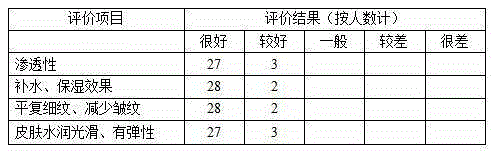
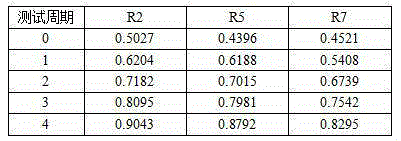
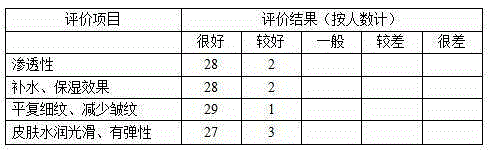
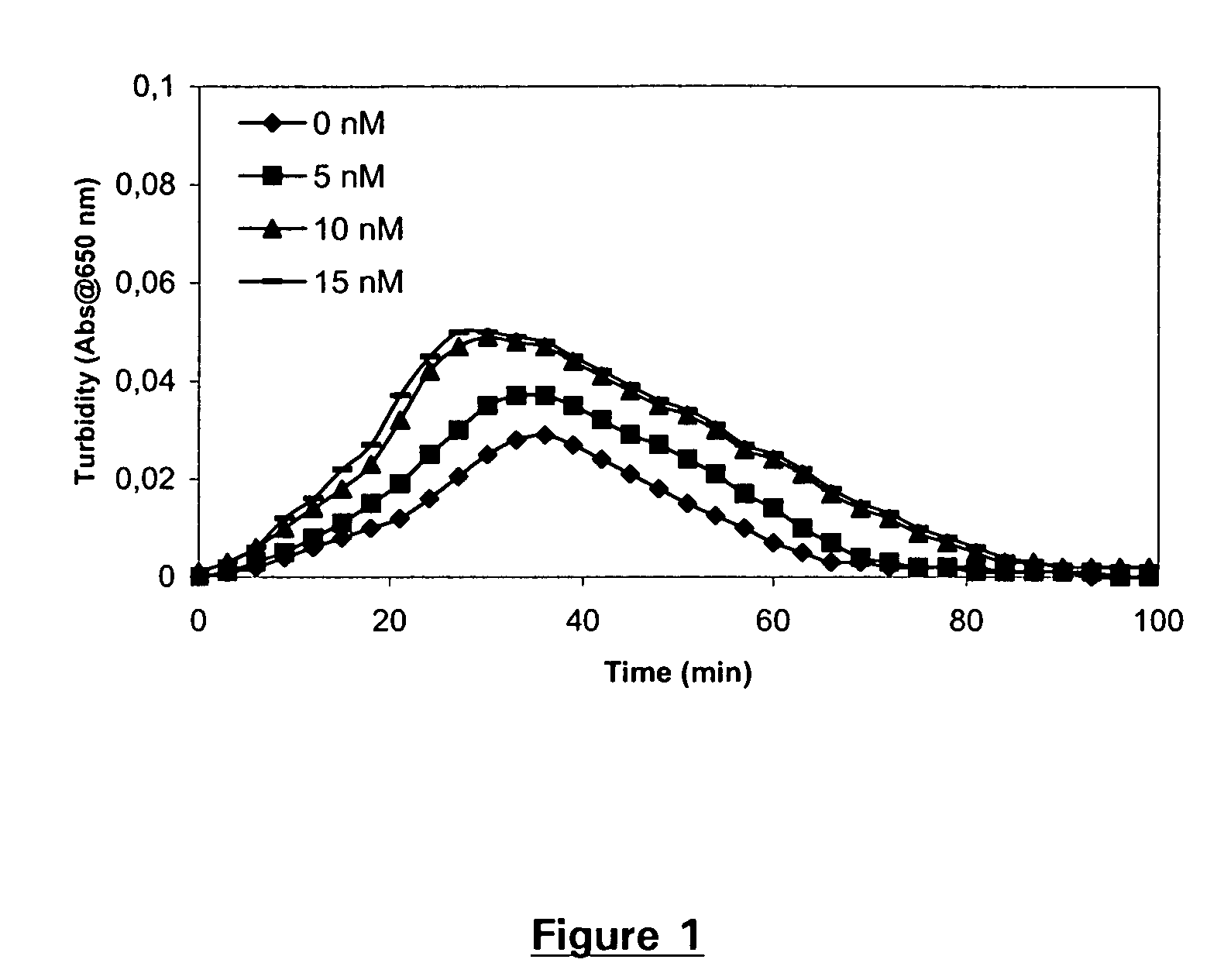
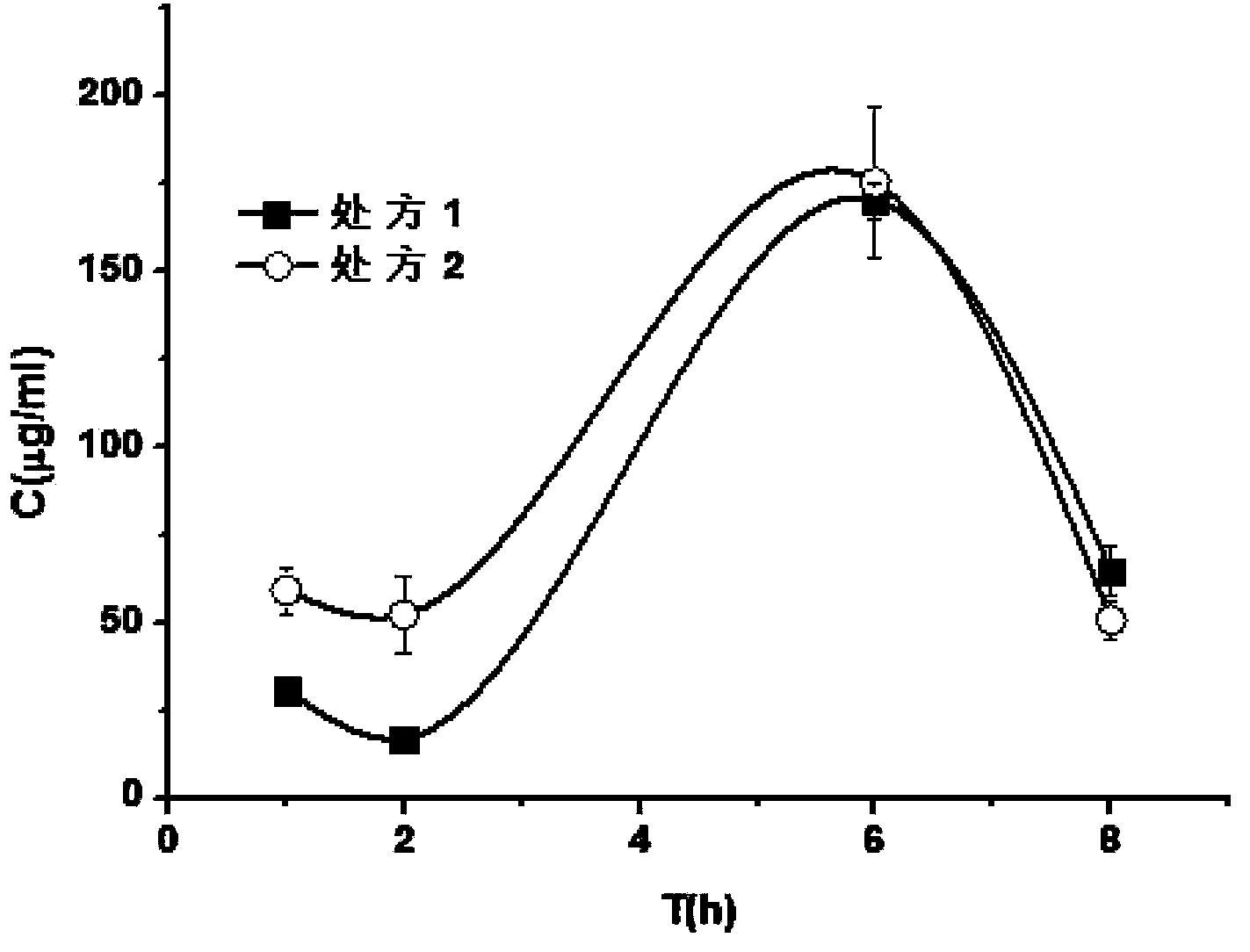
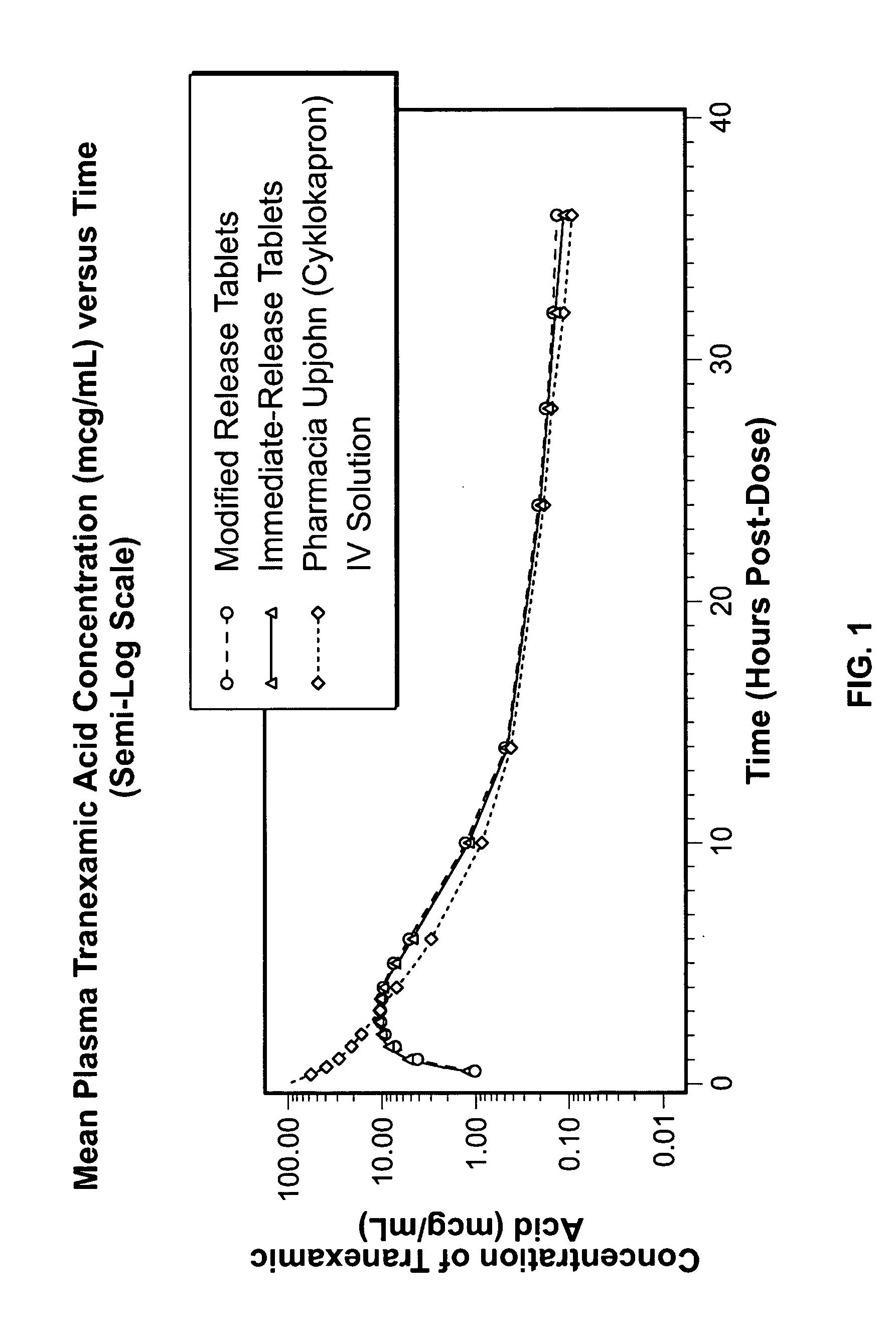
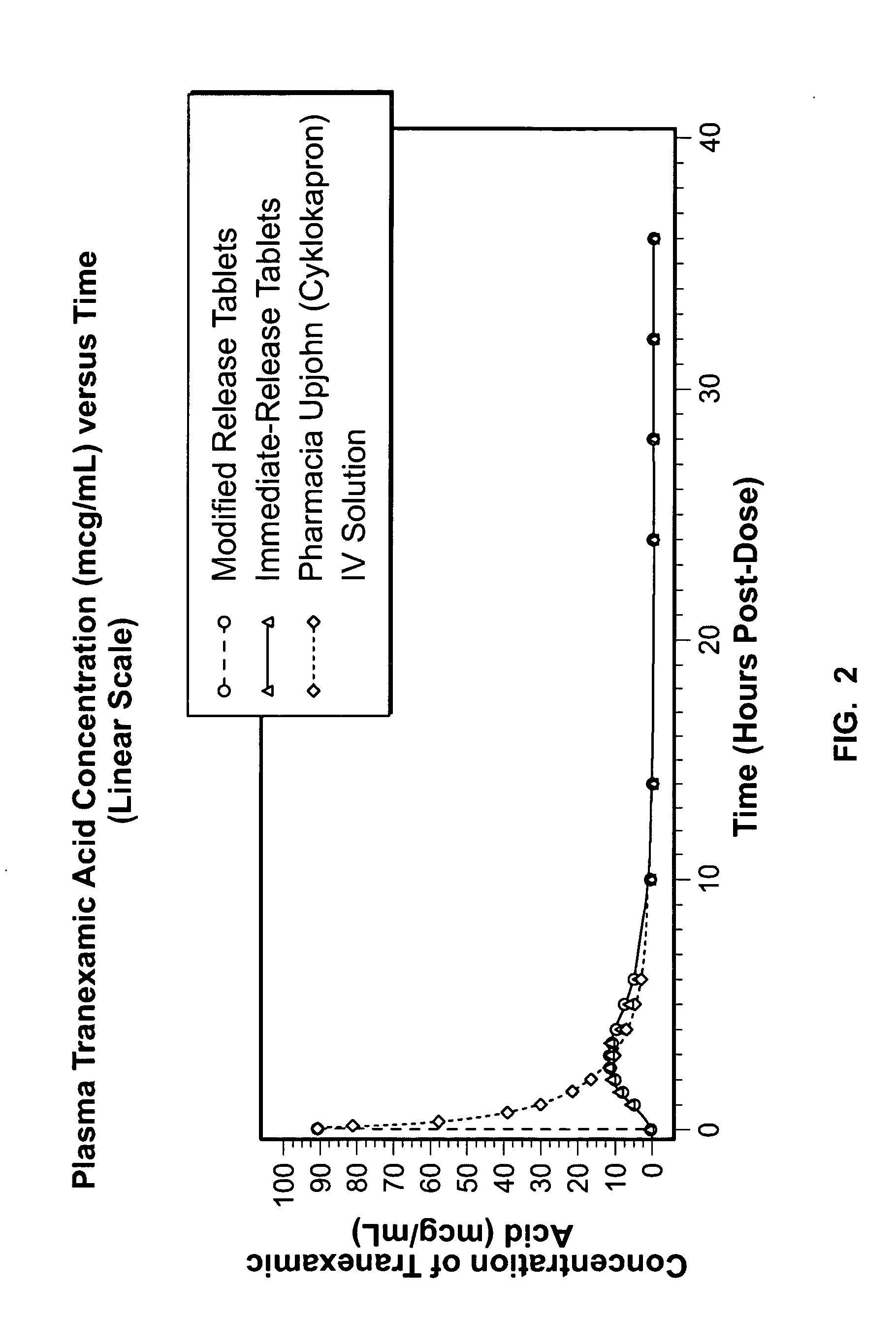
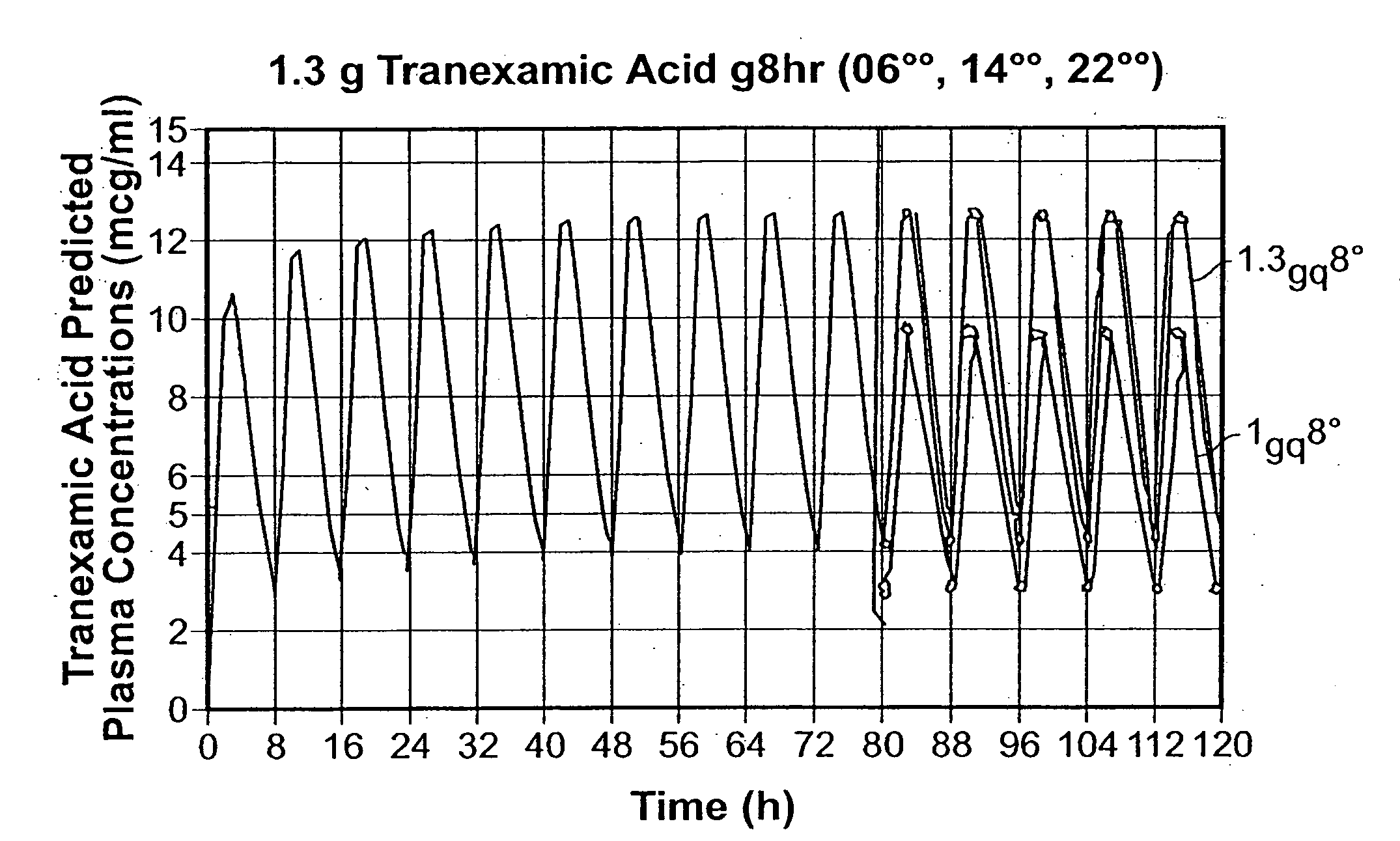
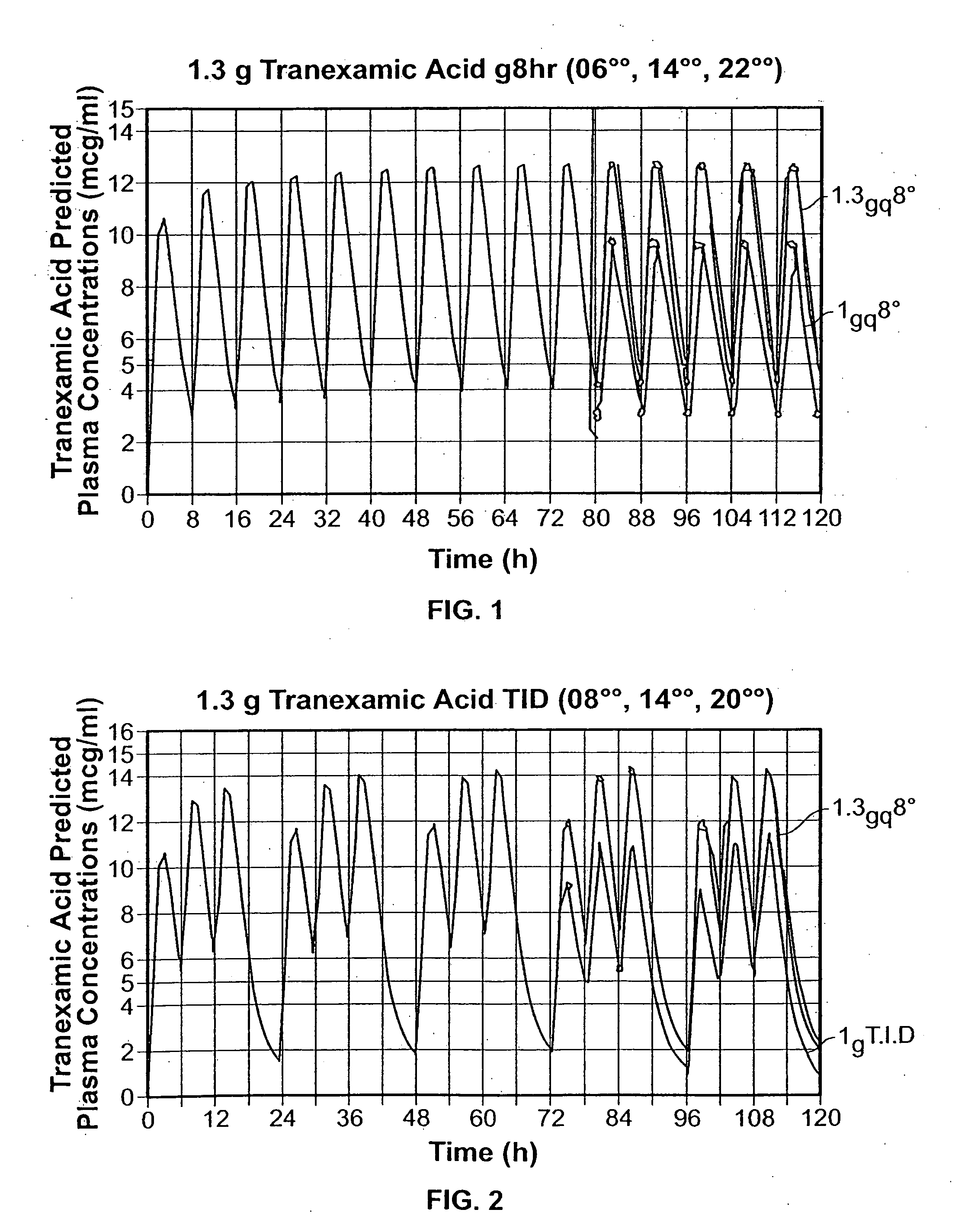
