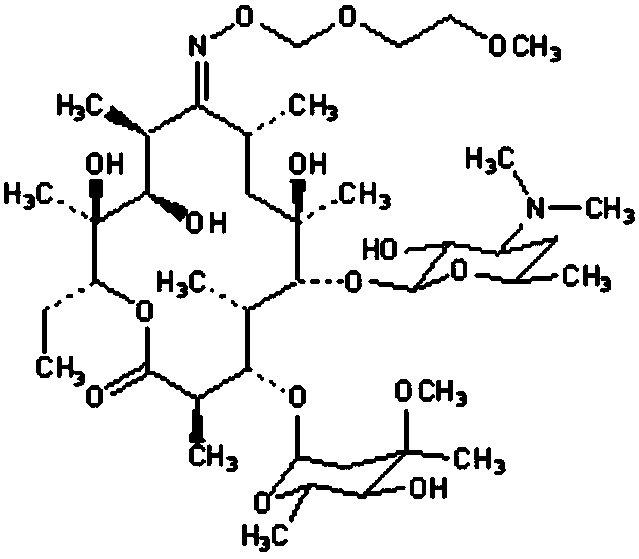
Roxithromycin capsule and preparation process thereof
A technology for roxithromycin and capsules, which is applied in the field of pharmaceutical preparations, can solve the problems of unstable dissolution curve, difficult drug detection work, influence of detection results, etc., and achieves the effect of simple and easy preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
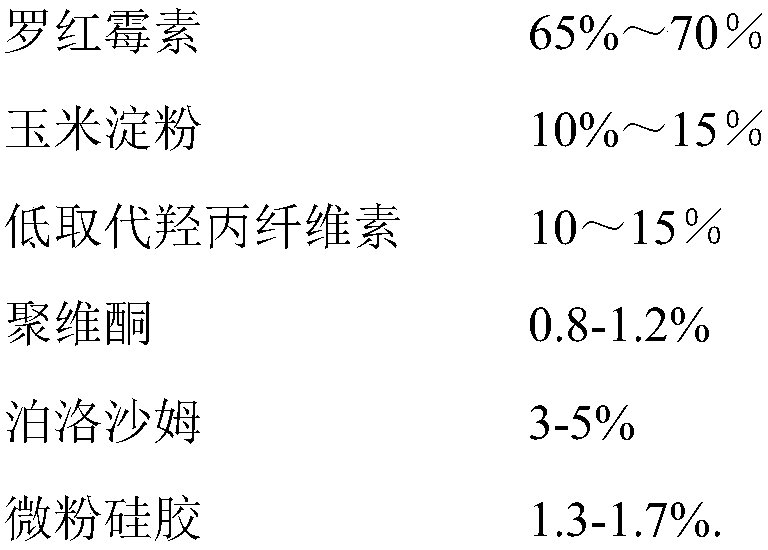
[0032] Example 1 Preparation of Roxithromycin Capsules of the Invention
[0033] Prepare according to the following formula:
[0034]
[0035]
[0036] Preparation:
[0037] a. Roxithromycin is crushed and sieved first, and then PVP K30 and poloxamer aqueous solutions are prepared for use;
[0038] b. Weigh roxithromycin, low-substituted hydroxypropyl cellulose, and corn starch, mix them evenly, and prepare soft materials with PVPK30 and poloxamer aqueous solution;
[0039] c. Put it directly in a drying oven to dry, and the dried particles are passed through a 20-mesh sieve to be granulated;
[0040] d. Weigh and mix finely powdered silica gel, magnesium stearate, talc and dry granules, and fill the capsules and pack them.
Embodiment 2
[0041] Example 2 Preparation of Roxithromycin Capsules of the Invention
[0042] Prepare according to the following formula:
[0043]
[0044] Preparation:
[0045] a. Roxithromycin is crushed and sieved first, and then PVP K30 and poloxamer aqueous solutions are prepared for use;
[0046] b. Weigh roxithromycin, low-substituted hydroxypropyl cellulose, and corn starch, mix them evenly, and prepare soft materials with PVPK30 and poloxamer aqueous solution;
[0047] c. Put it directly in a drying oven to dry, and the dried particles are passed through a 20-mesh sieve to be granulated;
[0048] d. Weigh and mix finely powdered silica gel, talcum powder and dry granules as additional auxiliary materials, fill the capsules and pack.
Embodiment 3
[0049] Example 3 Preparation of Roxithromycin Capsules of the Invention
[0050]
[0051] a. Roxithromycin is crushed and sieved first, and then PVP K30 and poloxamer aqueous solutions are prepared for use;
[0052] b. Weigh roxithromycin, low-substituted hydroxypropyl cellulose, and corn starch, mix them evenly, and prepare soft materials with PVPK30 and poloxamer aqueous solution;
[0053] c. Put it directly in a drying oven to dry, and the dried particles are passed through a 20-mesh sieve to be granulated;
[0054] d. Weigh out the finely powdered silica gel and dry granules, fill the capsules and pack.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com