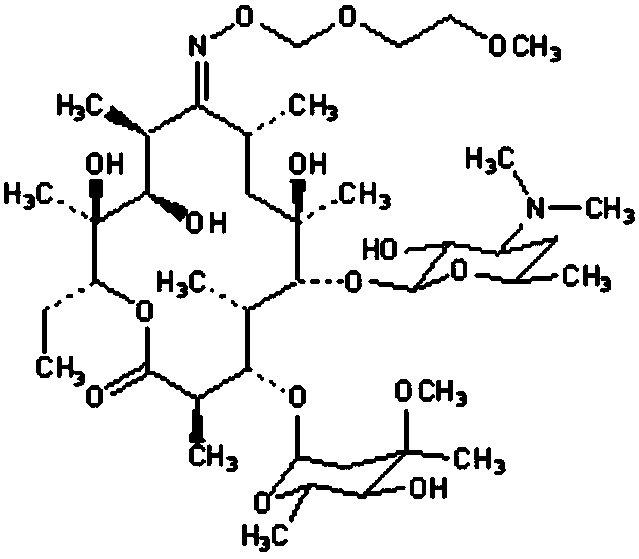
Roxithromycin capsule and preparation method thereof
ActiveCN103083278AImprove the disintegration effectHigh dissolution rateAntibacterial agentsOrganic active ingredientsRoxithromycinDissolution
The invention discloses a roxithromycin capsule and a preparation method thereof. The roxithromycin capsule pharmaceutical composition comprises the roxithromycin, silica powder, a disintegrating agent, a filler, a lubricant, etc. The preparation technology of the roxithromycin capsule comprises a direct filling technology and a wet granulation process. The method can satisfy demands of different equipment and is beneficial for production. The prepared roxithromycin capsule is good in dissolution and small in loading difference, and is a safe, stable and effective product.
Owner:SICHUAN KELUN PHARMA RES INST CO LTD
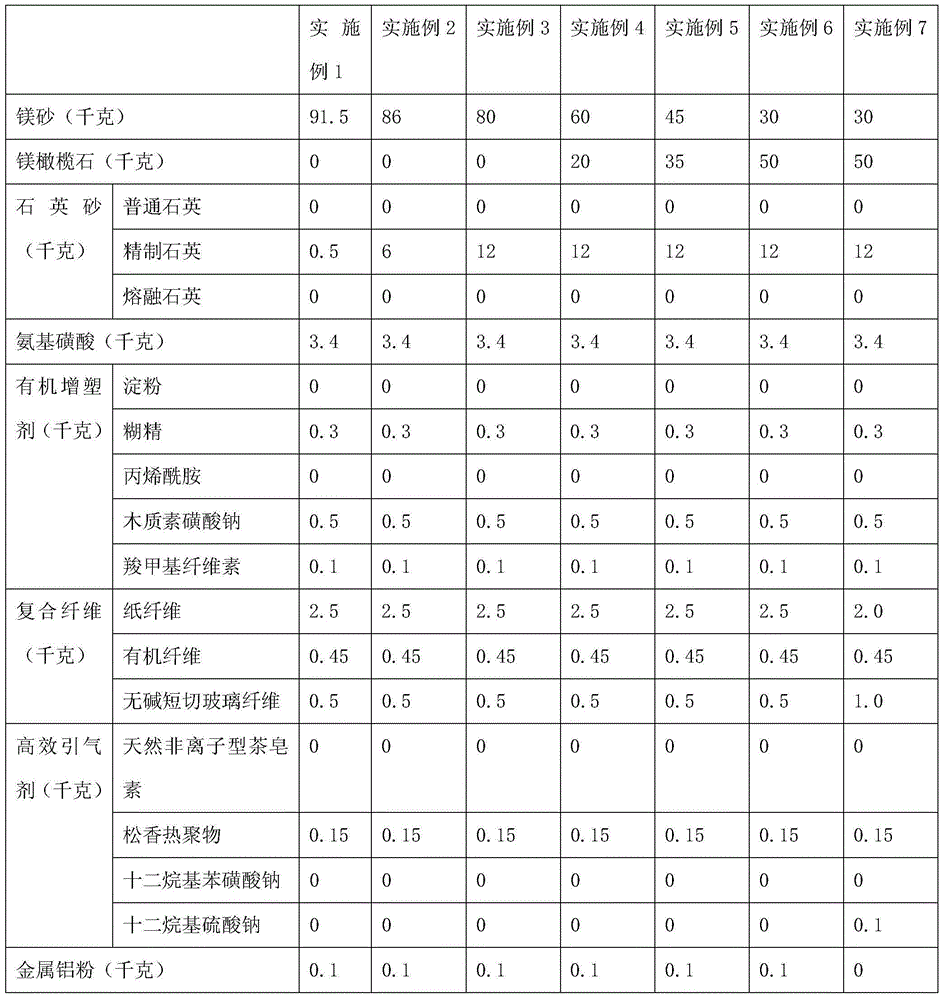
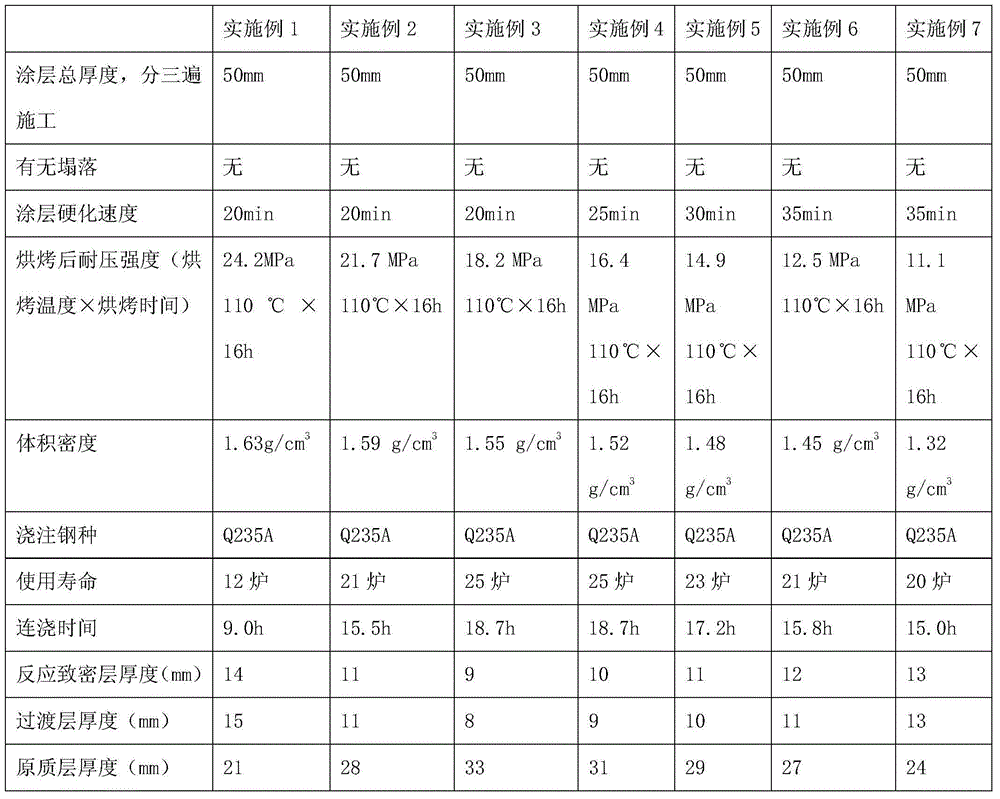
Steelmaking continuous casting tundish magnesium calcium paint
InactiveCN1459346AAbundant raw materialsReduce manufacturing costMelt-holding vesselsTundishDolomite
A Mg-Ca paint of the tundish for conticasting is prepared from 11 raw materials including natural dolomite, magnesium sand, clay, SiO2 powder, sodium hexametaphosphate, solid water glass, paper fibres, polyvinyl alkanol ether, tween -80, etc. in a certain proportion. Its advantages are low cost, high adhesion, high corrosion resistance, long service life, and sure effect to purity molten steel.
Owner:海城华宇耐火材料有限公司
Repaglinide troche and preparation method thereof
ActiveCN103610677AAvoid stickingGood dispersionOrganic active ingredientsMetabolism disorderMedicineDissolution
The invention relates to an oral troche which contains repaglinide or pharmaceutically acceptable derivatives of repaglinide as well as a preparation method of the oral troche. According to the preparation method, powder of repaglinide or pharmaceutically acceptable derivatives of repaglinide is directly pressed into troche, so that the production cost is remarkably lowered, and the disintegration and the dissolution rate are greatly improved. The bioavailability and the stability of the medicine can be improved, and the problem of low content uniformity of existing small-dose medicines formed by the direct pressing method is overcome, so that the quality of the troche is better guaranteed.