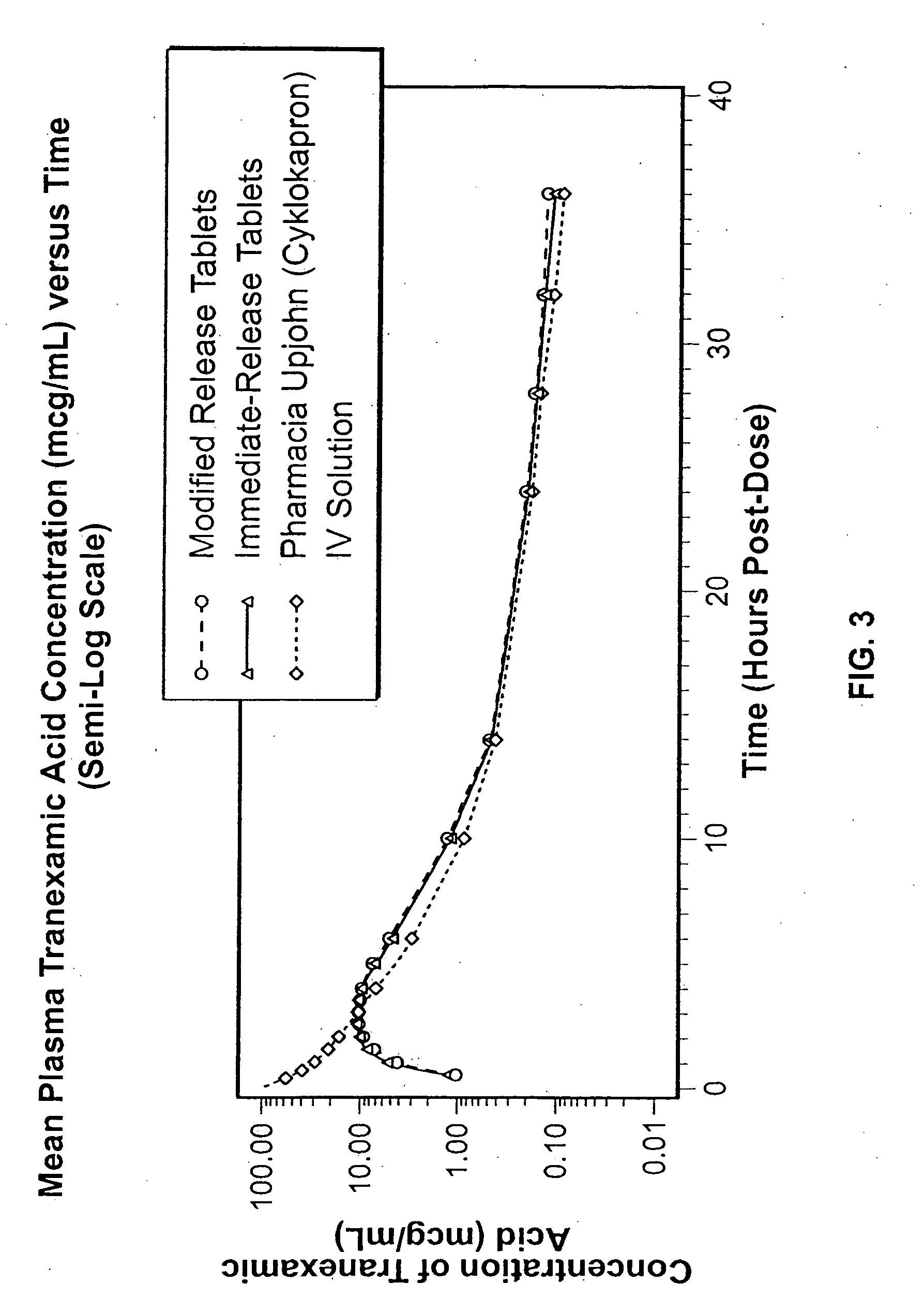
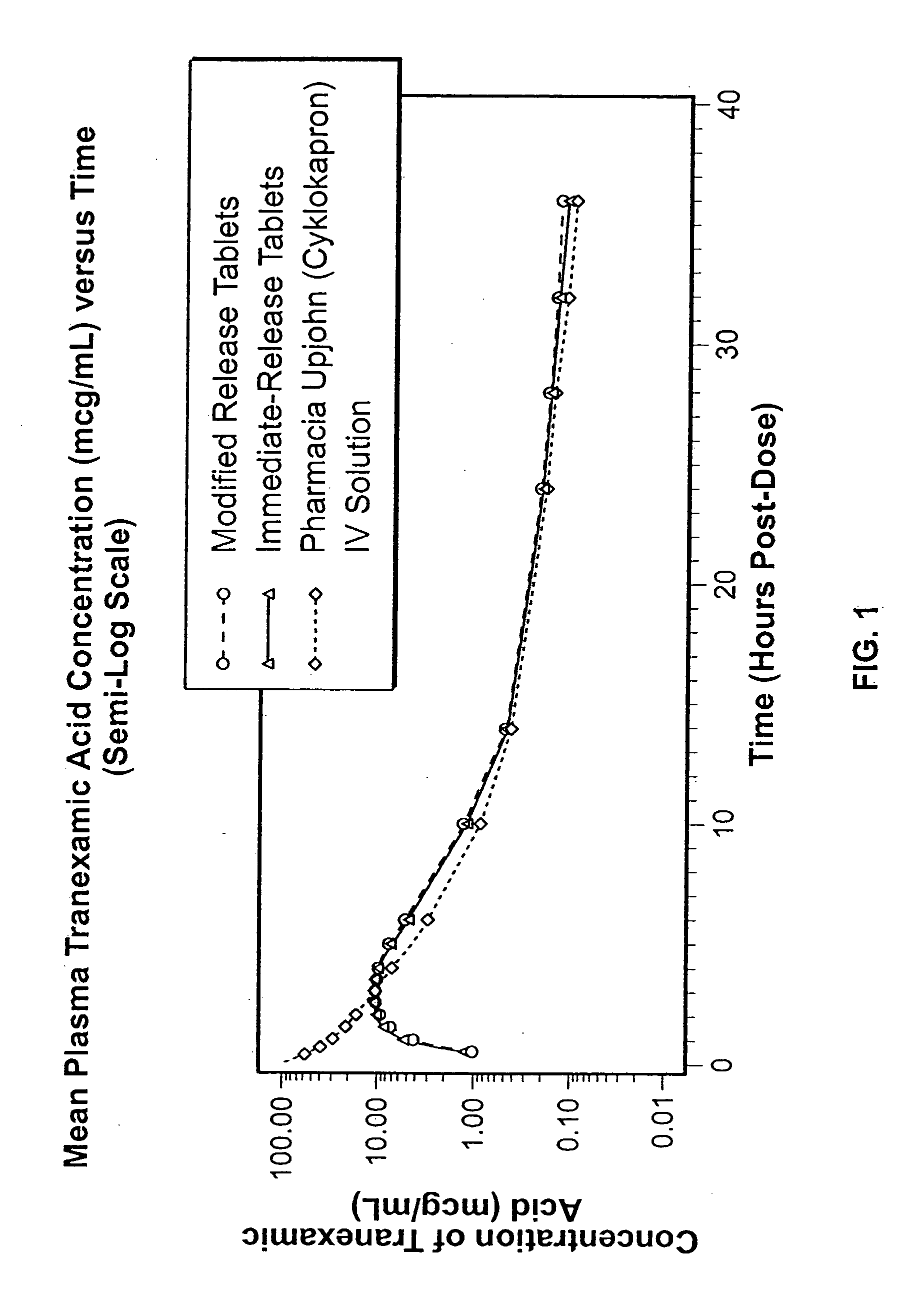
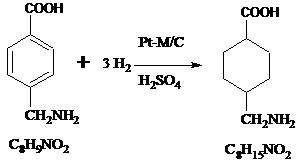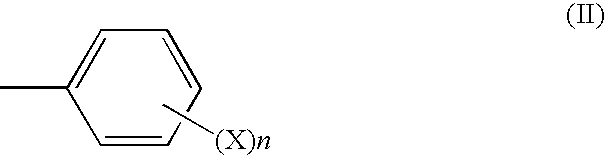Patents
Literature
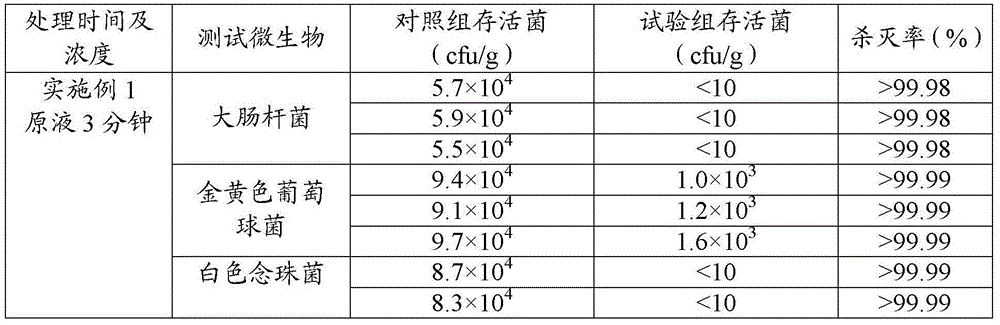
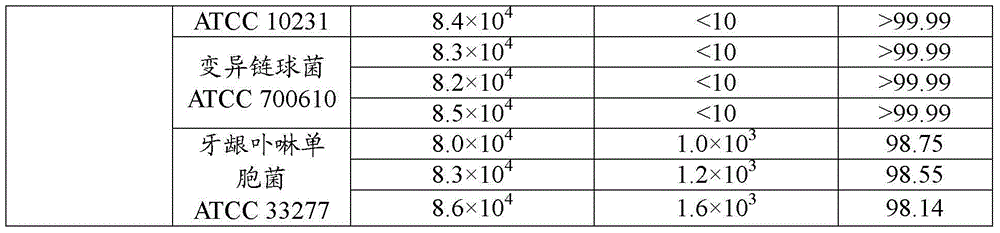
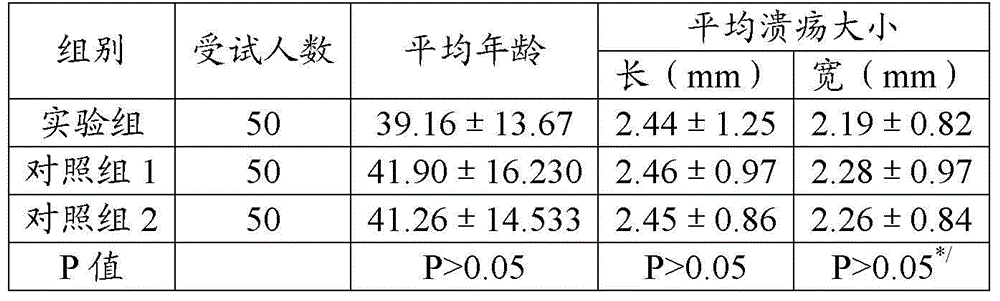
438 results about "Tranhexamic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
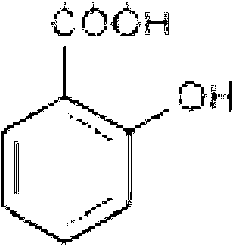
Tranexamic acid (TXA) is a medication used to treat or prevent excessive blood loss from major trauma, postpartum bleeding, surgery, tooth removal, nosebleeds, and heavy menstruation. It is also used for hereditary angioedema. It is taken either by mouth or injection into a vein.
