Intestinal disease treatment pharmaceutical composition and preparation method thereof
A technology of composition and prescription, which is applied in the field of pharmaceutical composition for treating intestinal diseases and its preparation, can solve problems such as incomplete disintegration of polycarbophil calcium, and achieve improved disintegration performance, good disintegration effect, and void space uniform effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
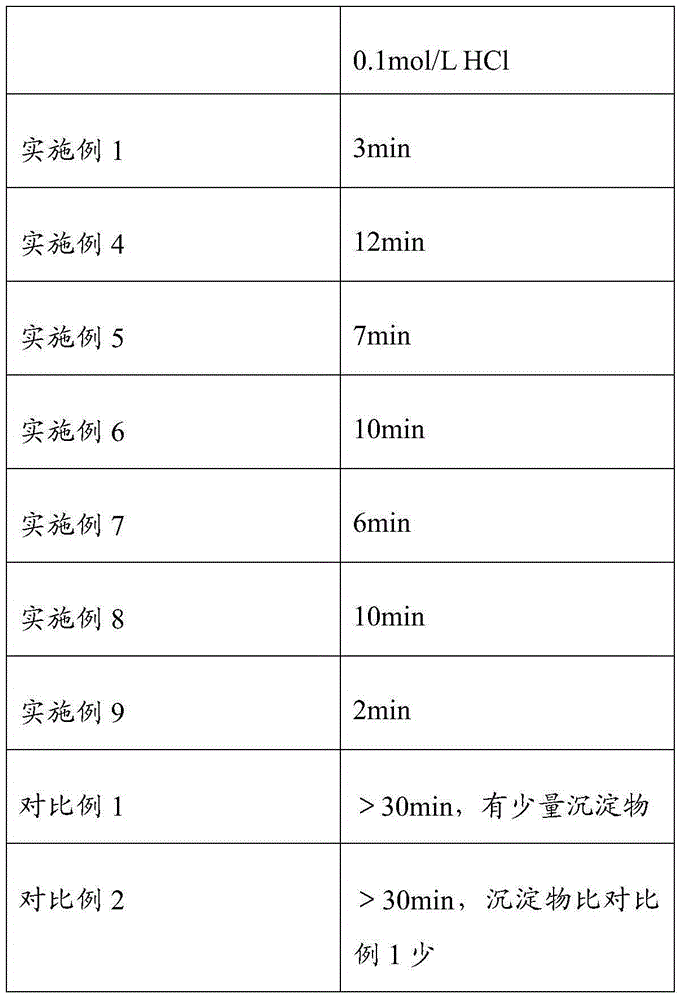
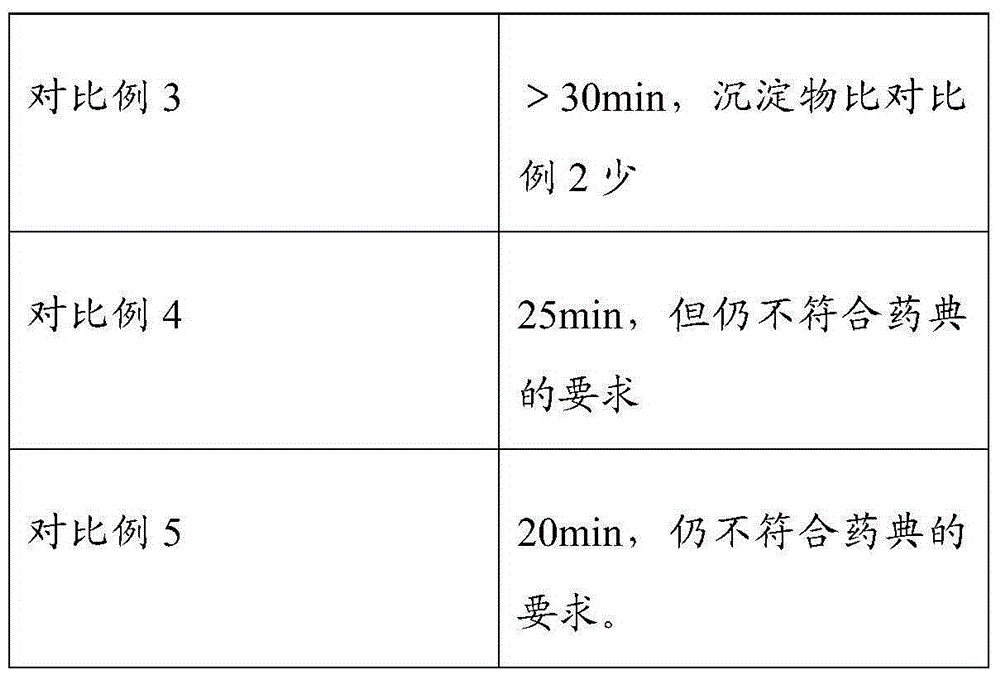
Embodiment 1
[0046] The formulation of polycarbophil calcium composition:
[0047] calcium polycarbophil
100g
16g
[0048] microcrystalline cellulose
33g
1g
6g
[0049] The preparation method of polycarbophil calcium composition tablet is:
[0050] The croscarmellose sodium and polycarbophil calcium of 50% recipe quantity are mixed at room temperature by equal volume increasing method; the preparation mass concentration is 10% propylene glycol aqueous solution, standby, and 10% propylene glycol aqueous solution is added to the above-mentioned mixture Medium granulation, drying and sizing at 60°C, then adding the remaining amount of croscarmellose sodium, prescription amount of microcrystalline cellulose, and magnesium stearate to it, and finally compressing into tablets to obtain tablets .