Owner:华益泰康药业股份有限公司
Tomato nutrient chewable tablet and preparation method thereof
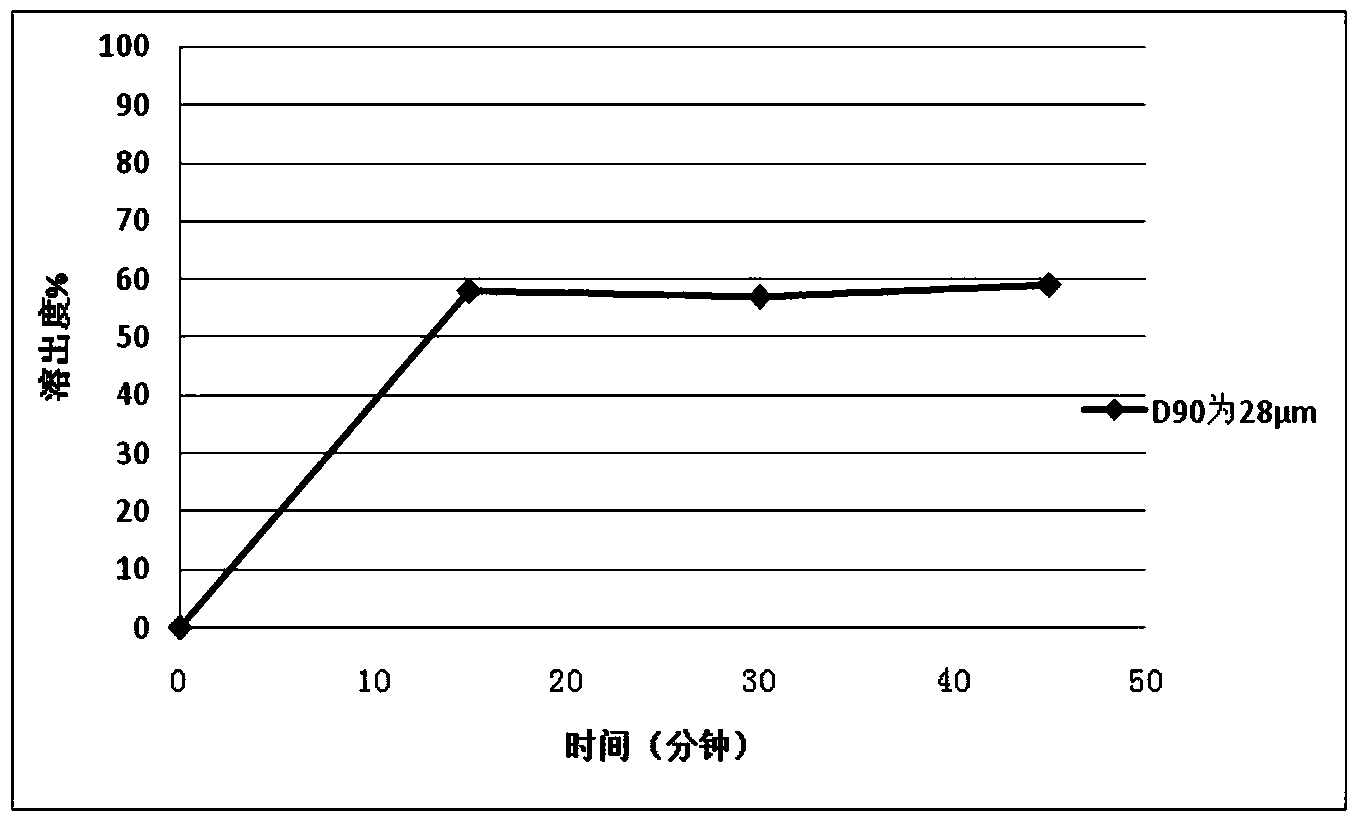
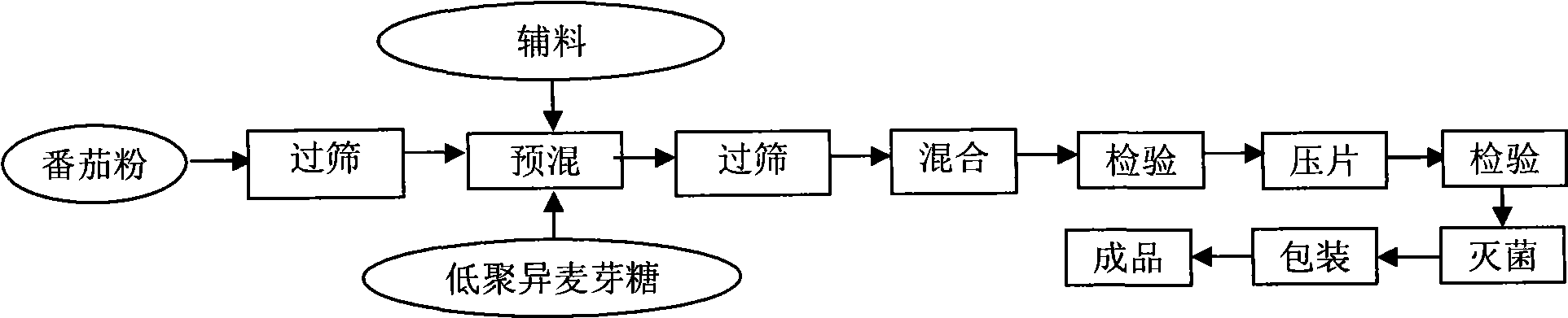
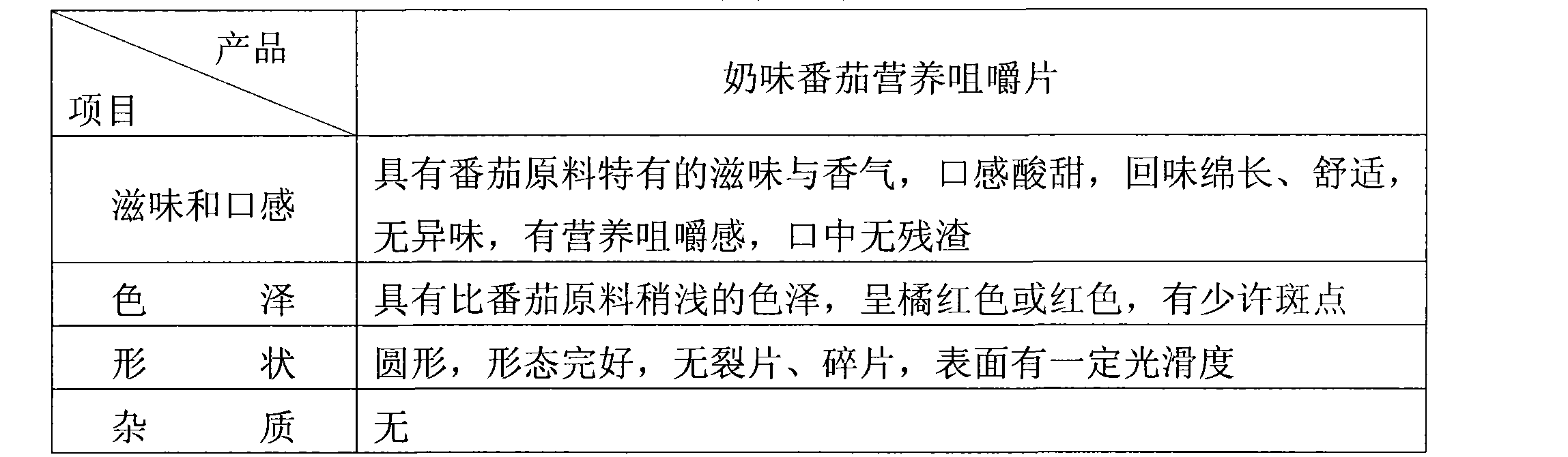
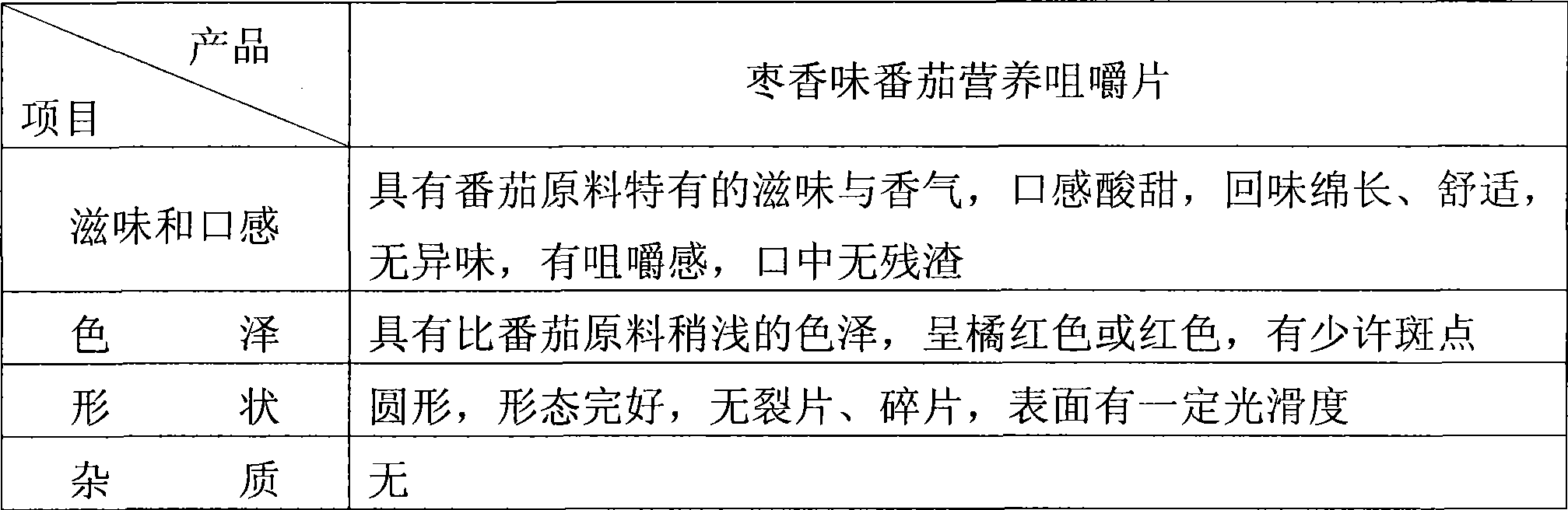
ActiveCN101530187AFull of nutritionRich tasteFood shapingFood preparationIsomaltooligosaccharideAdditive ingredient
The invention discloses a tomato nutrient chewable tablet and a preparation method thereof. The active ingredient of the tomato nutrient chewable tablet comprises tomato and oligoisomaltose. The tomato nutrient chewable tablet of the invention has high nutrition value and distinctive flavor, combines the dual health care efficacies of lycopene and oligoisomaltose, can be easily carried with and eaten anytime and anywhere. The invention adopts a method of rolling powder into tablet directly for preparing the tomato nutrient chewable tablet so that the problem that wet granulating is required before rolling during the production process of nutrient chewable tablet of the same category is solved and no rolling accessories or additives are necessary, thus simplifying the process procedure and improving the process efficiency.
Owner:INST OF AGRO FOOD SCI & TECH CHINESE ACADEMY OF AGRI SCI
Azithromycin oral disintegration tablet and its preparing method
ActiveCN1562067APromote dissolutionQuickly exert the therapeutic effect of the whole bodyAntibacterial agentsOrganic active ingredientsAzithromycinWater soluble
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD +1
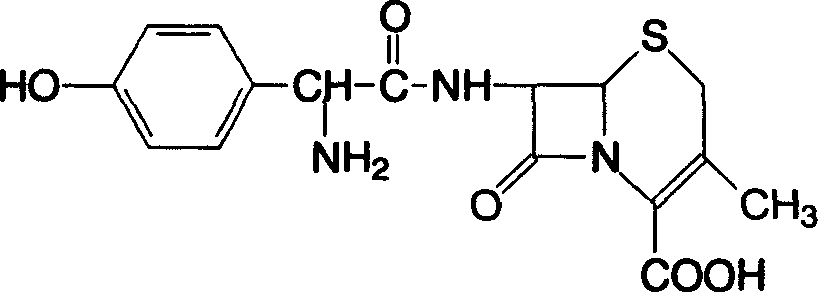
Cefadroxil oral disintegrant tablet, and its prepn. method
ActiveCN1618428APromote dissolutionQuickly exert the therapeutic effect of the whole bodyAntibacterial agentsOrganic active ingredientsWater solubleExcipient
An oral disintegrating tablet of dracefal contains dracefal and the medicinal excipient (water-soluble filler, disintegrant, lubricant, wetting agent, or adhesive). Its preparing process is also disclosed. Its advantages are high disintegrating speed, quickly taking its effect, less residue and low by-effect.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD +1
acidified bentonite
ActiveCN103979559BPromotes natural weatheringImprove the disintegration effectAluminium silicatesFiberSodium Bentonite
The invention discloses an acidified bentonite. The technical scheme is as follows: the acidified bentonite is composed of bentonite, sulfuric acid, instant sodium silicate, polyvinyl alcohol, hydroxypropyl methylcellulose and sodium carbonate. The production method of the acidified bentonite comprises the following steps: inputting acidified bentonite materials into a mill, and milling into powder, thereby obtaining the acidified bentonite. The composite proportioning is performed after acidification, thereby avoiding the chemical reaction between the sulfuric acid and the instant sodium silicate, polyvinyl alcohol, hydroxypropyl methylcellulose and sodium carbonate, fully displaying the respective characteristics of the raw materials in the formula, and ensuring the inherent quality of the acidified bentonite. The acidified bentonite has favorable adsorbability, high-temperature stability and lubricating property, and the water absorptivity, colloid index, plasticity, expansion coefficient, colloid viscosity and thixotropy are greatly enhanced. The acidified bentonite is suitable for producing products in the fields of paints, aviation, metallurgy, chemical fibers, petroleum, firefighting and the like.
