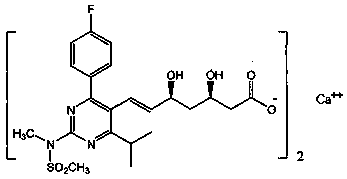
Stable rosuvastatin calcium pharmaceutical composition and preparation method thereof
A technology for rosuvastatin calcium and a composition is applied in the field of stable pharmaceutical composition and its preparation, and can solve the problems of product quality influence, large amount of lactone impurities, and inability to apply drug production, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
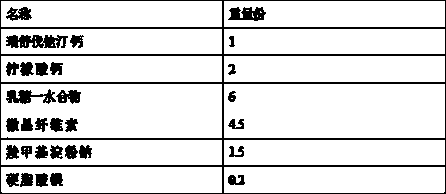
[0035] Name of raw material Dosage (g) Rosuvastatin Calcium 10 Calcium Citrate 20 lactose monohydrate 60 microcrystalline cellulose 45 Sodium carboxymethyl starch 15 Magnesium stearate 2 Opadry Film Coating Premix Appropriate amount
[0036] Pass the prescribed amount of rosuvastatin calcium and calcium citrate through a 80-mesh sieve, and mix the two thoroughly; , fully mixed uniformly; add the prescribed amount of magnesium stearate, fully mixed uniformly; the uniform mixed powder obtained was pressed into 1000 tablets; the tablets were coated with Opadry® 295K640007 PINK coating powder provided by Clorcon, Coating weight gain is 2~3% (w / w).
Embodiment 2
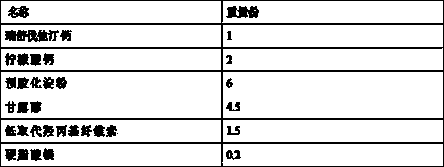
[0038] Name of raw material Dosage (g) Rosuvastatin Calcium 10 Calcium Citrate 20 pregelatinized starch 60 Mannitol 45 Low-substituted hydroxypropyl cellulose 15 Magnesium stearate 2 Opadry Film Coating Premix Appropriate amount
[0039]Pass the prescribed amount of rosuvastatin calcium and calcium citrate through a 80-mesh sieve, and mix the two thoroughly; add the prescribed amount of pregelatinized starch, mannitol, and low-substituted hydroxypropyl cellulose , fully mixed uniformly; add the prescribed amount of magnesium stearate, fully mixed uniformly; the uniform mixed powder obtained was pressed into 1000 tablets; the tablets were coated with Opadry® 295K640007 PINK coating powder provided by Clorcon, Coating weight gain is 2~3% (w / w).
Embodiment 3
[0041] Name of raw material Dosage (g) Rosuvastatin Calcium 10 Calcium Citrate 20 microcrystalline cellulose 60 Mannitol 45 Crospovidone 15 Magnesium stearate 2 Opadry Film Coating Premix Appropriate amount
[0042] The prescription amount of rosuvastatin calcium and calcium citrate are passed through 80 mesh sieve respectively, and the two are fully mixed evenly; Mix well; add the prescribed amount of magnesium stearate, mix well; press the obtained uniform mixed powder into 1000 tablets; use Opadry? 295K640007 PINK coating powder provided by Clorcon to coat the tablets, The weight gain is 2~3% (w / w).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com