Skin lightening composition
InactiveUS20060142382A1Good effectGood whitening effectBiocideCosmetic preparationsEngineeringTranexamic acid
A composition which comprises (i) tranexamic acid or a salt thereof, (ii) L-cysteine, a derivative thereof or a salt thereof and, as occasion demands, (iii) L-ascorbic acid, a derivative thereof or a salt thereof.
Owner:DAIICHI PHARMA CO LTD
Anti-wrinkle essence and preparing method thereof
ActiveCN105125476ATo promote metabolismAnti agingCosmetic preparationsToilet preparationsPolymer scienceGlycerol
The invention belongs to the technical field of cosmetics and particularly relates to anti-wrinkle essence and a preparing method thereof. The anti-wrinkle essence is prepared from cyclomethicone, butanediol, polydimethylsiloxane, poly(sodium L-glutamate), glycerinum, propylene glycol, polyglycerol-10, dipropylene glycol, erythritol, hydroxyethyl acrylate / acryloyl 2 methyl taurine sodium copolymer, bio-saccharide gum, xylitol, C20-22 alcohol phosphate / C20-22 alcohol, lactic acid bacillus / pomegranate fruit extracts of fermented products, arctic rock chlamydomonas essence, starfish essence, hexapeptide-3, orange flower essence, gentian root extracts, arbutin, milk protein, tranexamic acid and the like. The anti-wrinkle essence is good in permeability and easy to absorb, effective moisturizing is achieved, skin wrinkles are repaired and inhibited from being regenerated, and skin aging is delayed.
Owner:广州科玛生物科技股份有限公司
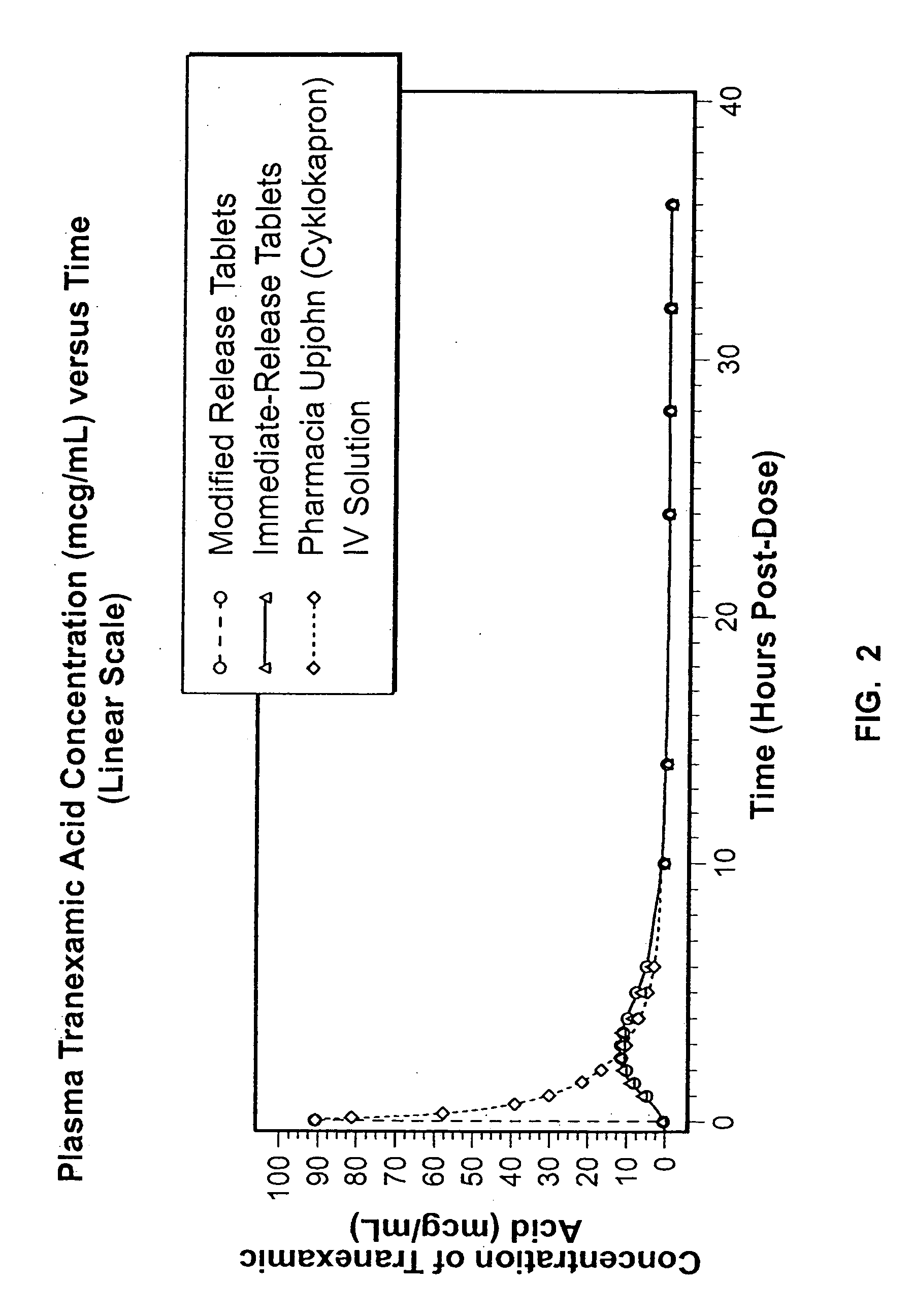
Tranexamic acid formulations with reduced adverse effects
InactiveUS20050025825A1Minimize and eliminate undesirable gastrointestinal side effectMinimize and prevent dissolutionBiocideOrganic active ingredientsIntestinal structureNausea sickness
Tranexamic acid formulated in an oral dosage form with at least one agent that decreases tranexamic acid release in the stomach. Such formulations minimize nausea, vomiting, and other adverse gastric effects that may accompany tranexamic acid therapy, for example, to treat heavy menstrual bleeding. One embodiment is an extended release formulation with waxes, polymers, etc. that prevent a bolus release of tranexamic acid in the stomach. An alternative embodiment is a delayed release formulation with polymers that prevent release of tranexamic acid in the acid environment of the stomach and delay its release until the formulation reaches the less acid environment of the intestines. Such formulations enhance patient compliance with therapy because adverse effects of tranexamic acid therapy are reduced.
Owner:XANODYNE PHARMACEUTICALS INC +1
Whitening mask and preparation method thereof
InactiveCN104971029APromote cell regenerationUV resistantCosmetic preparationsToilet preparationsGlycerolAloe Extract
The invention discloses a whitening mask which is prepared by dipping a liquid prepared from the following raw materials in parts by weight: 0.1-3 parts of micromolecular hyaluronic acid, 0.1-2.5 parts of macromolecular hyaluronic acid, 0.7-30 parts of synthetic egg white powder, 1-7 parts of an aloe extract, 0.7-9 parts of propylene glycol, 5-50 parts of 1,2-butanediol, 5-30 parts of glycerinum, 40-80 parts of a ginseng extract liquid, 0.1-1 part of glabridin, 0.3-2 parts of alpha-arbutin, 0.1-1 part of phloretin, 0.1-1 part of apple stem cells, 1-5 parts of raspberry ketone glucoside, 0.1-1 part of tetrahydropiperine, 1-10 parts of vitamin c ethyl ether, 1-5 parts of tranexamic acid, 1-10 parts of hydrolyzed pearl and added to 1000 parts of water on mask paper. The whitening mask has the efficacies of promoting skin absorption of various effective components, repairing damaged cells, activating resting cells, promoting cell regeneration, moisturizing the skin, rapidly and efficiently whitening the skin and removing freckles, and is free of irritation, allergy or damage to the skin.
Owner:GUILIN HONGXU BIOTECH CO LTD
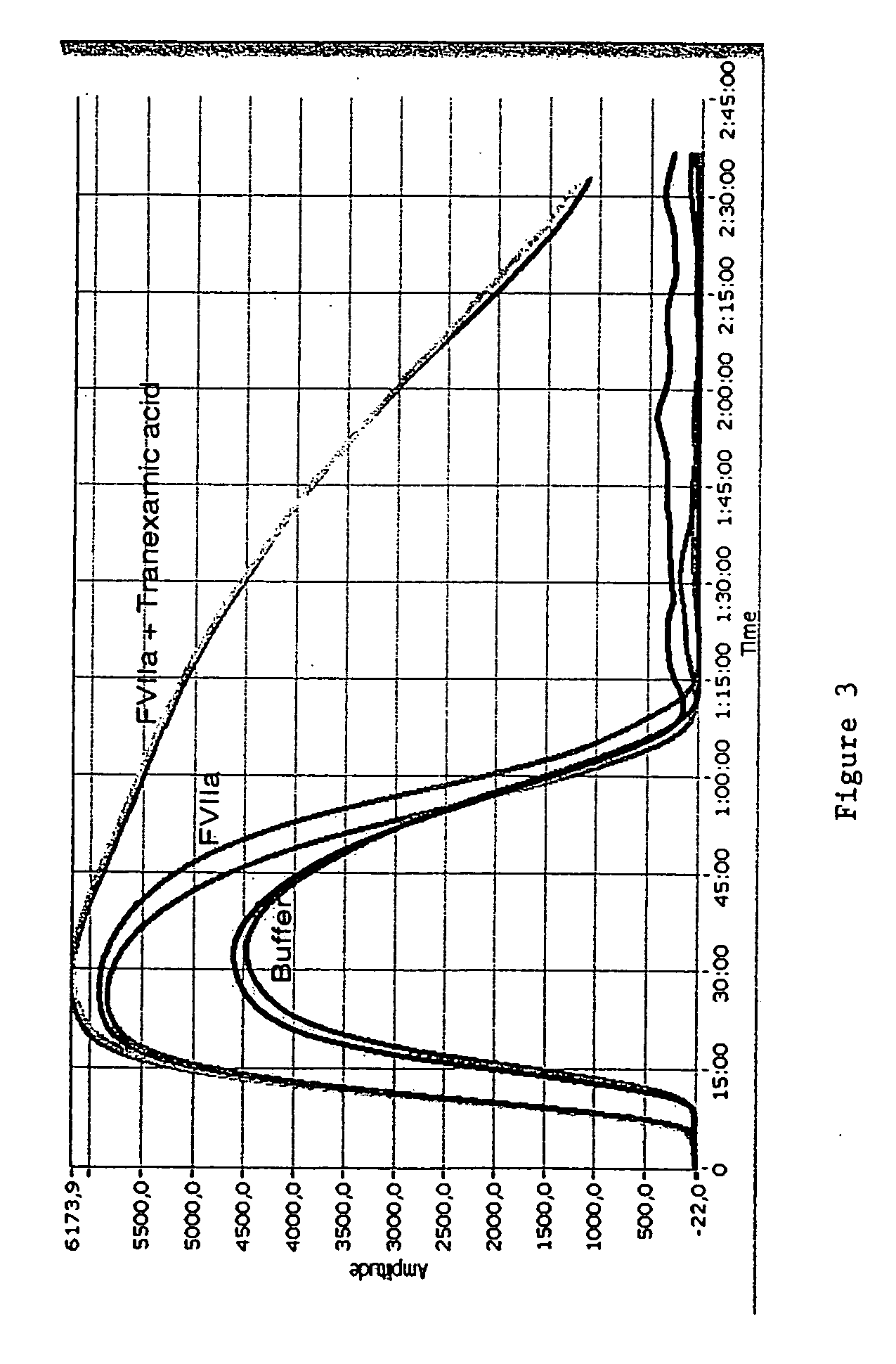
Pharmaceutical composition comprising factor VII polypeptides and tranexamic acid
The present invention relates to compositions comprising factor VII or a factor VII-related polypeptide and tranexamic acid, and the use thereof for treating bleeding episodes.