Owner:江苏世澳非金属应用科技有限公司
Hydraulic pulper
The invention discloses a hydraulic pulper. The hydraulic pulper comprises a tank body, rotors, a screen plate, a bracket, a first support column and a second support column, wherein the screen plate is positioned at the lower end of the tank body, and is used for supporting the tank body; a material dropping opening and a water spray pipe are arranged at the top part of the tank body, the bottom part of the tank body is provided with a slag outlet and a pulp outlet, the slag outlet is positioned above the screen plate, and the pulp outlet is positioned under the screen plate; the cross section of the tank body is in a splay shape, and the tank body comprises a first cavity and a second cavity; the first support column is arranged in the middle of the first cavity, and the second support column is arranged in the middle of the second cavity; the rotors comprise a first rotor and a second rotor, motors are respectively arranged at the lower parts of the first rotor and the second rotor, and the rotation directions of the two motors are opposite; the first rotor is sheathed on the first support column, the second rotor is sheathed on the second support column, and the first rotor and the second rotor are not positioned on the same horizontal surface. Compared with the prior art, the hydraulic pulper has the advantages that the dead corner is avoided, the utilization rate of energy is higher, and the pulping efficiency is higher.
Owner:江苏富星纸业有限公司
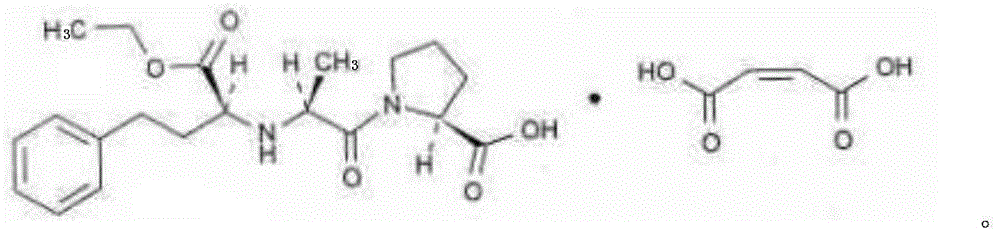
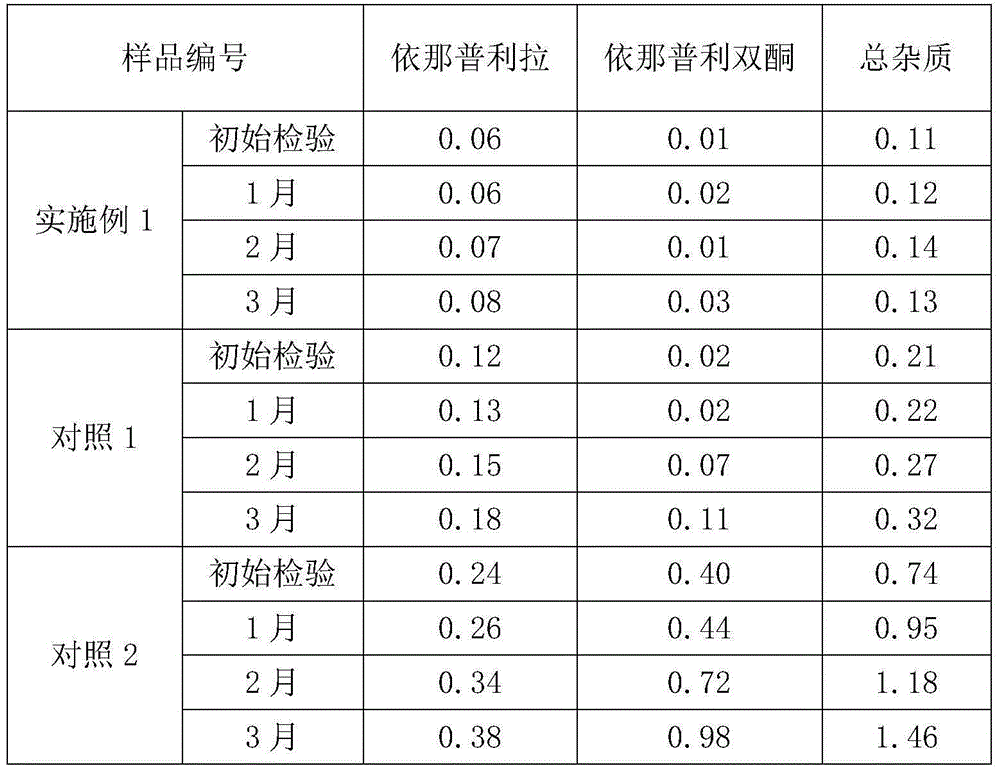
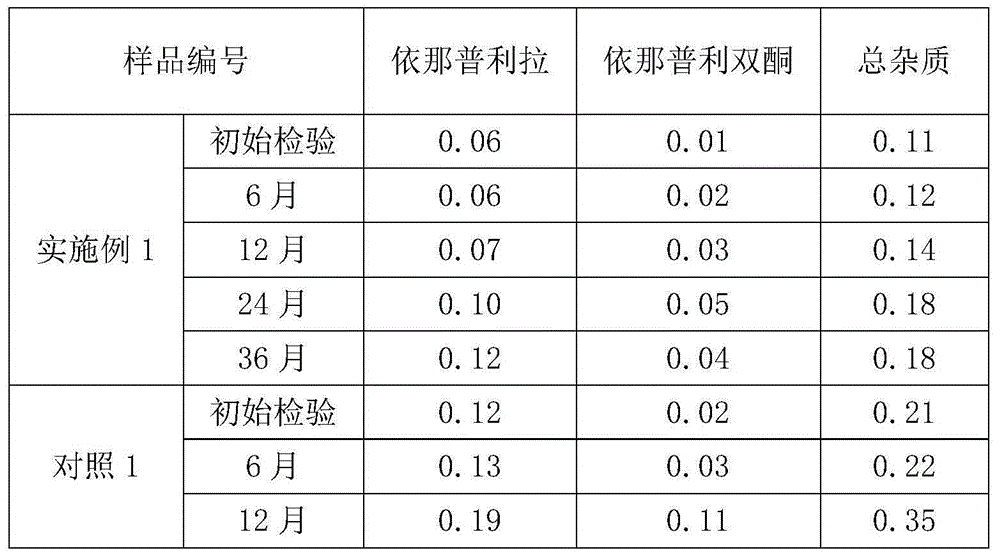
Stable enalapril maleate capsules and preparation method thereof
InactiveCN105125514AHigh hardnessImprove the disintegration effectPill deliveryPharmaceutical non-active ingredientsLACTOSE MONOHYDRATEArginine
The invention relates to stable enalapril maleate capsules and a preparation method thereof, and belongs to the technical field of medicine. Enalapril maleate, lactose monohydrate, microcrystalline cellulose, startch, hydroxypropyl methyl cellulose, sodium alga acid, poloxamer 407, polyethylene glycol 4000 and L-arginine are preferably used as medical components of the stable enalapril maleate capsules, the degradation speed of the enalapril maleate is remarkably decreased through the mutual synergistic effect, the stability of the enalapril maleate capsules is greatly improved, and it is ensured that clinical medicine is safe, effective and capable of being stored for a long time.
Owner:CSPC OUYI PHARM CO LTD
Flame-retardant urea-formaldehyde resin adhesive
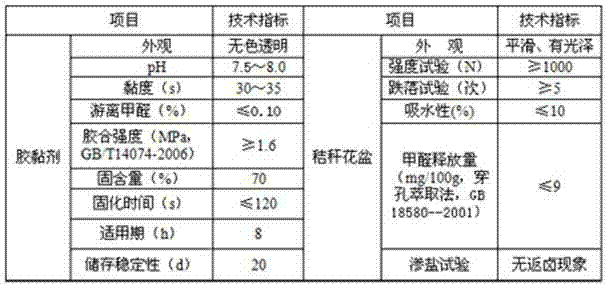
InactiveCN104531018AGood cross-linkingStable structureNon-macromolecular adhesive additivesAldehyde/ketone condensation polymer adhesivesChemistryAging property
The invention discloses a flame-retardant urea-formaldehyde resin adhesive which contains the following raw materials in parts by weight: 40-60 parts of formaldehyde, 120-140 parts of urea, 2-3 parts of polyvinyl alcohol, 5-7 parts of melamine, 0.2-0.4 parts of sodium myrastate, 2-4 parts of decabromodiphenyl ethane, 1-2 parts of ammonium dihydrogen phosphate, 0.6-1 part of tricresyl phosphate, 2-3 parts of tackifying aid, 0.1-0.3 parts of calcium acetylacetonate, 0.3-0.5 parts of 3,2-thiolbenzimidazole, 0.4-1 part of sodium dimercaptosulphonate and 0.1-0.3 parts of silane coupling agent KH560. According to the invention, formaldehyde, urea, melamine and aids are blended and modified, the finished product has good crosslinking property, stable structure, perfect water resistance, anti-aging property, flame retardance and the like and long service life; and the added tackifying aid can be effectively combined into a copolymer adhesive molecular network, so that the tackiness and the biodegradability and disintegration of subsequent products are enhanced, and the comprehensive performance is excellent.
Owner:李成功
Anti-permeation type tundish light coating and preparation method thereof
The invention discloses an anti-permeation type tundish light coating and a preparation method thereof. The anti-permeation type tundish light coating comprises the following components in parts by weight: 73-99 parts of magnesite and / or forsterite with the granularity less than or equal to 1mm, 0.1-15 parts of quartz sand, 0.2-4 parts of sulfamic acid, 0.1-3.0 parts of an organic plasticizer agent, 0.5-5 parts of composite fiber, 0.02-0.25 part of high-efficiency air entraining agent and 0.01-0.15 part of metal aluminum powder. The coating disclosed by the invention is small in volume density, high in pressure resisting intensity after being baked, small in low melt and capable of effectively preventing slag permeation and solving the problems of a conventional light coating in construction, baking and use.
