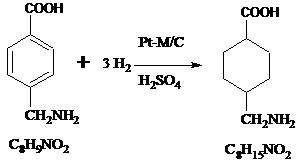A method for preparing tranexamic acid by catalytic hydrogenation of p-aminomethylbenzoic acid
A technology of aminomethylbenzoic acid and tranexamic acid, which is applied to the preparation of cyanide reaction, chemical instruments and methods, and the preparation of organic compounds, can solve the problems of harsh reaction conditions, increased production costs, and unsuitability for industrial production, and achieves The preparation method is simple, the utilization rate is improved, and the effect of reducing the amount of use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Take the preparation of 1 gram of catalyst as an example: 0.135g H 2 PtCl 6 and 0.05g La(NO 3 ) 3 Dissolve in 30ml of water, impregnate 1g of activated carbon in this solution for 12 hours, heat to 100°C, add hydrazine hydrate dropwise for reduction, cool after reduction, filter, and wash with water to obtain Pt-La / C catalyst.
[0025] Catalytic hydrogenation: 0.3 g Pt-La / C catalyst, 1 g p-aminomethylbenzoic acid, 30 ml deionized water, 0.6 g H 2 SO 4 , airtight, vacuumed to -0.08 Mpa, and then replace the air with nitrogen twice, nitrogen twice with hydrogen, heat the reaction temperature to 60°C, hydrogen pressure 0.5 MPa, reaction time 4 h, the conversion of p-aminomethylbenzoic acid is 99.3%; the prepared p-aminomethylcyclohexanecarboxylic acid undergoes conformational conversion under alkaline earth metal catalysis at 220°C and 2.0MPa to obtain tranexamic acid.
Embodiment 2
[0027] Catalyst preparation: 0.135g H 2 PtCl 6 and 0.4g Ni(NO 3 ) 2 Dissolve in 30ml of water, soak 1g of activated carbon in this solution for 12 hours, heat to 100°C, add KBH dropwise 4 The solution is reduced, and after the reduction is completed, it is cooled, filtered, and washed with water to obtain a Pt-Ni / C catalyst.
[0028] Catalytic hydrogenation: Add 0.3 g of the above catalyst, 1 g p-aminomethylbenzoic acid, 30 ml deionized water, 0.6 g H 2 SO 4 , sealed, vacuumed to -0.06 Mpa, and then replaced the air with nitrogen twice, replaced nitrogen twice with hydrogen, heated the reaction temperature to 50 ° C, hydrogen pressure 0.6 MPa, reaction time 3 h, the conversion of p-aminomethylbenzoic acid was 98.6%; the prepared p-aminomethylcyclohexanecarboxylic acid undergoes conformational conversion under alkaline earth metal catalysis at 220°C and 2.5MPa to obtain tranexamic acid.
Embodiment 3
[0030] Catalyst preparation: 0.135g H 2 PtCl 6 and 0.4g Co(NO 3 ) 2 Dissolve in 30ml of water, impregnate 1g of activated carbon in this solution for 12 h, heat to 100°C, add CH 3 COONa solution, cooled after reduction, filtered and washed with water to prepare Pt-Co / C catalyst.
[0031] Catalytic hydrogenation: Add 0.3 g of the above catalyst, 1 g p-aminomethylbenzoic acid, 30 ml deionized water, 0.6 g H 2 SO 4, airtight, vacuum to -0.07 Mpa, and then replace the air with nitrogen twice, nitrogen twice with hydrogen, heat the reaction temperature to 40 ° C, hydrogen pressure 0.5 MPa, reaction time 4 h, the conversion of p-aminomethylbenzoic acid is 97.6%; the prepared p-aminomethylcyclohexanecarboxylic acid undergoes conformational transformation under the catalysis of alkaline earth metals at 220°C and 2.2MPa to obtain tranexamic acid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com