Owner:NOVO NORDISK AS
Tranexamic acid skin externally applied nano-preparation, as well as preparation method and use thereof
InactiveCN103565743AIncrease percutaneous permeabilityGood curative effectPeptide/protein ingredientsEmulsion deliveryUse medicationMedicine
The invention relates to a tranexamic acid skin externally applied nano-preparation, as well as a preparation method and a use thereof. The nano-preparation is prepared from tranexamic acid, medicated oil, an emulsifier, pharmaceutical additives, a thickener and pure water. The tranexamic acid skin externally applied nano-preparation provided by the invention has good physiological skin compatibility and can effectively promote the percutaneous absorption of tranexamic acid, improve the effect of preventing or treating pigmentation of the tranexamic acid and avoid toxicity and side effects, which are caused by systemic administration of the tranexamic acid.
Owner:CENT HOSPITAL XUHUI DISTRICT SHANGHAI CITY +1
Preparation method of polypeptide silk mask
InactiveCN105878048AEfficient hydrationEfficient AntioxidantCosmetic preparationsToilet preparationsCentella asiatica extractBetaine
The invention discloses a preparation method of a polypeptide silk facial mask, which comprises the steps of fully mixing polypeptide powder and silk protein nutrient solution and cooling. Polypeptide powder is mainly composed of aloe vera, carnosine, oligopeptide, soluble collagen, 2‑o‑ethyl ascorbic acid, betaine, trehalose, niacinamide, allantoin; the protein nutrient solution of silk is mainly composed of water, glycerin, Propylene glycol, butylene glycol, malto-oligosaccharide glucoside, hydrogenated starch hydrolyzate, glycerol polymethacrylate, PVM / MA copolymer, carbomer, sodium hyaluronate triethanolamine, baobab pulp extract, tranexamic acid , Centella asiatica extract, dipotassium glycyrrhizate and other skin conditioners or moisturizers. Through the combination of polypeptide powder and silk protein liquid, it can effectively give fresh nutrition to the skin, promote the skin repair process, strengthen the nutrient absorption capacity, deeply nourish, smooth and rejuvenate the skin, and make the skin elastic.
Owner:韩玉逍
Tranexamic acid formulations
Owner:XANODYNE PHARMACEUTICALS INC +1
External-use and oral-use composition comprising tranexamic acid
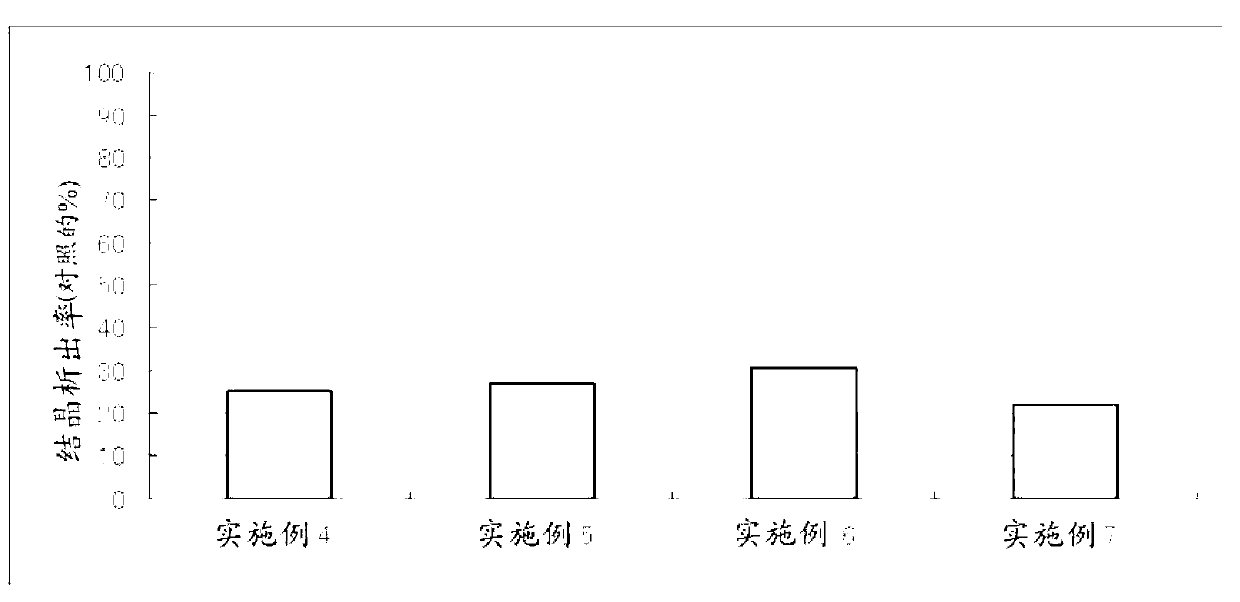
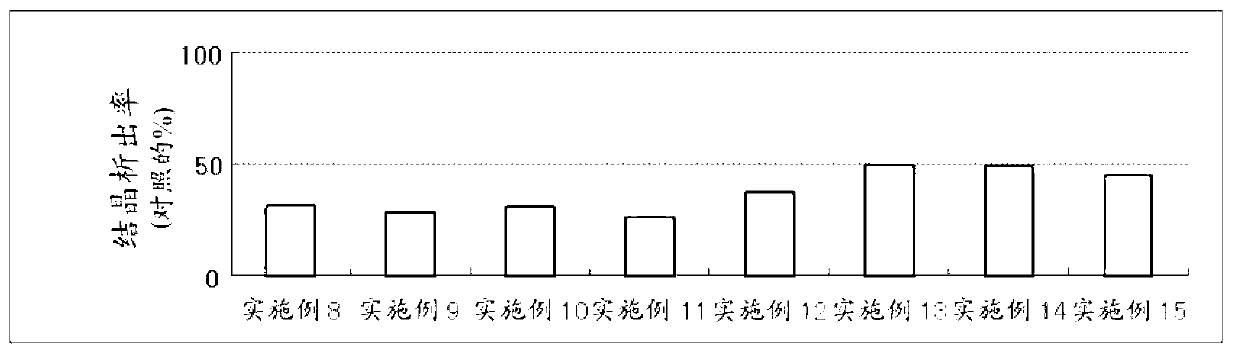
ActiveCN103099800APrevent crystallizationCosmetic preparationsPeptide/protein ingredientsAlcoholWater soluble
The invention discloses an external-use and oral-use composition. The composition comprises a tranexamic acid or a pharmaceutically-acceptable salt thereof, and a polyhydroxy acid or a pharmaceutically-acceptable salt, and also comprises polyhydric alcohol and / or a water-soluble polymer according to the condition.
Owner:ROHTO PHARM CO LTD
Skin whitening and tendering facial mask and preparation method thereof
ActiveCN103767971ARelieve sensitive stateImprove skin qualityCosmetic preparationsToilet preparationsVitamin CAllergic state
The invention provides a facial mask, which contains high active ingredients, can whiten skin and improve skin tone and can sooth allergic state of skin. The facial mask comprises the following components in percentage by weight: 1-5 percent of centella, 1-5 percent of tranexamic acid, 0.2-0.8 percent of vitamin B5, 1-5 percent of vitamin B3, 1-3 percent of vitamin C, 0.2-0.8 percent of allantoin, 4-8 percent of licoflavone, 1-3 percent of glucan, 0.2-0.8 percent of sodium hyaluronate, 10-20 percent of propylene glycol, 5-15 percent of 1,3-butanediol, 0.5-1.5 percent of collagen, 1-5 percent of amino acid, 2-8 percent of witch hazel, 1-5 percent of palmitoyl pentapeptide-3, 2-8 percent of hydroxyethylurea, 0.1-0.3 percent of hydroxyethyl cellulose, 0.2-0.8 percent of azone, and 31.1-40.5 percent of deionized water.
Owner:上海典雅生物科技有限公司
Full-function facial mask and preparation method thereof
ActiveCN103932898ANo burdenReduces and improves pigmentationCosmetic preparationsToilet preparationsVitamin CLiposome
The invention provides a full-function facial mask. The facial mask comprises the following components in percentage by mass: 0.01-2 percent of hyaluronic acid, 0.01-1 percent of reduced glutathione, 0.01-2 percent of L-vitamin C, 0.01-2 percent of tranexamic acid, 0.01-2 percent of liquiritigenin, 0.01-5 percent of vitamin E, 0.01-1 percent of EGF lyophilized powder, 0.01-2 percent of active peptide, 0.01-3 percent of SOD, 0.01-2 percent of arbutin, 0.1-2 percent of allantoin, 0.5-5 percent of glycerinum, 0.5-5 percent of propylene glycol and the balance of water, wherein the effective components are independently embedded with liposome or coated with nano-microcapsules before being mixed. The activated effective components of the facial mask are independently coated or embedded with transporters, and are delivered to the corium layer or the superficial corium layer by virtue of transportation of the transporters to achieve the effects of quadruple whitening, triple repairing and double moistening.
Owner:BEIJING BIOSIS HEALING BIOLOGICAL TECH
A method for preparing tranexamic acid by catalytic hydrogenation of p-aminomethylbenzoic acid
ActiveCN102276490AEasy to prepareReduce usageOrganic compound preparationAmino-carboxyl compound preparationHydrogen pressureReaction temperature
The invention relates to a method for preparing tranexamic acid from para-aminomethylbenzoic acid by catalytic hydrogenation. A Pt-M / C catalyst (M is selected from Ru, La, Ce, Co and Ni) is used for carrying out catalytic hydrogenation on the para-aminomethylbenzoic acid, and has the advantages of mild reaction conditions, reaction temperature of 30-60 DEG C, hydrogen pressure of 0.3-0.5MPa, high activity, reaction time of 2-4 hours, para-aminomethylbenzoic acid conversion rate up to 99 percent, tranexamic acid yield up to 95 percent, shortened reaction time, improvement of unit yield and high industrial application value.
Owner:CHANGZHOU YINSHENG PHARMA
Tranexamic acid formulations with reduced adverse effects
ActiveUS20060127476A1Minimize and eliminate undesirable gastrointestinal side effectMinimize and prevent dissolutionBiocidePeptide/protein ingredientsNausea sicknessPatient compliance
Tranexamic acid formulated in an oral dosage form with at least one agent that decreases tranexamic acid release in the stomach. Such formulations minimize nausea, vomiting, and other adverse gastric effects that may accompany tranexamic acid therapy, for example, to treat heavy menstrual bleeding. One embodiment is an extended release formulation with waxes, polymers, etc. that prevent a bolus release of tranexamic acid in the stomach. An alternative embodiment is a delayed release formulation with polymers that prevent release of tranexamic acid in the acid environment of the stomach and delay its release until the formulation reaches the less acid environment of the intestines. Such formulations enhance patient compliance with therapy because adverse effects of tranexamic acid therapy are reduced.
Owner:AMRING PHARM INC
Whitening cream and preparing method thereof
InactiveCN106309272AInhibitory activityCatalytic action to inhibit hydrolysisCosmetic preparationsToilet preparationsMethoxylaricinolic acidGlycyrrhiza glabra Root
The invention discloses a whitening cream and a preparing method thereof, which aims to provide a stable whitening cream with high safety, good skin whitening effect; the technical scheme is: the whitening cream is made from, by weight percentage, 0.5-0.3% of tranexamic acid, 0.5-3.5% of nicotinamide,4- 0.2-1.5% of potassium methoxysalicylate, 0.005-0.04% of glycyrrhiza glabra root extract, 0.5-2% of leucojum extract , 0.1-1.5% of saxifraga stolonifera extract, 3-6% of emulsifier, 2-5% of co-emulsifier, 10.1-23.2% of grease, 0.05-0.1% of antioxidant, 0.05-0.1% of disodium EDTA, 0.2-0.5% of allantoin, 4-10% of polyhydric alcohols, 2.25-8.55% of humectant, 0.2-0.5% of aqueous thickener, 0.5-1% of thickened stabilizer, 0.5-3% of anti-allergic conditioner, 0.8-1% of preservative, 0.5-1.2% of pigment and the margin is water; the invention belongs to the field of cosmetics technology.