Owner:PUYANG REFRACTORIES GRP CO LTD
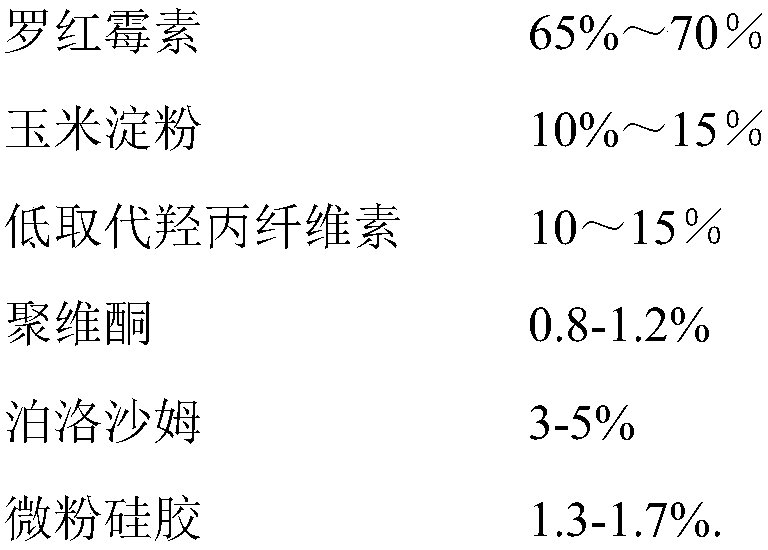
Roxithromycin capsule and preparation process thereof
ActiveCN109248155ASimple manufacturing processExcellent qualityAntibacterial agentsOrganic active ingredientsRoxithromycinMedicine
The invention relates to a roxithromycin capsule and a preparation method of the roxithromycin capsule. The roxithromycin capsule is prepared from the following constituents in percentage by weight:50-75 % of roxithromycin, 5-25 % of corn starch, 10-30 % of low-substituted hydroxypropyl cellulose, 0.5-6 % of a wetting agent, 0.5-3 % of a binder, and 0.5-3 % of a lubricant, wherein the binder ispolyvinylpyrrolidone; the wetting agent is selected from sodium dodecyl sulfate, Tween 80 and poloxamer; and the lubricant is selected from one or more of micropowder silica gel, magnesium stearate and talcum powder.
Owner:北京鑫开元医药科技有限公司
Fluidized bed device
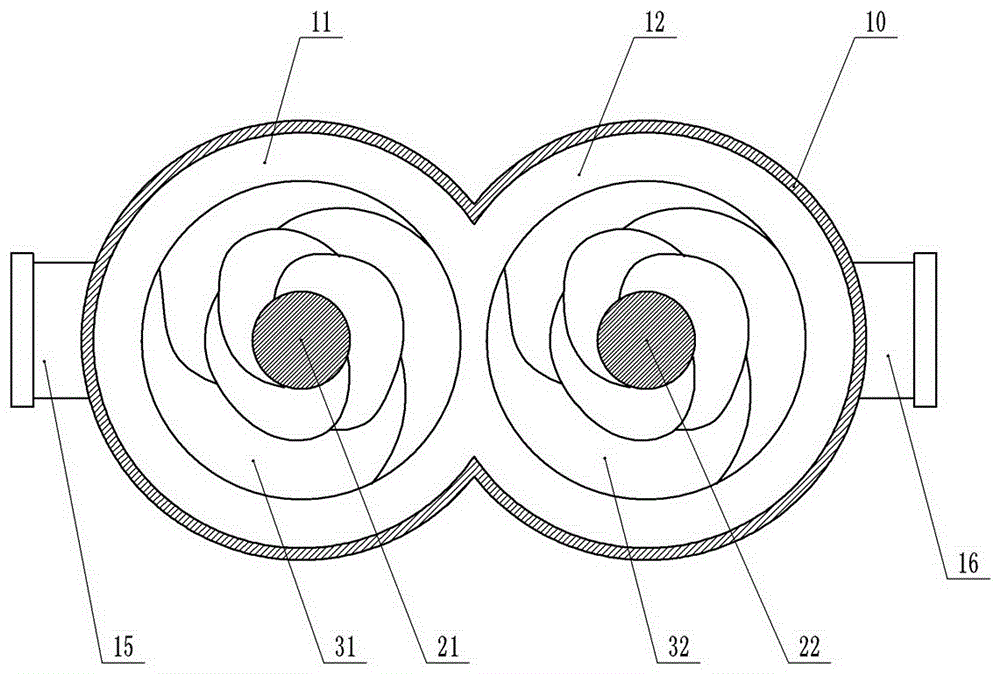
InactiveUS20060104871A1Little generationImprove production yieldGranule coatingDrying solid materials with heatFluidized bedEngineering
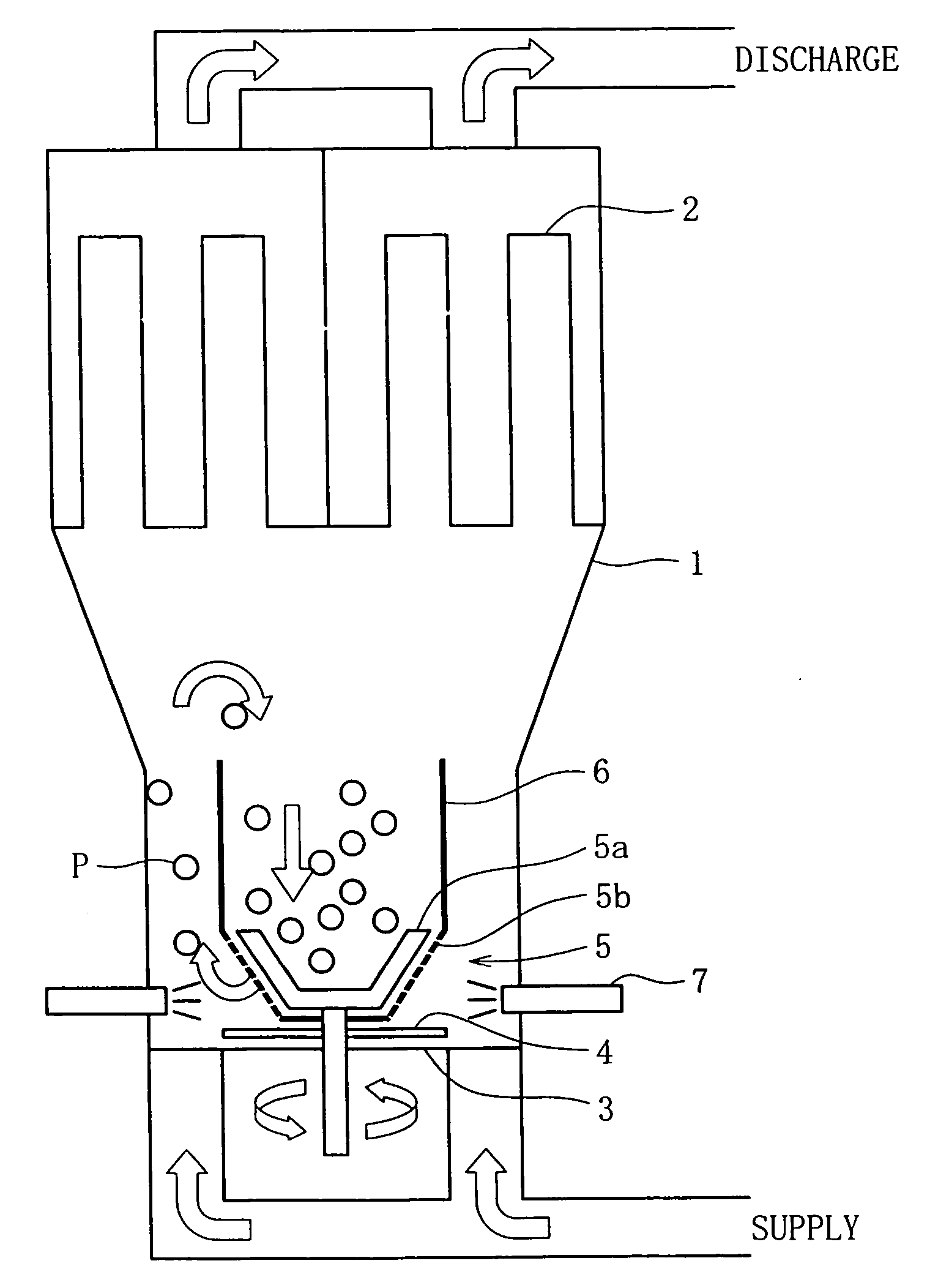
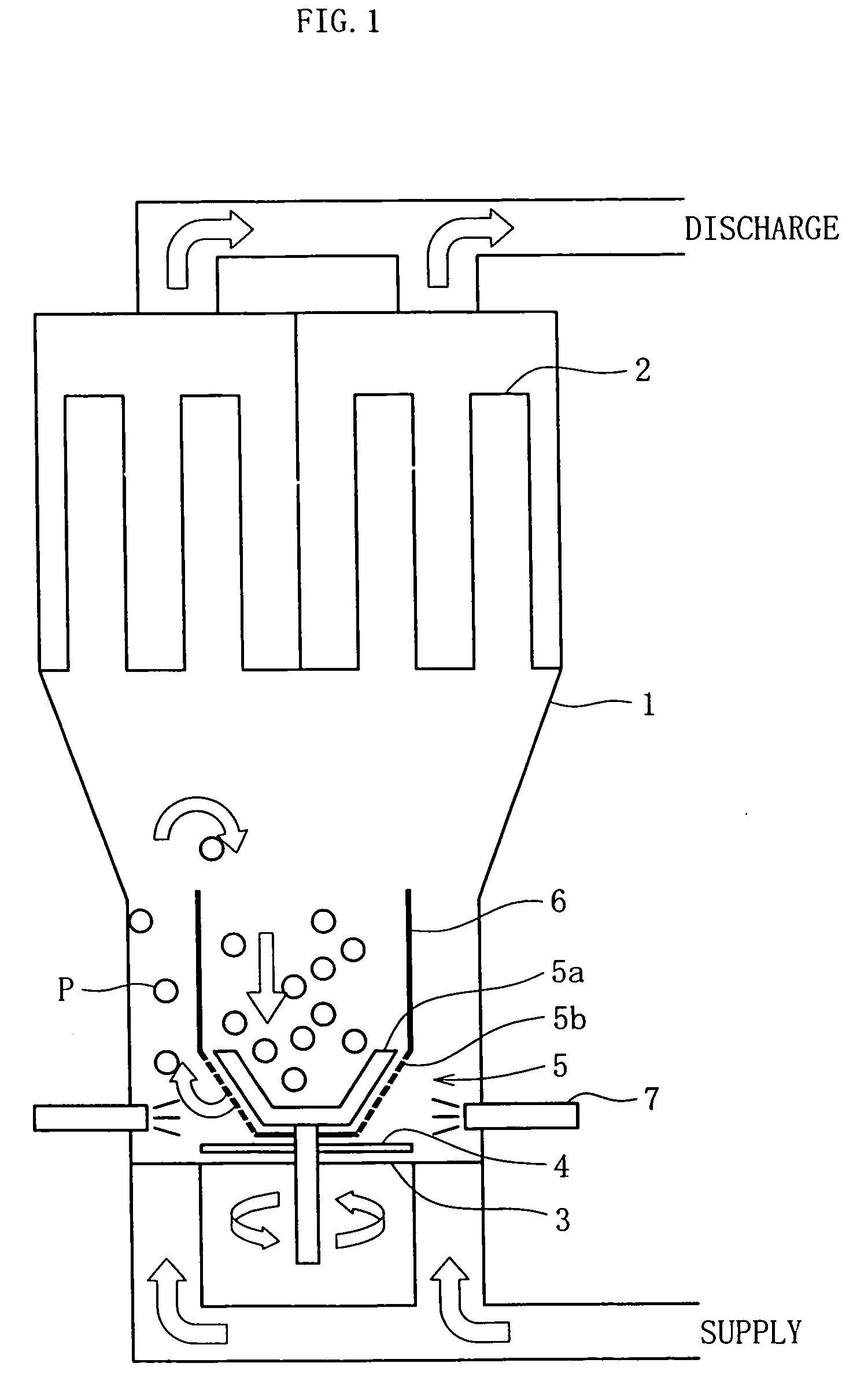
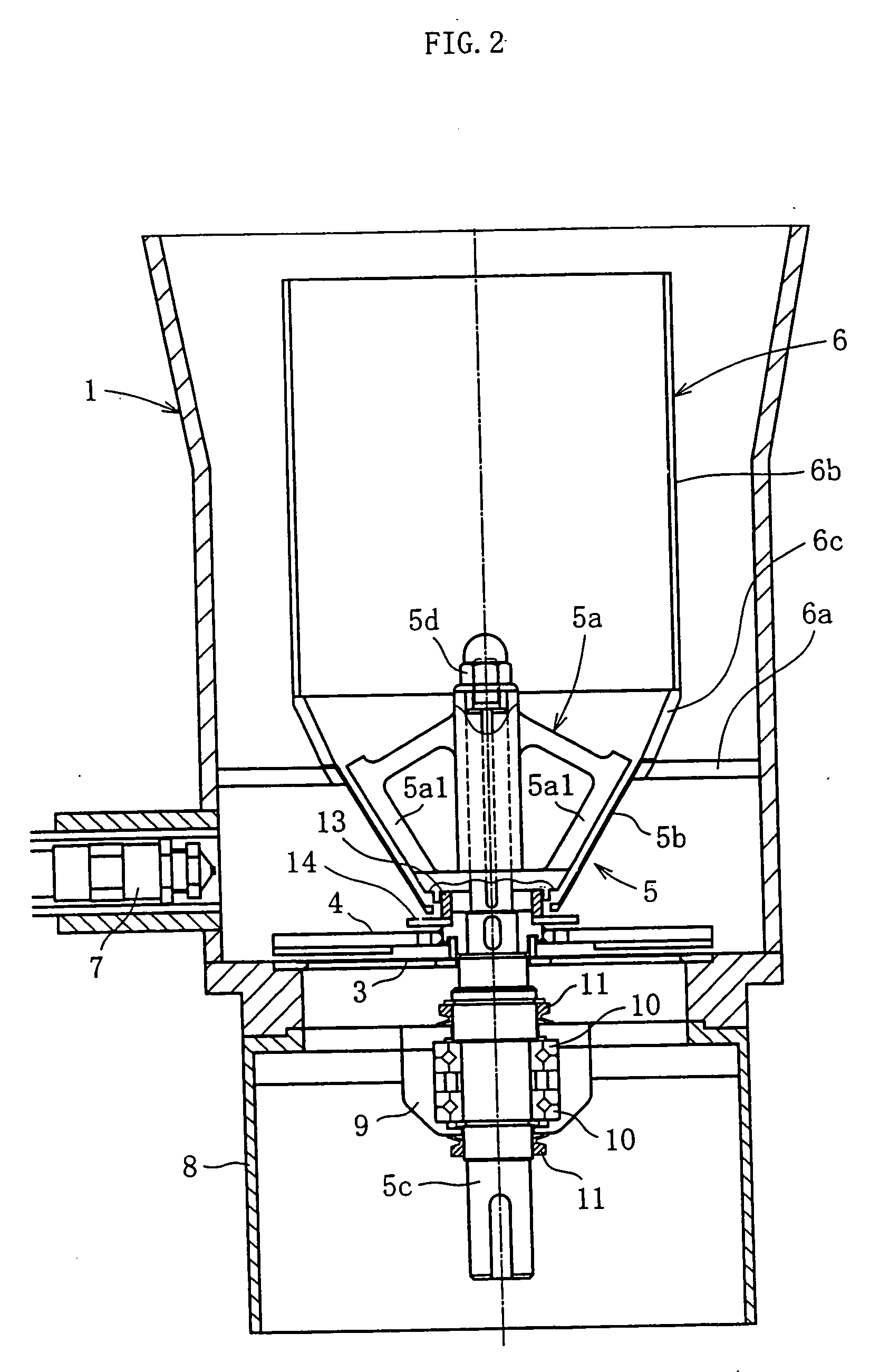
A fluidized bed apparatus has a rotary rotor 4 arranged at the bottom center thereof, a disintegrator mechanism 5 arranged above the rotary rotor 4, and a cylindrical draft tube 6 installed above the disintegrator mechanism 5. A fluidizing gas jetted from a gas dispersion plate 3 causes powder particles P in a processing container 1 to form a fluidized bed in which they circulate so as to ascend through the gap between the outer periphery of the rotary rotor 4 and the bottom inner wall of the processing container 1, the space between the disintegrator mechanism 5 and the inner wall of the processing container 1, and the space between the outer periphery of the draft tube 6 and the inner wall of the processing container 1, and descend through the inner portion of the draft tube 6.
Owner:POWREX
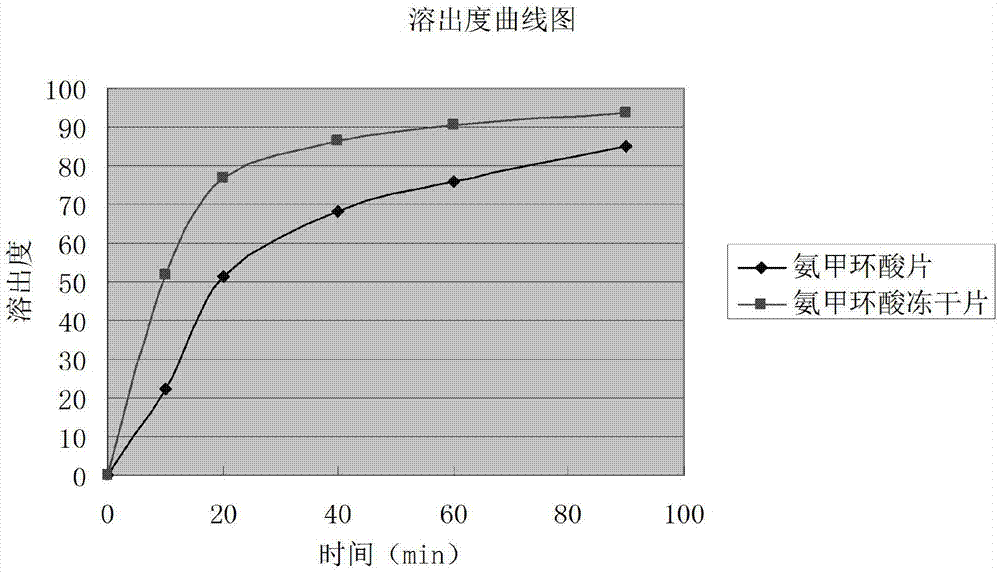
Tranexamic acid composition lyophilized tablet and preparation method thereof
InactiveCN104490752AImprove adhesionImprove the disintegration effectPeptide/protein ingredientsPharmaceutical delivery mechanismTranhexamic acidCorn starch
