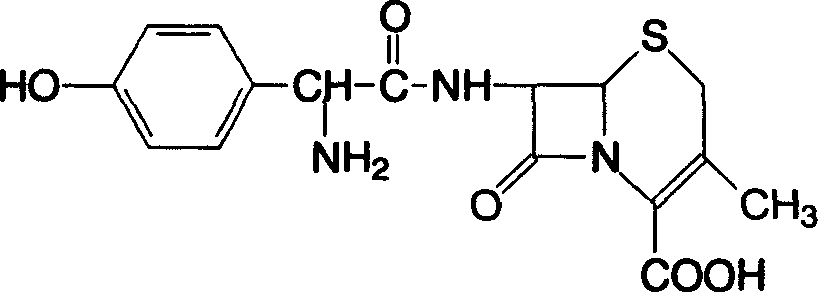
Cefadroxil oral disintegrant tablet, and its prepn. method
A cefadroxil oral cavity and cefadroxil technology are applied in the field of cefadroxil oral disintegrating tablets and their preparation fields, and can solve the problems of not being able to fully meet the medication needs of all patients, difficulty in swallowing patients taking the medicine, and children being unable to take it by themselves, and the like. The effect of shortening the dissolution time, increasing the absorption point, and good compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1: Cefadroxil orally disintegrating tablet
[0047] Component Weight Weight %
[0048] Cefadroxil 132g (equivalent to cefadroxil 44% (equivalent to cefadroxil
[0049] Ampicillin Anhydrate 125g) Ampicillin Anhydrate 41.7%)
[0050] Mannitol 93g 31%
[0051] Aspartame 1.5g 0.5%
[0052] Menthol 1g 0.33%
[0053] Sweet Orange Powder Flavor 6g 4.55%
[0054] 5% povidone 95% ethanol appropriate amount
[0055] the solution
[0056]Low substituted hydroxypropyl cellulose 12g 4%
[0057] Microcrystalline Cellulose 48g 16%
[0058] Tartaric acid 1.5g 0.5%
[0059] Sodium bicarbonate 1.5g 0.5%
[0060] Magnesium Stearate 3g 1%
[0061] Total weight 300g
[0062] A total of 1000 pieces were made
[0063] Concrete preparation method is as follows:
[0064] Weigh cefadroxil, mannitol, aspartame, menthol, sweet orange powder essence and part of low-substituted hydroxypropyl cellulose that have passed through a 100-mesh sieve, mix well and a...
Embodiment 2
[0065] Embodiment 2: Cefadroxil orally disintegrating tablet
[0066] Component Weight Weight %
[0067] Cefadroxil 132g (equivalent to cefadroxil 26.4% (equivalent to cefadroxil
[0068] Amoxycillin Anhydrous 125g) Amoxycillin Anhydrous 25%)
[0069] Erythrose 180g 60%
[0070] Aspartame 2.5g 0.5%
[0071] Menthol 1g 0.2%
[0072] Sweet Orange Powder Flavor 12g 2.4%
[0073] 50% ethanol appropriate amount
[0074] Mannitol Powder 60g 12%
[0075] Low-substituted hydroxypropyl cellulose 20g 4%
[0076] Microcrystalline Cellulose 80g 16%
[0077] Citric acid 5g 1%
[0078] Sodium bicarbonate 2.5g 0.5%
[0079] Magnesium Stearate 5g 1%
[0080] Total weight 500g
[0081] A total of 1000 pieces were made
[0082] Concrete preparation method is as follows:
[0083] Weigh cefadroxil, erythrose, aspartame, menthol, sweet orange powder essence and part of low-substituted hydroxypropyl cellulose that have passed through a 100-mesh sieve, mix well, add ...
Embodiment 3
[0084] Embodiment 3: Cefadroxil orally disintegrating tablet
[0085] Component Weight Weight %
[0086] Cefadroxil 264g (equivalent to cefadroxil 44% (equivalent to cefadroxil
[0087] Ampicillin Anhydrate 250g) Ampicillin Anhydrate 41.7%)
[0088] Erythrose 184g 30.7%
[0089] Aspartame 3g 0.5%
[0090] Menthol 2g 0.33%
[0091] Sweet Orange Powder Flavor 12g 2%
[0092] 50% ethanol appropriate amount
[0093] Crospovidone 40g 6.7%
[0094] Microcrystalline Cellulose 80g 13.3%
[0095] Citric acid 6g 1%
[0096] Sodium bicarbonate 3g 0.5%
[0097] Magnesium Stearate 6g 1%
[0098] Total weight 600g
[0099] A total of 1000 pieces were made
[0100] Concrete preparation method is as follows:
[0101] Weigh cefadroxil, erythrose, aspartame, menthol, sweet orange powder essence and part of cross-linked povidone that passed through a 100-mesh sieve, mix well, add an appropriate amount of 50% ethanol to granulate, and mix the rest Crospovidone, citric ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com