Owner:GUANGDONG BAWEI BIOLOGICAL TECH CO LTD
Blood cell separation membrane and blood retention tool including the same
InactiveUS20060188392A1Avoid hemolysisImprove accuracyAnalysis using chemical indicatorsMaterial analysis by observing effect on chemical indicatorTranhexamic acidCells isolation
The present invention provides a blood cell separation membrane for separating blood into serum or plasma and blood cells while preventing hemolysis of the blood cells. Such a blood cell separation membrane can be obtained by impregnating a porous membrane for separating blood cells from blood supplied thereto with a solution containing a hydrophobic aminocarboxylic acid, a protein derived from silk, Tris, TES, ε-aminohexanoic acid, tranexamic acid, or heparin and then drying the membrane.
Owner:ARKRAY INC
Methods to enhance a non-surgical medical treatment
ActiveUS20200046663A1Convenient medical treatmentMinimizing bruisingCosmetic preparationsOrganic active ingredientsNon surgical treatmentAntifibrinolytic agent
In an embodiment, a method to enhance a non-surgical medical treatment, the method including applying a composition having an antifibrinolytic agent to an area of skin for non-surgical medical treatment, where the applying is at least one of before, during, and after the non-surgical medical treatment, beginning the non-surgical medical treatment to the area of skin, and continuing the non-surgical medical treatment until the non-surgical medical treatment is completed. In some embodiments, the antifibrinolytic agent is tranexamic acid in an amount of about 20% (w / v). In some embodiments, the method further includes minimizing, by the antifibrinolytic agent, bruising caused by the non-surgical medical treatment. In an additional embodiment, the present disclosure relates to a composition to enhance a non-surgical medical treatment, the composition having an antifibrinolytic agent. In a further embodiment, the present disclosure relates to a kit having a carrier and an antifibrinolytic agent.
Owner:ANTI PLASMIN TECH LLC
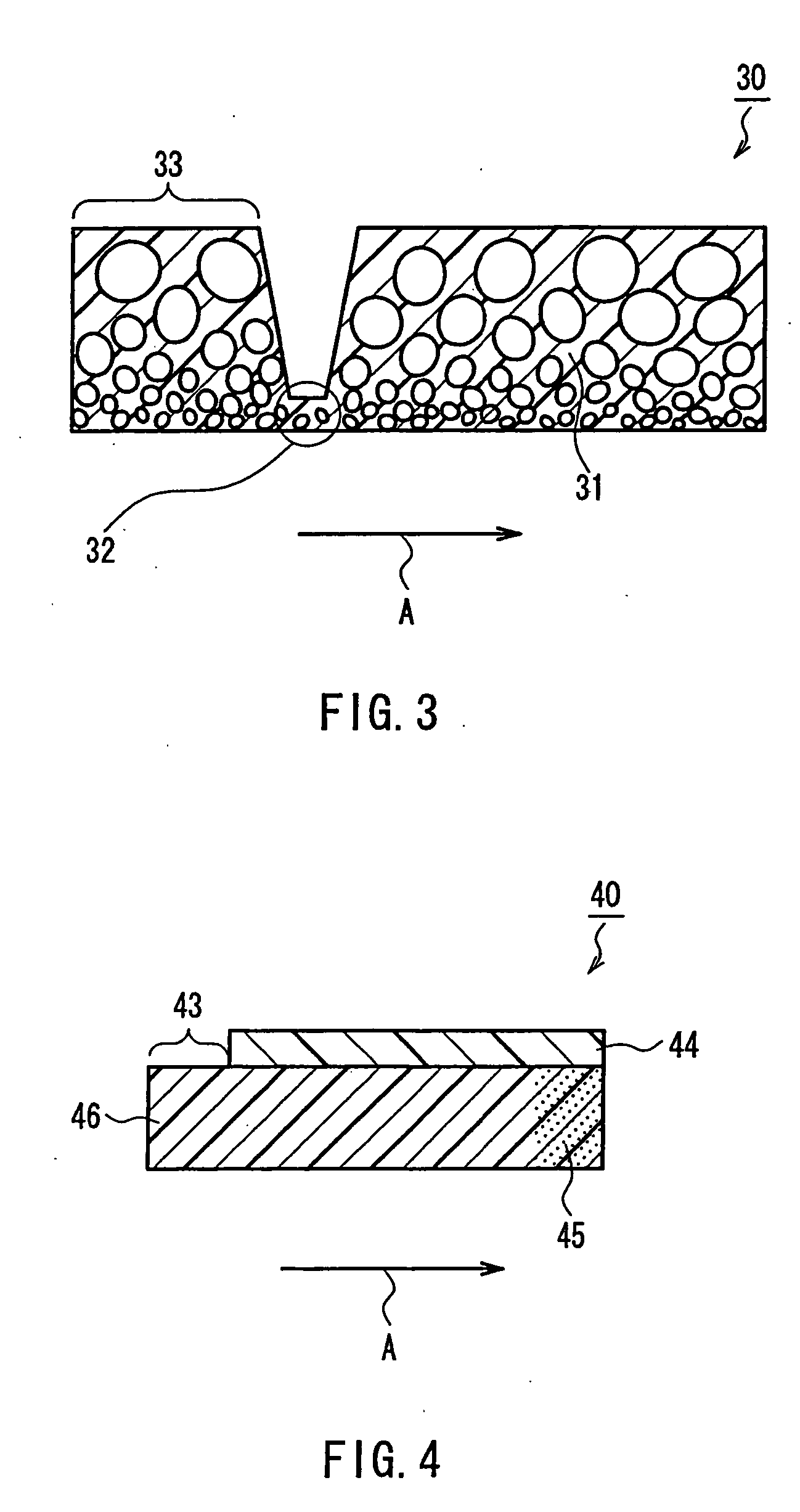
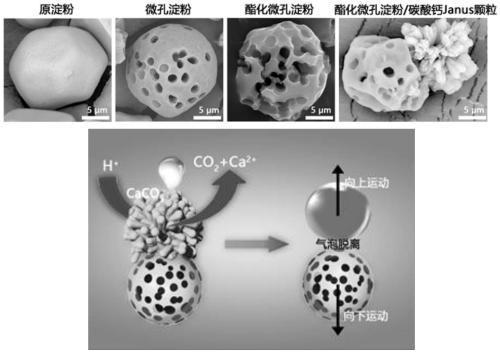
Preparation method for janus structure based rapid hemostatic agent having directional propulsion function
ActiveCN111135339AEffective hemostasisGood biocompatibilitySurgical adhesivesPharmaceutical delivery mechanismJanus particlesDeep wounds
For defects that existing hemostasis research and products cannot intelligently perform self-propelling or cannot be driven to forward deep in the wound under the action of external field force, the invention provides a preparation method for a janus structure based rapid hemostatic agent having a directional propulsion function. The preparation method includes performing esterification on microporous starch to unidirectionally grow calcium carbonate particles so as to obtain esterified microporous starch / calcium carbonate Janus particles; fixedly assembling thrombin on the surfaces of the esterified microporous starch / calcium carbonate Janus particles to obtain esterified microporous starch / calcium carbonate Janus particles assembled with the thrombin; mixing the esterified microporous starch / calcium carbonate Janus particles assembled with the thrombin and protonated tranexamic acid powder so as to obtain the janus structure based rapid hemostatic agent having a directional propulsion function. Through the formation of the biphasic heterogeneous Janus structure, the unidirectional and intelligent self-propulsion of hemostatic starch can be realized by cooperating with the protonated tranexamic acid, so that rapid three-dimensional hemostasis on deep wounds, penetrating wounds and irregular wounds such as aortic / venous rupture can be realized.
Owner:SOUTHWEST UNIVERSITY
Composition for external use preparation with improved transdermal permeability
InactiveUS20160058723A1High skin permeationNo skin irritationOrganic active ingredientsCosmetic preparationsSkin permeabilityIrritation
Provided is a skin external composition, which includes tranexamic acid or a salt thereof and a skin penetration enhancer, thereby showing remarkably increased skin permeability and improved sense of use, skin irritation, and storage stability.
Owner:HYUNDAI PHARMA
Composition for Prevention or Alleviation of Pigmentation
InactiveUS20080260878A1Effectively prevent and improvePreventing and improving pigmentation of skinBiocideCosmetic preparationsArbutinAdenosine
A composition for the prevention or alleviation of pigmentation which can produce the higher effect of preventing or alleviating pigmentation. The composition for the prevention or alleviation of pigmentation comprises a combination of (A) at least one member selected from the group consisting of adenosine 5′-monophosphate and salts thereof with (B) at least one member selected from the group consisting of arbutin, ellagic acid, 4-alkylresorcinols, linoleic acid, tranexamic acid, salts of these, Chamomilla recuita extract, and Ubiquinone.
Owner:OTSUKA PHARM CO LTD
Moisturizing, whitening, acne removing, grease controlling and relieving skin beautifying composition and preparation method thereof
InactiveCN105434211ABalance secretionReduce generationCosmetic preparationsToilet preparationsPhenolic content in teaTremella
The invention discloses a moisturizing, whitening, acne removing, grease controlling and relieving skin beautifying composition. The composition is mainly prepared from the following raw materials: raspberry ketone glucoside, arbutin, matrine, fructo-oligosaccharide, tea tree oil, dipotassium glycyrrhizinate, an extracting solution of leaf of Tasmanian bluegum, tea polyphenol, a peony root extract, tremella polysaccharide, hyaluronic acid with low molecular weight, hyaluronic acid with high molecular weight, transparent xanthan gum, zinc gluconate, Carbomer 940, hydroxyethyl cellulose, 1,3-butanediol, nicotinamide, D-panthenol, tranexamic acid, vitamin C ethyl ether, triethanolamine, an emulsifier, water, and the like. The moisturizing, whitening, acne removing, grease controlling and relieving skin beautifying composition has the beneficial effects that the composition is rich in plant essence, is reasonable in proportioning and can achieve the effects of effectively comforting and smoothening pores, cleaning hair follicles, balancing grease excretion, promoting wound healing and ensuring no pockmarks after healing and low recurrence probability; besides, the composition is also rich in moisturizing ingredients, so that the skins can be hydrated and bright, fine lines can be reduced and smooth and fair skins can be restored after the composition is used for a long term.
Owner:马南行 +1
Tranexamic acid formulations
ActiveUS20090048341A1Minimize and eliminate undesirable gastrointestinal side effectReduce concentrationBiocidePeptide/protein ingredientsTranexamic acidPharmacology
Disclosed are modified release oral tranexamic acid formulations and methods of treatment therewith.