The invention provides a tranexamic acid composition lyophilized tablet and a preparation method thereof, and relates to the technical field of medicines and medicine production. The tablet is prepared from tranexamic acid, starch and can sugar, wherein starch and cane sugar are used as auxiliaries, common corn starch is subjected to heating treatment to improve the adhering and disintegrating effects of the starch in the tablet, so that the moldability of the tablet can be improved. The tranexamic acid composition lyophilized tablet only needs starch and cane sugar two auxiliaries. According to the preparation method, a twice-cooling twice-heating lyophilizing process is adopted for the tranexamic acid composition lyophilized tablet, the moldability of the tablet can be better by means of twice cooling and twice heating, the solubility of the tablet can be improved, and the bioavailability of the tablet can be improved; the tablet is capable of overcoming the defect of conventional tranexamic acid tablets and reducing the types and usage of auxiliaries in the tranexamic acid tablets, is high in solubility and high in bioavailability, and can be used for guaranteeing the curative effect and safety of clinical administration.
Owner:HAINAN WEI KANG PHARMA QIANSHAN
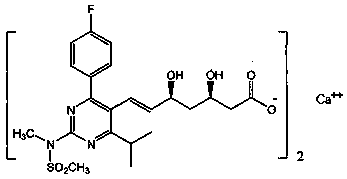
Stable rosuvastatin calcium pharmaceutical composition and preparation method thereof
InactiveCN103961354AGood disintegrationImprove featuresOrganic active ingredientsMetabolism disorderRosuvastatin CalciumMedicinal chemistry
The invention provides a stable rosuvastatin calcium pharmaceutical composition. The composition has high stability. A tablet containing the pharmaceutical composition has total impurity content of lower than 1.0% after accelerated testing, wherein lactone individual impurity content is lower than 0.10%.
Owner:SHENZHEN SALUBRIS PHARMA CO LTD
Cross-linked pachyman, its prepn. and uses
InactiveCN1834109AImprove liquidityStrong water absorptionOrganic active ingredientsAlcoholPhysical chemistry
This invention relates to cross-linking pachyman, its preparation method and application. The cross-linking pachyman is prepared as follows: pachyman is mixed with two to five-fold mass of water or alcohol to form a mixture and the pH value is adjusted to 9~11. Epichlorohydrin with a mass 0.1~0.5% of pachyman is added and the cross-linking reaction is kept at 35~45 deg. C for 6~10 hours. The mixture is then neutralized, filtered, washed and lyophilized to obtain cross-linking pachyman. The cross-linking pachyman in this invention has good fluidity, strong water-absorption, high bulk density and dilatability and can be used as efficient disintegrating reagent. The cross-linking pachyman is homogeneously dispersed into powders during disintegration so that the disintegrating ability of the troches is enhanced and the disintegrating efficiency is promoted.