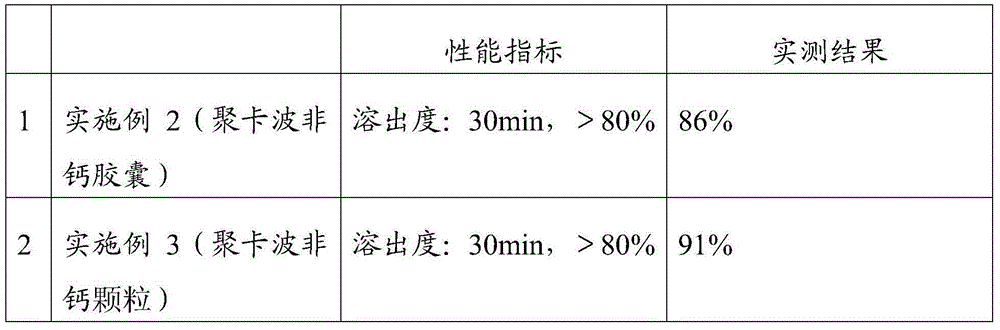
Embodiment 2
[0052] The prescription is the same as in Example 1, and the preparation method is: the croscarmellose sodium and polycarbophil calcium of 50% recipe quantity are mixed at room temperature by an equal increase method; the preparation mass concentration is 10% propylene glycol aqueous solution, Standby, add 10% propylene glycol aqueous solution to the above mixture to granulate, dry and granulate at 60°C, then add the remaining amount of croscarmellose sodium and the prescribed amount of microcrystalline cellulose, hard Magnesium steatate, filled capsules, to obtain calcium polycarbophil capsules.
Embodiment 3
[0054] The prescription is the same as in Example 1, and the preparation method is: the croscarmellose sodium and polycarbophil calcium of 50% recipe quantity are mixed at room temperature by an equal increase method; the preparation mass concentration is 10% propylene glycol aqueous solution, Standby, add 10% propylene glycol aqueous solution to the above mixture to granulate, dry and granulate at 60°C, then add the remaining amount of croscarmellose sodium and the prescribed amount of microcrystalline cellulose, hard Magnesium fatty acid, finally divided into granules.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com