Owner:AMRING PHARM INC
Drug-delivery absorbable hemostatic sponge and method for preparing same
InactiveCN101590288ARapid hemostasisImprove sexual functionAbsorbent padsBandagesSocial benefitsMedicine
The invention discloses a drug-delivery absorbable hemostatic sponge, which belongs to the technical field of novel hemostatic materials and comprises drug-loaded chitosan microsphere and collagen protein, wherein the drug-loaded chitosan microsphere comprises medicinal grade chitosan and medicinal grade tranexamic acid. The weight ratios of the medicinal grade chitosan to the medicinal grade tranexamic acid are (0.01-100):1 respectively. The invention also discloses a method for preparing the drug-delivery absorbable hemostatic sponge. By selecting collagen with good hemostatic function and biocompatibility as the main hemostatic substrate, compounding the drug-delivery chitosan microsphere and the medicinal grade tranexamic acid, and making use of the special properties of the chitosan including hemostatic function and drug-delivery function, a novel hemostatic sponge with rapid hemostasis and lasting and steady drug delivery in a period of time can be prepared, and has considerable economic and social benefits.
Owner:深圳市朗天文化创意有限公司 +1
Tranexamic acid formulations
InactiveUS20080280981A1Few adverse effectBiocidePeptide/protein ingredientsTranexamic acidPharmacology
Owner:AMRING PHARM INC
Anti-allergic facial mask fluid for promoting skin regeneration
InactiveCN104644510AActivate repair abilityImprove erythemaCosmetic preparationsToilet preparationsIrritationLophatherum
The invention discloses anti-allergic facial mask fluid for promoting skin regeneration. According to the anti-allergic facial mask fluid, a variety of pure natural plant extracting solutions are in combined use to achieve a certain synergistic effect, immunoregulation and inflammation prevention can be really achieved endogenously, the self-repairing ability of the skin can also be activated, and after the anti-allergic facial mask fluid is used, the skin is comfortable, no irritation can be caused, and erythema can be improved. Relative to the total mass of the facial mask fluid, the anti-allergic facial mask fluid comprises the following components in percentage by mass: 0.1-0.2% of hyaluronic acid, 0.5-1% of tranexamic acid, 0.05-0.1% of methyl hesperidin, 0.01-0.03% of carboxymethyl dextran, 2-3% of a wheat protein hydrolysate, 0.5-0.8% of a common lophatherum herb extracting solution, 1-1.5% of a baikal skullcap root extracting solution, 2.3-3% of a danshen root extracting solution, 0.3-0.5% of a fresh ginger extracting solution, 0.5% of an aloe extracting solution, 2-2.5% of a wild chrysanthemum flower extracting solution, 0.2-0.8% of a largehead atractylodes rhizome extracting solution and 0.2-0.3% of 1,2-hexanediol.
Owner:北京铂润天和生物科技有限公司
Fair-skinned, bright and white facial mask and preparation method thereof
ActiveCN107213063AFully nourishedBrighten upCosmetic preparationsToilet preparationsBetainePurslane extract
The invention belongs to the technical field of cosmetics and in particular relates to a fair-skinned, bright and white facial mask and a preparation method thereof. The fair-skinned, bright and white facial mask contains propylene glycol, hydroxyethyl urea, betaine, 3-O-ethyl ascorbic acid, arbutin, purslane extract, thyme extract, peony skin whitening essence, panthenol, nicotinamide, jojoba wax PEG-120 esters, tranexamic acid, carbomer, triethanolamine, sodium hyaluronate, PEG-40 hydrogenated castor oil, methyl hydroxybenzoate, phenoxyethanol / ethylhexyl glycerol, p-hydroxyacetophenone, essence and water. The fair-skinned, bright and white facial mask provided by the invention is natural and safe, contains multiple active ingredients, is good in permeability, can go deep into muscle bottom and supplement massive moisture and nutrients and can moisturize, nourish and repair, whiten skin, remove stains and alleviate a dark yellow state of skin, and fair-skinned and transparent skin is restored.
Owner:广州科玛生物科技股份有限公司
Whitening essence and preparation method thereof
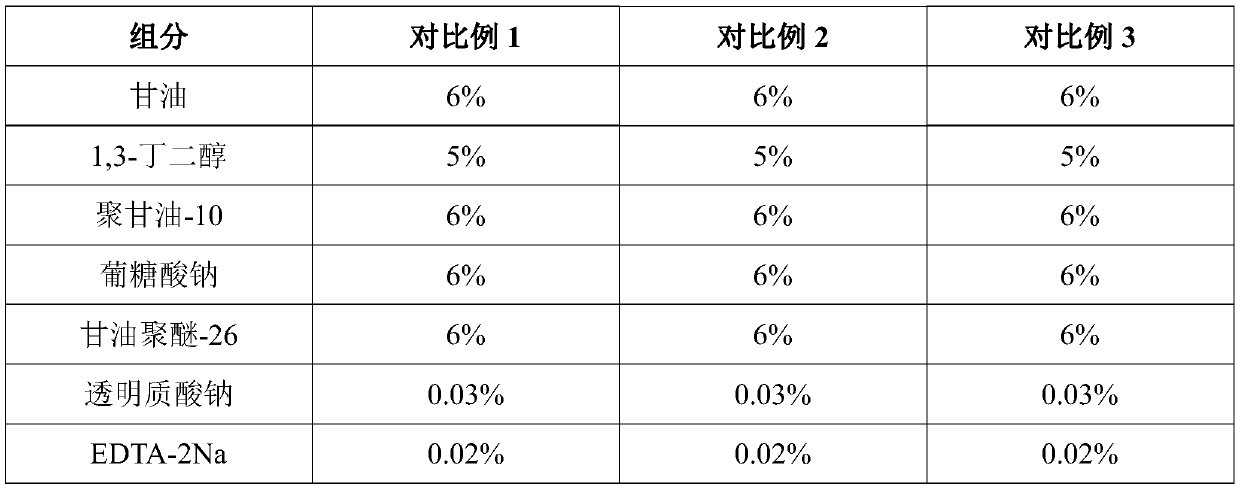