Owner:WUHAN UNIV
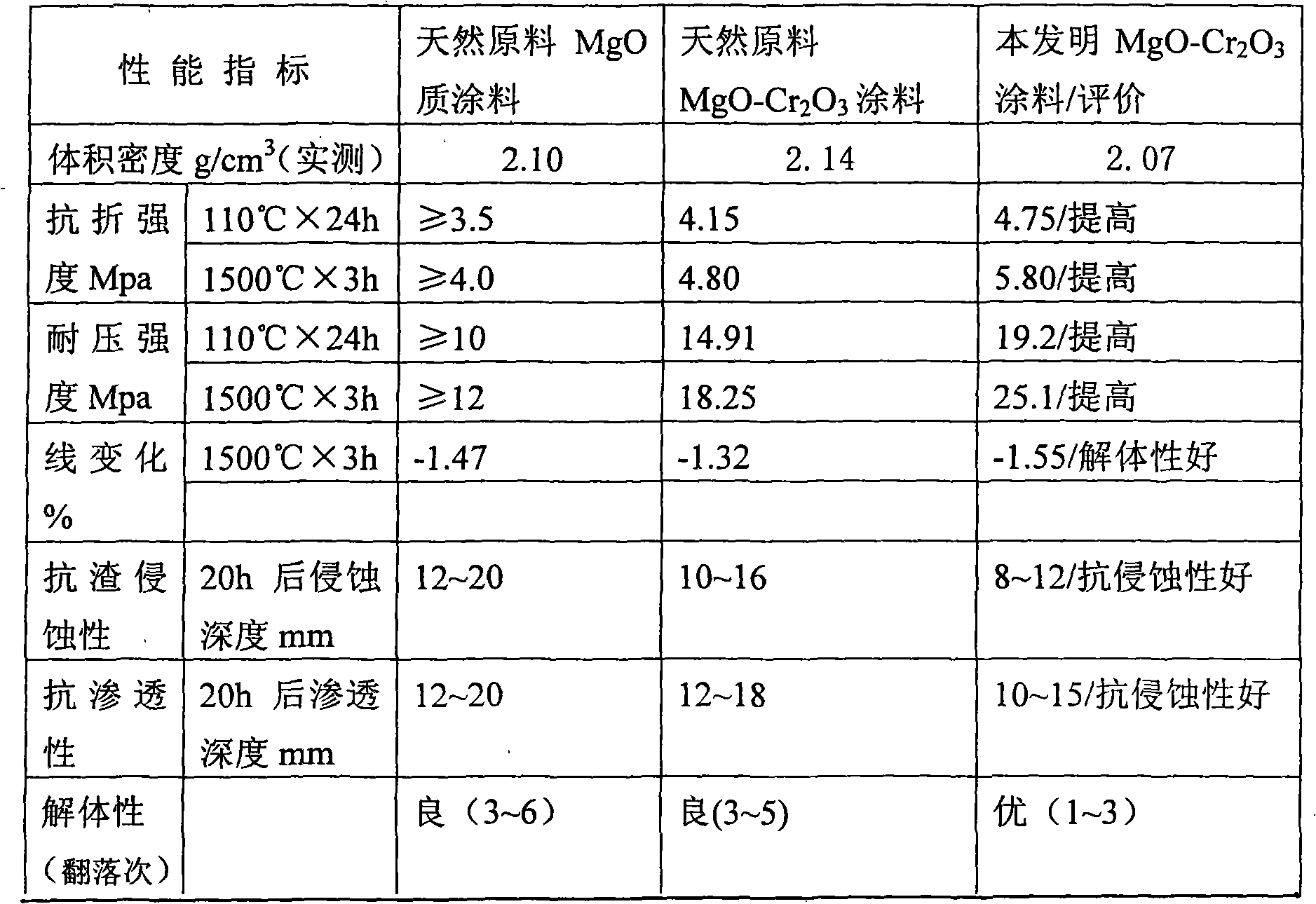
MgO-Cr2O3 paint
InactiveCN101342572AConserve natural mineral resourcesLow costFoundry mouldsFoundry coresCorrosionVolume concentration
The present invention relates to the improvement of magnesite-chrome coating that is used for wrapping a working layer, which is characterized in that the coating at least comprises MgO particle and Cr2O3, wherein, the content of the used MgO is more than or equal to 80 percent, the content of Cr2O3 is more than or equal to 8 percent, and the MgO-Cr2O3 brick waste material with the volume concentration being more than or equal to 3 g / cm<3>. Compared with the prior art adopting the natural raw materials, not only a great amount of natural mineral resource is saved, but also different performances of the coating are better than the coating that is made of the natural raw materials; moreover, the price is cheap, the quality is stable, and the coating of the present invention has good plasticity, and is easy to be coated, and is free of fracture, dropping off and explosion, and has favorable corrosion-resistance and long service life; the remained coating is separated from the permanent layer, so that the coating is easy to be sprayed again, thereby having better disassembling property.
Owner:YIXING XINDA REFRACTORY
Orally dispersible tablet
InactiveUS20120244223A1Good disintegrationDisintegrates quicklyBiocideNervous disorderAlcohol sugarsPharmaceutical Substances
The present invention provides a preparation with improved disintegration property, a preparation showing improved bioavailability of a medicament, production methods thereof and the like. A rapidly disintegrating preparation comprising granules comprising a medicament coated with a coating layer containing sugar or sugar alcohol; and a disintegrant. A production method of a rapidly disintegrating preparation including a step of producing granules comprising a medicament, a step of forming a coating layer containing sugar or sugar alcohol on the obtained granules and a step of mixing the coated granules with a disintegrant and molding the mixture.
Owner:TAKEDA PHARMA CO LTD
Intestinal disease treatment pharmaceutical composition and preparation method thereof
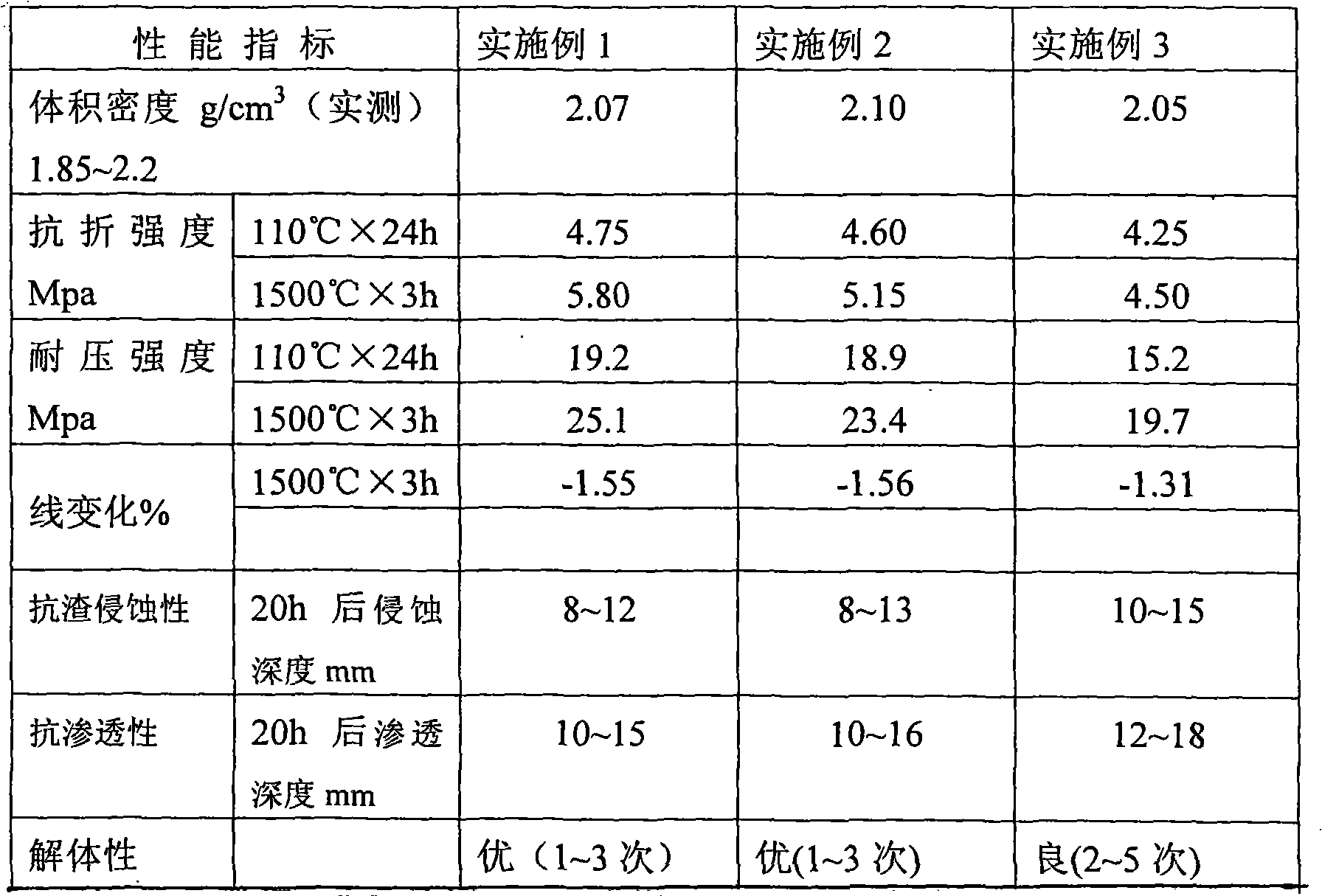
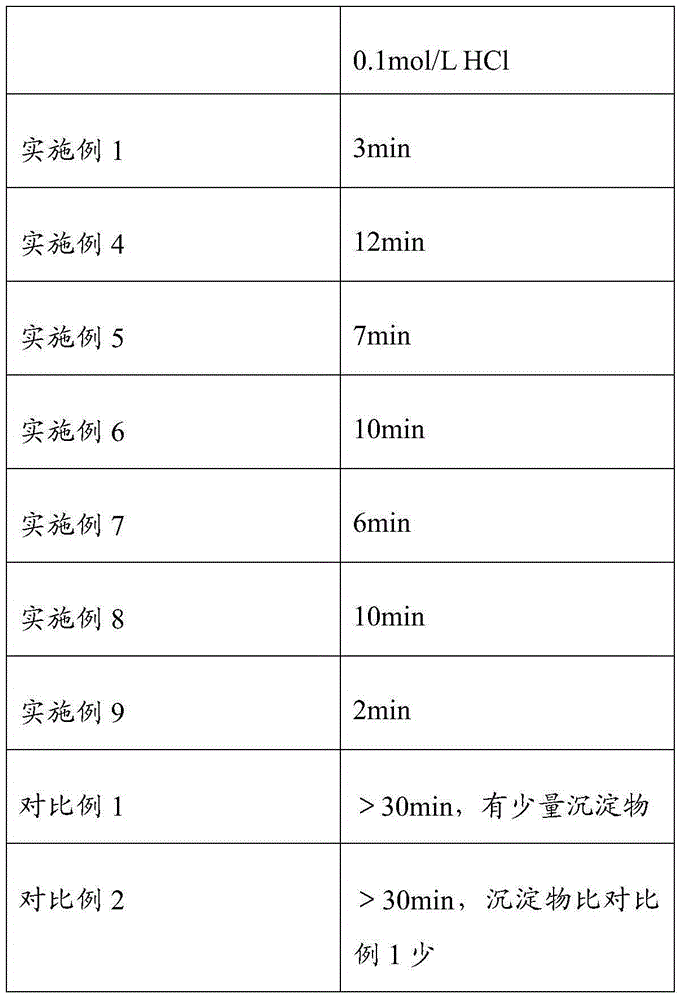
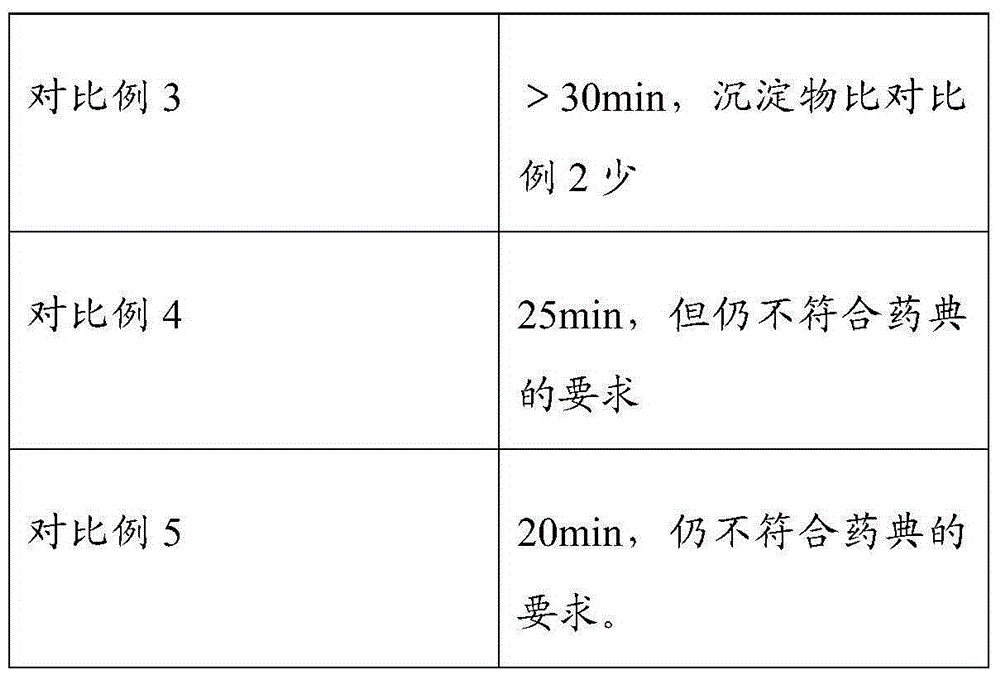
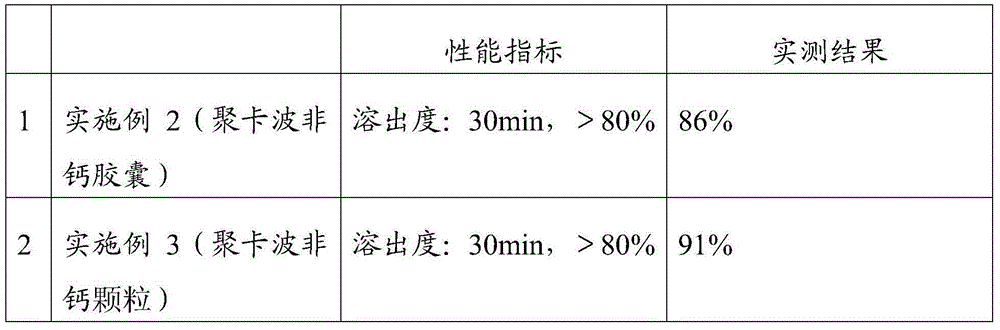
ActiveCN105012256AImprove organizational structureImprove the disintegration effectDigestive systemPill deliveryDiseaseCalcium Polycarbophil
The invention discloses an intestinal disease treatment pharmaceutical composition and a preparation method thereof. The intestinal disease treatment pharmaceutical composition comprises, by weight, 100 parts of calcium polycarbophil, 7.5-30 parts of disintegrant, 19-48 parts of stuffing, 0.5-4 parts of lubricant and 2.5-8 parts of propylene glycol. The propylene glycol added into the intestinal disease treatment pharmaceutical composition has function of improving tissue structure of the composition and making gaps among particles more uniform so as to quicken disintegration of the particles in an acid environment. As the intestinal disease treatment pharmaceutical composition is prepared by a way of adding the disintegrant in granulation and mixing the disintegrant after granulation, the intestinal disease treatment pharmaceutical composition tablet has good disintegration effect.
Owner:SUZHOU CHUNGHWA CHEM & PHARMA IND
Method for preparing baicalin monolayer osmotic pump tablets
InactiveCN107028910ATake a lotReduce fluctuations in blood concentrationOrganic active ingredientsDigestive systemSolventChemistry
The invention relates to a method for preparing baicalin monolayer osmotic pump tablets. The method includes steps of preparing baicalin solid dispersion; preparing tablet cores; preparing coating solution; carrying out coating and drying, and the like. The solid dispersion is prepared by the aid of mixture carriers of PVPk30 and polysorbate-80 by the aid of solvent-spray drying techniques. The method has the advantages that absolute ethyl alcohol is used as a reagent, is convenient to recycle and extremely low in influence on environments and human bodies, is inexpensive and is easily available; coating membranes PEG 400 are low in dosage, accordingly, the production costs of the baicalin monolayer osmotic pump tablets can be reduced, environmental pollution can be abated, and economic burden on patients can be relieved; the baicalin monolayer osmotic pump tablets are prepared by the aid of direct tabletting techniques, technological processes are simple, the medicine stability, the medicine dissolution and the disintegration can be improved, and accordingly the method is obviously superior in economical efficiency.