PendingCN111067818AInhibit transferPhotoprotectiveCosmetic preparationsToilet preparationsAcropustulosisButanediol
The invention provides whitening essence and a preparation method thereof. The whitening essence consists of the following components of glycerine, 1,3-butanediol, sodium hyaluronate, EDTA-2Na, polyglycerol-10, sodium polyacrylate, xanthan gum, hydroxyacetophenone, dipotassium glycyrrhizinate, hydrolyzed sclerotium gum, gluconic acid sodium salt, glycereth-26, caprylhydroxamic acid, glycerol caprylate, 3-o-ethyl ascorbic acid, tranexamic acid, ceramide, phenethyl resorcinol, diglucosyl gallic acid and water. The whitening essence disclosed by the invention can refrain conversion of tyrosine tomelanin before the tyrosine is in preparation of conversion to the melanin, so that the activity of the tyrosinase is also high, generation of more melanin cannot be caused, and transfer of skin melanin can also be restrained. Besides, the whitening essence has powerful antioxidant ability, has light protection effects on skin, can control dermatitis, and is a whitening product most comprehensivein action mechanism at present, so that whitening effects can be achieved.
Owner:广州市柏姿生物科技有限公司
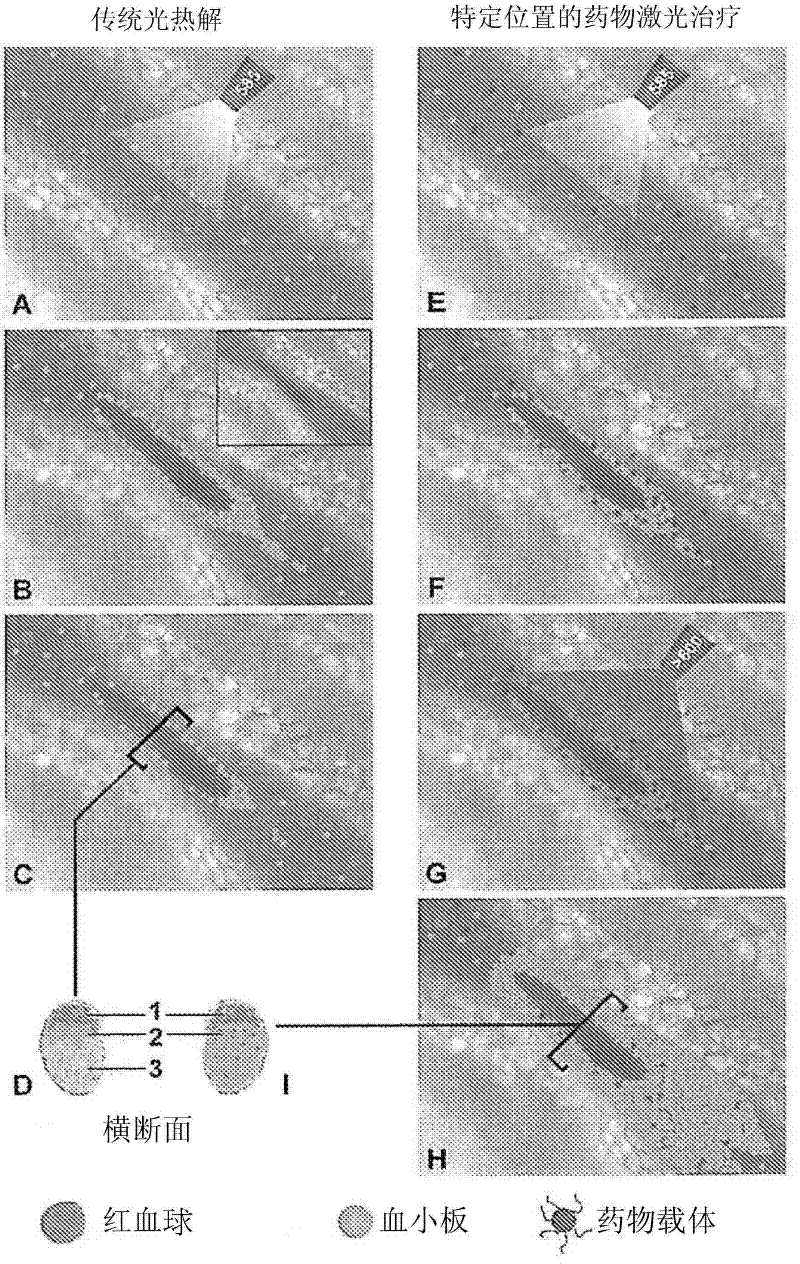
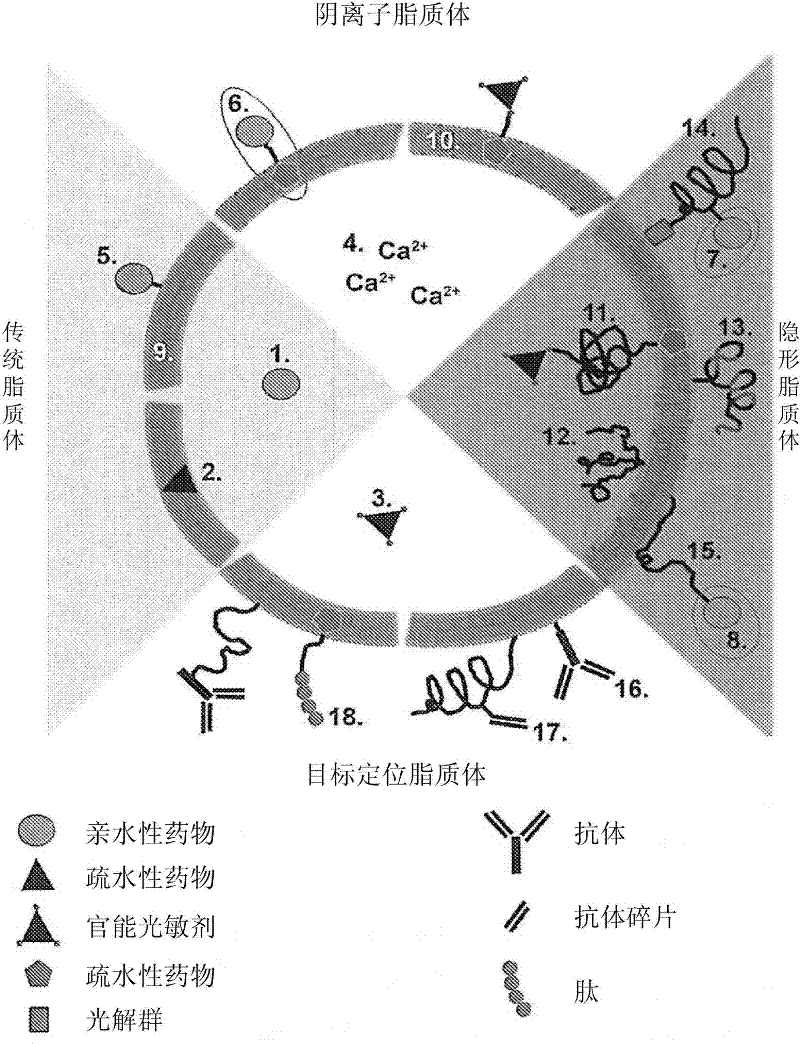
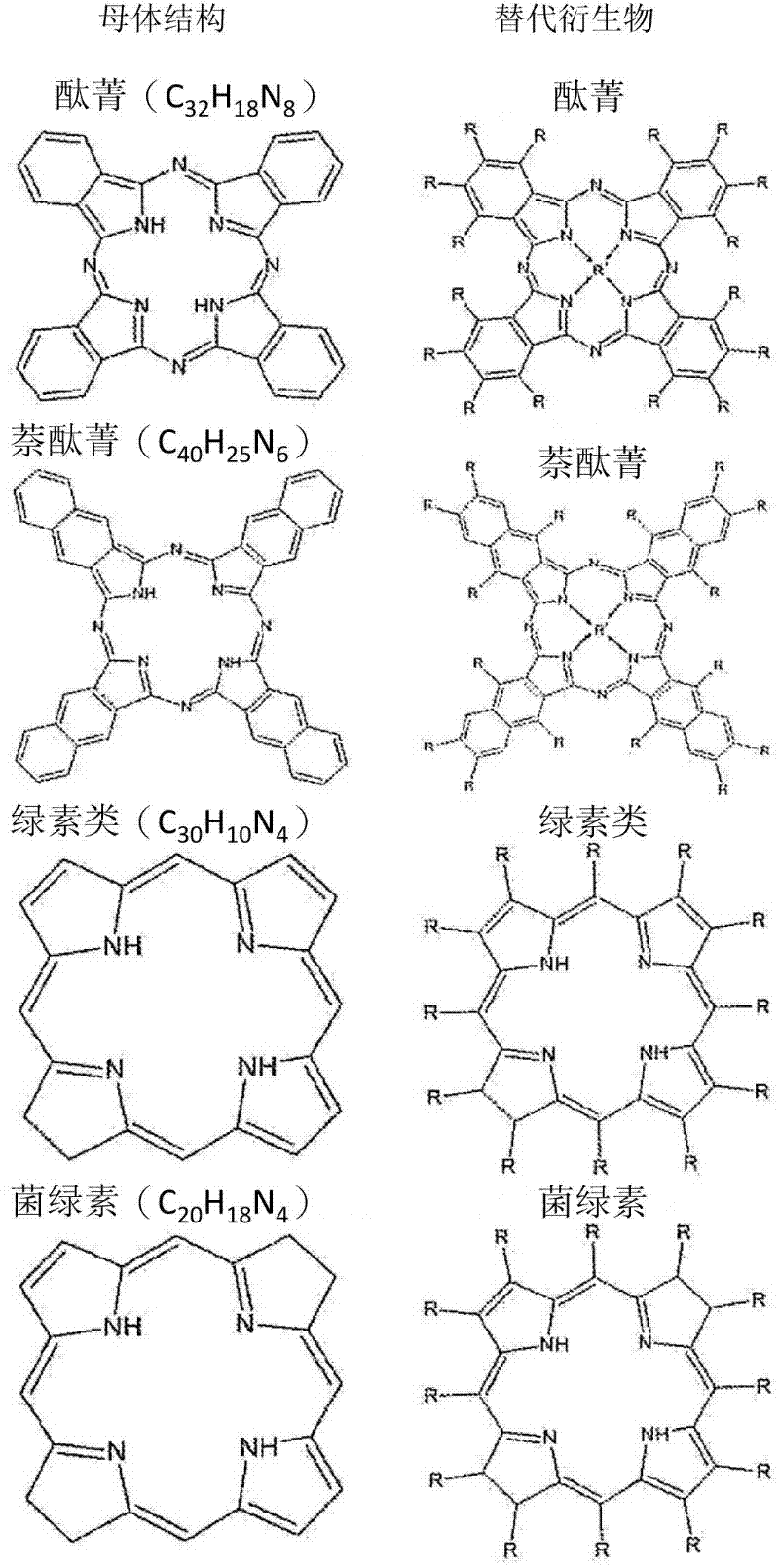
Drug delivery system for the treatment of vascular diseases
InactiveCN102300557AOrganic active ingredientsPharmaceutical non-active ingredientsTranhexamic acidDrug delivery
The present invention relates to a drug delivery system for use in the treatment of vascular and vessel-related pathologies, comprising a drug delivery platform that comprises at least one compound capable of exerting an effect on the formation and / or maintenance of a thrombus in the vessel to be treated. The platform is preferably formed by liposomes that are sterically stabilized by grafting of poly(ethylene glycol) onto the liposome surface. The liposomes may further comprise photosensitizers and targeting molecules. The liposomes may be thermosensitive. The compound is suitably tranexamic acid. The drug delivery system is preferably used for the treatment of port wine stains.
Owner:ACADEMISCH MEDISCH CENT BIJ DE UNIV VAN
Medicinal toothpaste composition and preparation method and application thereof
InactiveCN104644460AReduce inflammationShorten the course of the diseaseCosmetic preparationsAntipyreticOral ulcersToothpaste
The invention discloses a medicinal toothpaste composition which comprises the following raw materials in percentage by weight: 0.01%-0.05% of sodium guaiazulene sulfonate, 0.1%-0.5% of paeonol, 0.1-0.5w / w% of allantoin, 0.1-0.5w / w% of tranexamic acid, and toothpaste matrix on the basis of the total weight. In addition, the invention further discloses a preparation method of the medicinal toothpaste composition and applications in resisting bacteria, diminishing inflammation, easing pain, shortening the treatment course of dental ulcer, and promoting oral wound healing.
Owner:北京华素制药股份有限公司
Inhibitor for melanin, and cosmetic composition containing same
InactiveCN102821742APrevent or improve hyperpigmentationReduce stimulationCosmetic preparationsToilet preparationsNiacinamideBULK ACTIVE INGREDIENT
The present invention relates to an inhibitor for melanin which contains tranexamic acid and niacinamide as active ingredients for inhibiting the formation of melanin cells in the skin, and a cosmetic composition containing the inhibitor for melanin as an active ingredient for relieving liver spots, blemishes, freckles and inflammatory hyperpigmentation, improving skin tone and texture, and skin whitening.
Owner:AMOREPACIFIC CORP
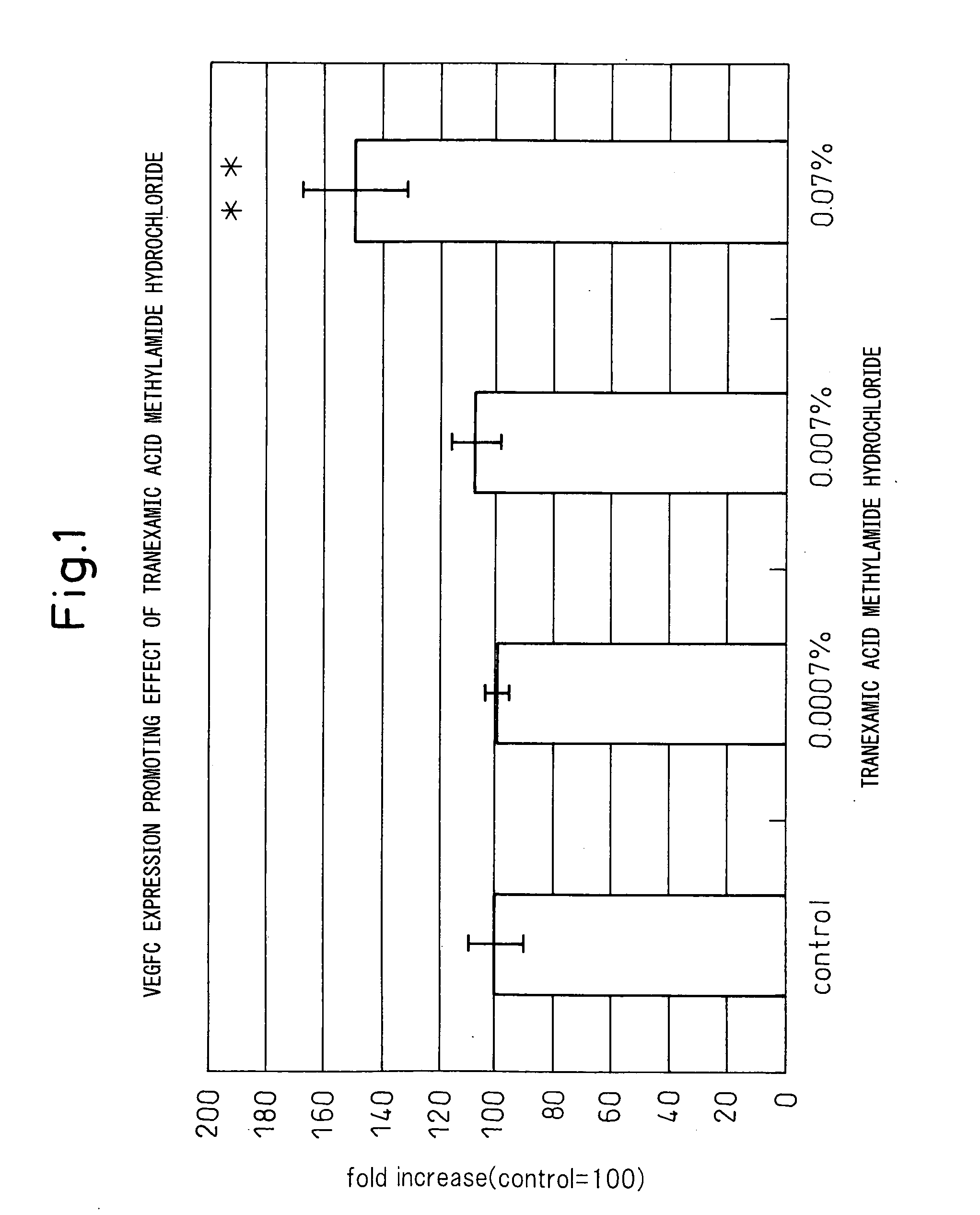
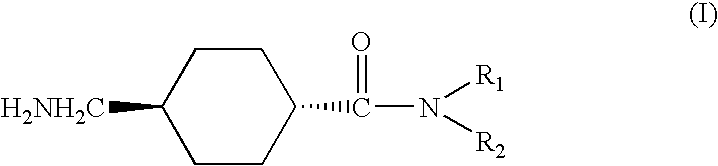
Vegfc production promoter
InactiveUS20100292509A1Promote activationPreventing or inhibiting bloating, lymphedema, wrinkle formationCosmetic preparationsOrganic active ingredientsStereochemistryTranexamic acid
A bloating ameliorant, a lymphatic vessel activator and a VEGFC production promoter comprising a tranexamic acid amide derivative and / or salt thereof.
Owner:SHISEIDO CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com