Owner:CHENGDE MEDICAL UNIV
Oral film-form base and preparation
InactiveCN103202822APromote dissolutionFull film strengthInorganic non-active ingredientsSheet deliverySolubilityFoaming agent
The present invention provides an oral film-form base which has a rapid dissolution profile in the mouth and sufficient film strength, and gives an improved taking property by foaming in the mouth. The oral film-form base includes an edible polymer soluble both in water and in an organic solvent having a solubility parameter of 9.7 or higher, a foaming agent, and an auxiliary foaming agent, wherein the foaming agent is foamable in the presence of water, and the foaming agent and the auxiliary foaming agent each are insoluble in the organic solvent, have an average particle size of 0.1 to 60 [mu]m, and are included in particle states.
Owner:NITTO DENKO CORP
Erythromycin ethylsuccinate chewable tablet and preparation method thereof
InactiveCN104434830AGood molding effectEasy to operateOrganic active ingredientsPharmaceutical non-active ingredientsSucroseErythromycin Ethylsuccinate
The invention provides an erythromycin ethylsuccinate chewable tablet and a preparation method thereof, and relates to the technical field of medicine production. The erythromycin ethylsuccinate chewable tablet consists of erythromycin ethylsuccinate, starch and sucrose. The tablet disclosed by the invention is capable of overcoming the shortcomings of a normal chewable tablet, and reducing the variety and dosage of accessories in the erythromycin ethylsuccinate chewable tablet; and the medicine preparation is excellent in performance, high in bioavailability, good in stability, high in patient acceptability and free from grittiness, and has important clinical application value.
Owner:HAINAN WEI KANG PHARMA QIANSHAN
Dimemorfan phosphate tablet composite and preparation method thereof
ActiveCN103169681AGood disintegrationHigh hardnessOrganic active ingredientsPharmaceutical non-active ingredientsCarboxymethyl starchCalcium bicarbonate
The invention relates to the field of pharmaceutical preparation, and specifically relates to a dimemorfan phosphate tablet composite and a preparation method thereof. The composite comprises the following active ingredients in percentage by weight: 8-8.2% of dimemorfan phosphate tablets, 72-73% of microcrystalline cellulose, 9-10% of calcium bicarbonate, 6-7% of sodium carboxymethyl starch, and 0.7-1% of magnesium stearate. The method for preparing a dimemorfan phosphate tablet composite provided by the embodiment of the invention comprises the steps of sieving, mixing, granulating, total mixing, pelleting and the like. A dimemorfan phosphate tablet disclosed by the embodiment of the invention has the advantages of low cost and good pharmaceutical effect, becomes one of the domestic low-cost and high-efficiency antibechic, and has an extremely good promotion value; and the preparation method of dimemorfan phosphate tablets provided by the embodiment of the invention has the advantages of simple preparation process and high production efficiency.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
Emamectin-benzoate water dispersible granules and preparation method thereof
InactiveCN108684689AImprove adhesionAvoid it happening againBiocideAnimal repellantsWater dispersibleAdhesive
The invention discloses emamectin-benzoate water dispersible granules and a preparation method thereof. The formula comprises the following components in percent by weight: 0.1-30% of emamectin-benzoate raw drug, 5-10% of adhesive, 7-13% of dispersing agent, 1-3% of wetting agent and the balance of filler. The preparation method comprises the following steps of: fully and uniformly mixing the emamectin-benzoate raw drug, the dispersing agent, the wetting agent and the filler, crushing to target particle diameter, spraying aqueous solution of the adhesive to carry out kneading, carrying out granulation, drying and sieving after kneading to prepare a finished product of the emamectin-benzoate water dispersible granules. The emamectin-benzoate water dispersible granules and the preparation method disclosed by the invention have the beneficial effects that the drug effect is not influenced, meanwhile the finished-product yield is greatly increased to 95-97% or above, the dust generation amount is reduced, the loss is reduced and the cost is reduced.
Owner:山东百农思达生物科技有限公司
Flow-mixing type rotating wheel capable of preventing blockages
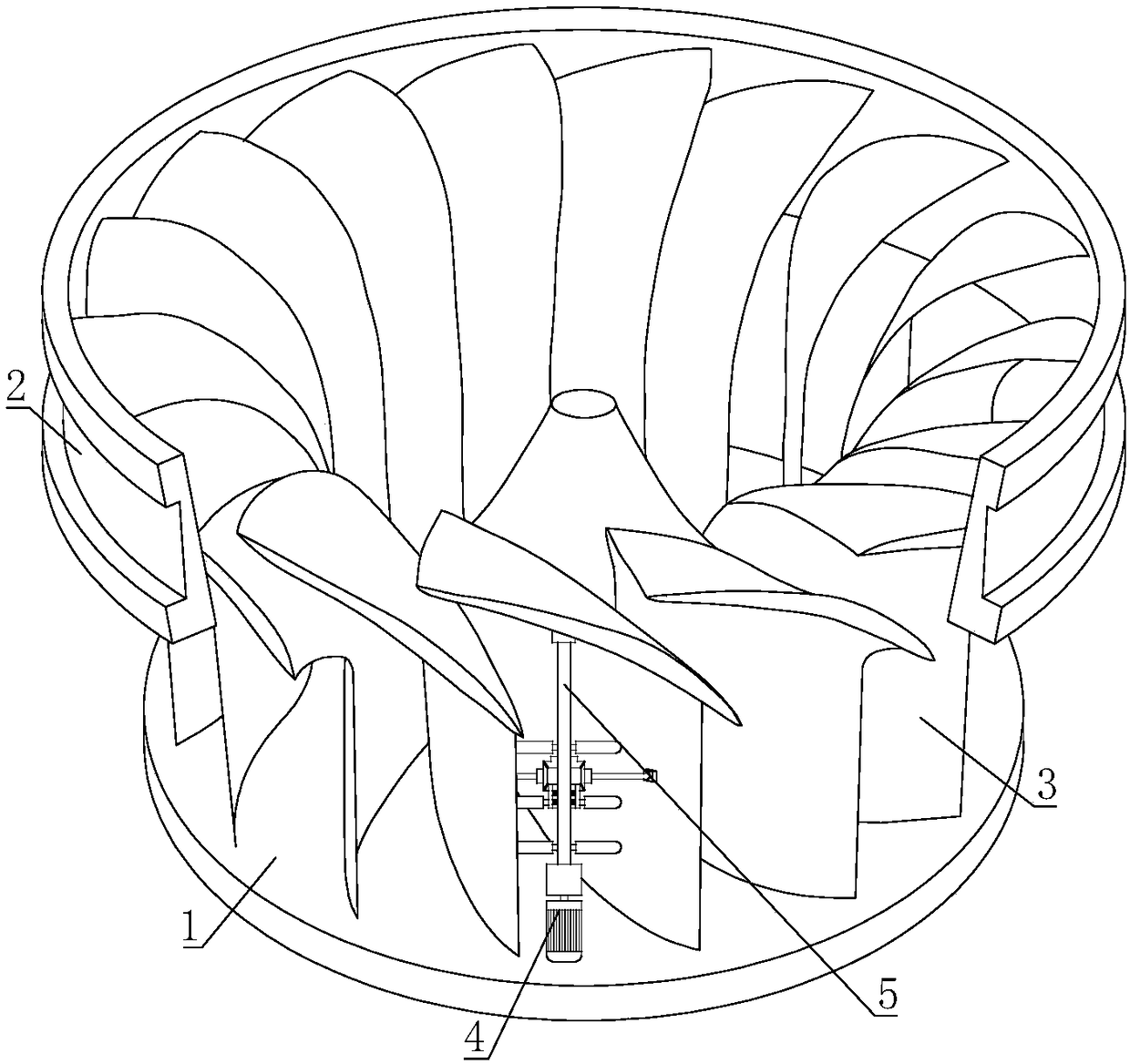
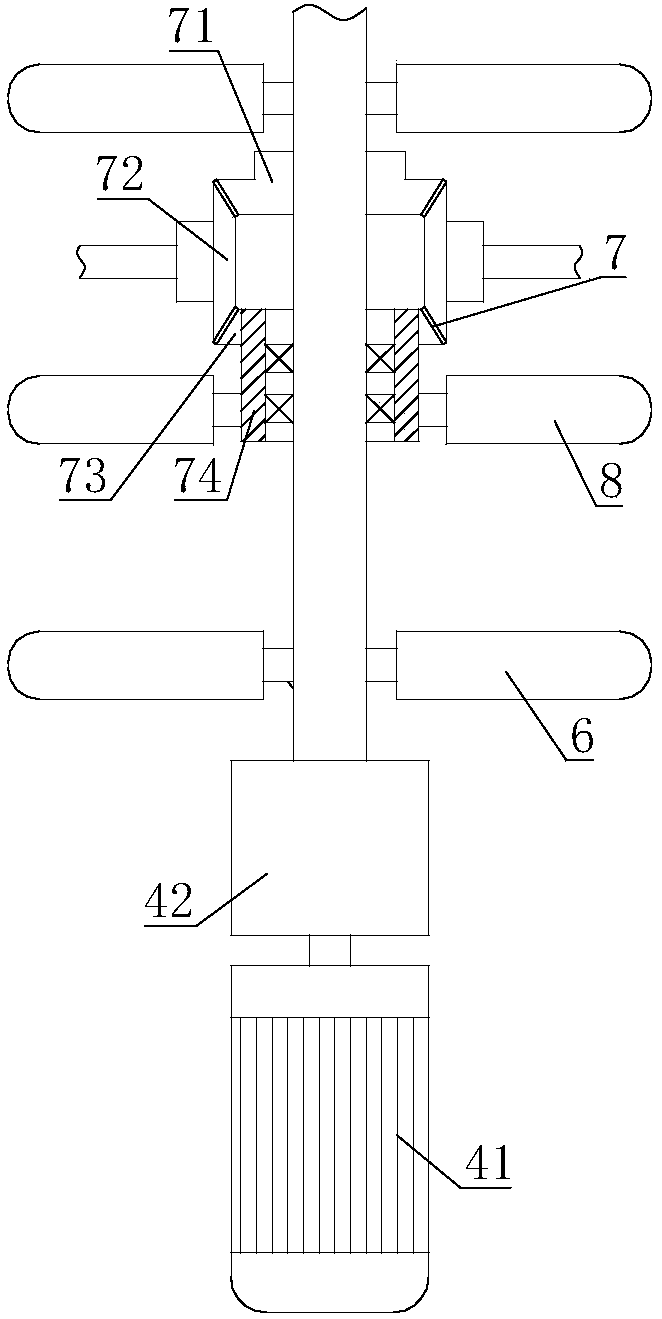
ActiveCN108425778AAvoid excessive vibrationImprove power generation efficiencyEngine fuctionsHydro energy generationForeign matterWater turbine
The invention relates to the field of water turbines, in particular to a flow-mixing type rotating wheel capable of preventing blockages. According to the technical scheme, the flow-mixing type rotating wheel capable of preventing the blockages comprises an upper crown, a lower ring and a plurality of flow-mixing blades, wherein the plurality of flow-mixing blades are connected between the upper crown and the lower ring; a driving mechanism is arranged between every two adjacent flow-mixing blades, the driving mechanisms are arranged on the upper crown, the output ends of the driving mechanisms are connected to rotating shafts, and the other ends of the rotating shafts are connected to the flow-mixing blades through bearings, and the rotating shafts are connected to crushing devices used for crushing sundries between adjacent flow-mixing blades. According to the flow-mixing type rotating wheel, the blockages are prevented, and the problem that the flow-mixing type rotating wheel of anexisting water turbine is prone to being blocked by foreign matters is solved.
Owner:SICHUAN HAOYUAN ELECTROMECHANICAL CO LTD
Molding material mixtures containing an oxidic boron compound and method for the production of molds and cores
ActiveUS20160361756A1High strengthImprove the disintegration effectFoundry mouldsFoundry coresShell moldingBoron
The invention relates to molding material mixtures containing a molding base material, water glass, amorphous silicon dioxide and an oxidic boron compound, and the production of molds and cores, in particular for metal casting.
Owner:ASK CHEM LP
Halogen seepage-free adhesive and its preparation method and use
InactiveCN102516711AOvercoming the problem of brine seepageOvercome water resistanceReceptacle cultivationPolymer scienceAdhesive
The invention relates to a halogen seepage-free adhesive and its preparation method and use. The halogen seepage-free adhesive is a melamine-urea-formaldehyde copolymer containing low-free urea and low-free formaldehyde. Urea, melamine and formaldehyde undergo a polymerization reaction in the presence of a thickening agent as an auxiliary material and one or more free formaldehyde catching agentsto produce the halogen seepage-free adhesive, wherein a mole ratio of the formaldehyde to the urea is (1.3 to 1.5): 1; a mole ratio of the formaldehyde to the melamine is (11 to 14): 1; the mass of the thickening agent is 1.0 to 3.0% of the total mass of the urea; the mass of the one or more free formaldehyde catching agents is 8.0 to 12.0% of the total mass of the urea; and the halogen seepage-free adhesive has a pH value of 7.5 to 8.0. The preparation method adopts a weak base-weak acid synthesis technology and comprises the following steps of adding urea into a reaction system by two steps, adding melamine into the reaction system by three steps, adding the thickening agent into the reaction system in an early stage of synthesis and adding the one or more free formaldehyde catching agents into the reaction system in a later stage of the synthesis. In use, the halogen seepage-free adhesive and straw powder are mixed and are processed into a halogen seepage-free straw flowerpot.
Owner:JIANGSU ACAD OF AGRI SCI
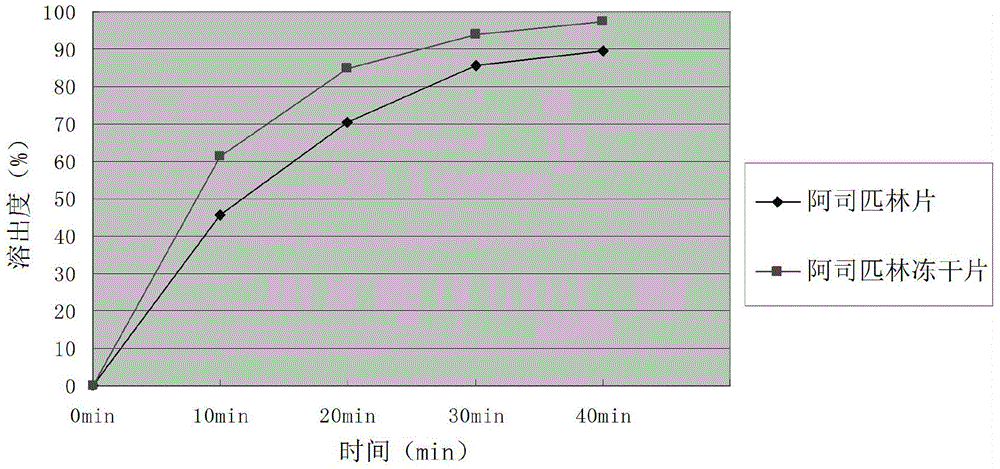
Aspirin composition freeze-dried tablets and preparation method thereof
InactiveCN104546680AReduce typesReduce dosageOrganic active ingredientsNervous disorderSucroseFreeze-drying
The invention provides aspirin composition freeze-dried tablets and a preparation method thereof, and relates to the technical field of medicine and medicine production. The aspirin composition freeze-dried tablets comprise aspirin, starch and sucrose, wherein starch and sucrose are used as accessories. Ordinary corn starch is treated by a heating process, so that the bonding and disintegration effects of starch in the tablets can be improved, and the forming property of the tablets can be improved; only starch and sucrose are required to be used as the accessories of the aspirin composition freeze-dried tablets. A two-cooling two-heating freeze-drying process is adopted for the aspirin composition freeze-dried tablets, and by twice cooling and twice heating, better forming property of the tablets can be achieved, and the dissolubility of the tablets is improved, so that the bioavailability of the tablets is improved. According to the tablets, the defects of ordinary aspirin tablets are overcome, the sort and using amounts of the accessories in the aspirin tablets are reduced, the tablets are high in dissolubility and bioavailability, and the curative effects and safety of clinical mediation are ensured.
Owner:HAINAN WEI KANG PHARMA QIANSHAN
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com